Abstract
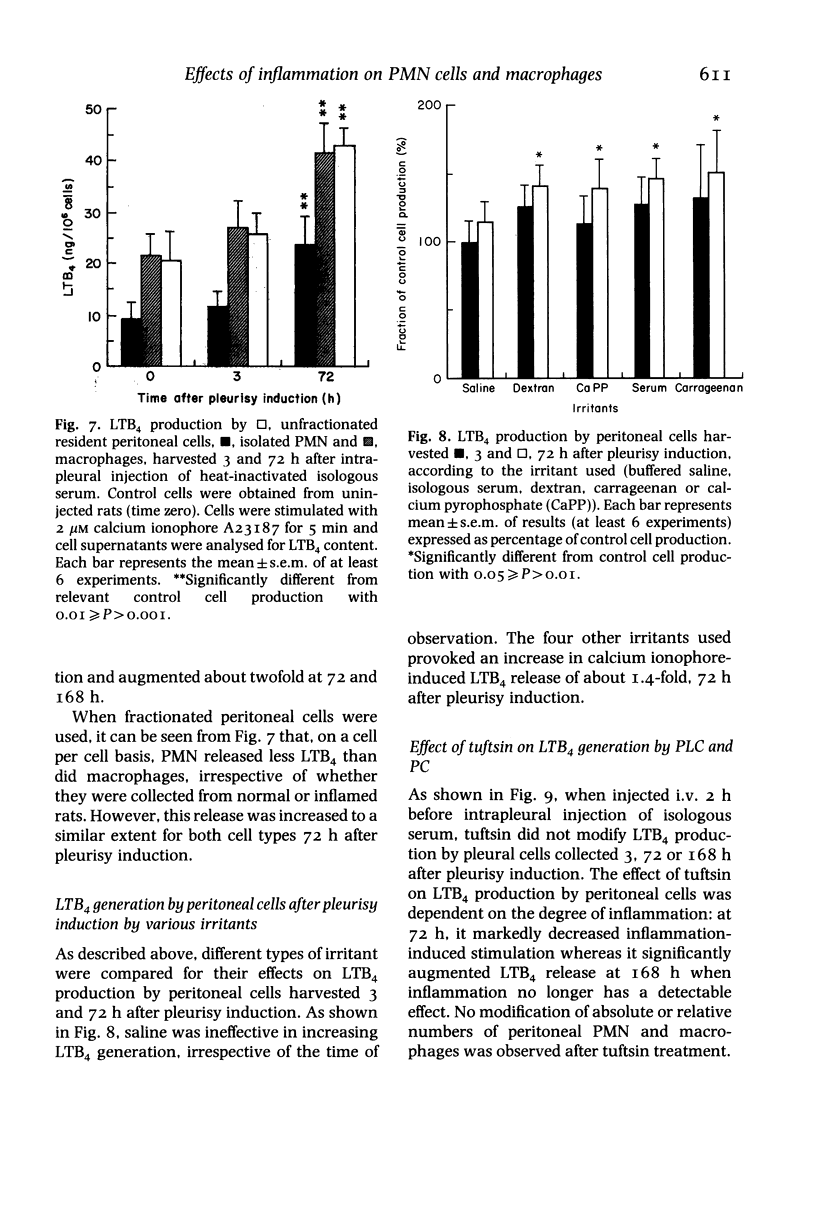
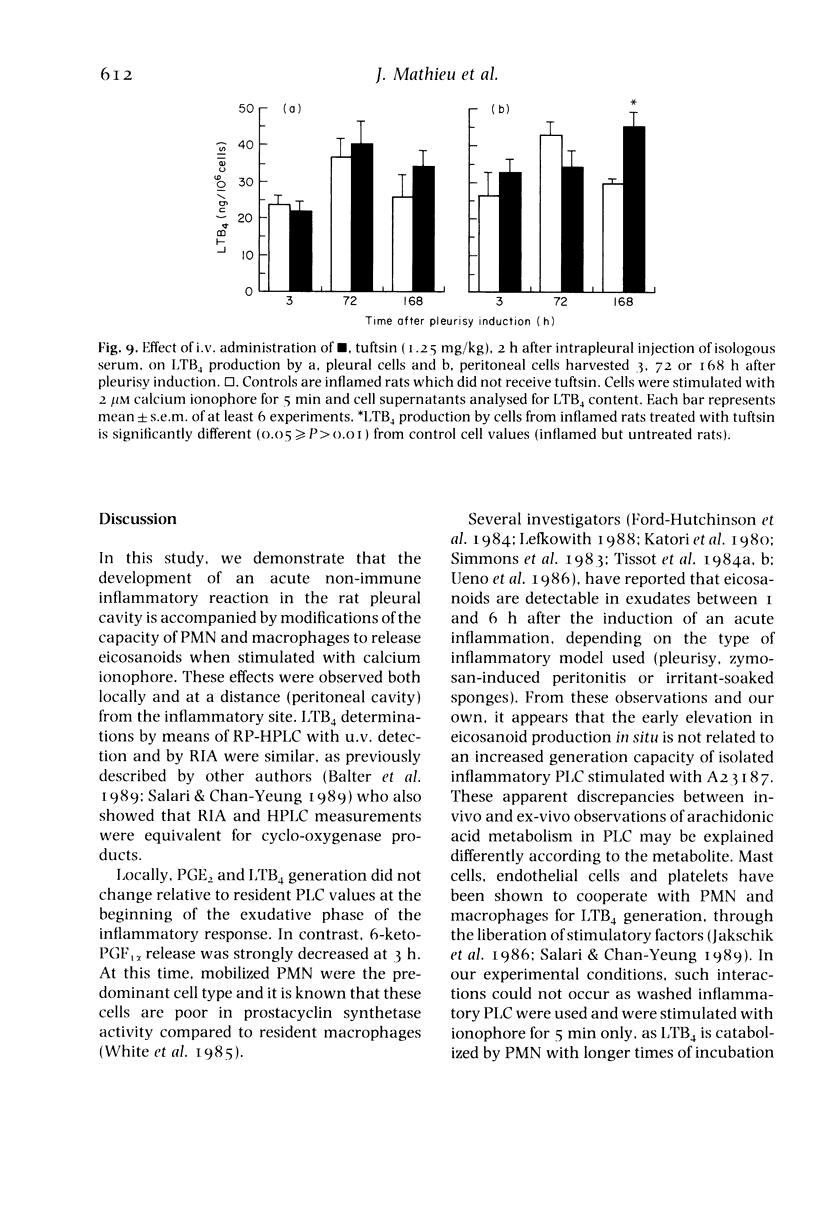
Acute non-specific inflammation was induced in rats by injection of isologous serum into the pleural cavity. Pleural and peritoneal cells were collected at various times after pleurisy induction and tested for production of leukotriene B4 (LTB4), prostaglandin E2 (PGE2) and prostacyclin (PGI2) after in-vitro stimulation with calcium ionophore A23187. Cells obtained by lavage of pleural and peritoneal cavities of normal rats were used as controls. Increased production of LTB4, PGE2 and PGI2 by pleural cells was observed 3 days after pleurisy induction, but with a significant depression of PGI2 release at 3 h. As the relative proportions of polymorphonuclear cells (PMN) and macrophages in the inflammatory exudate varied during the development of inflammation, these cells were examined separately for LTB4 production. PMN and macrophages contributed equally to the liberation of this mediator in normal and inflamed rats. Similar qualitative and quantitative changes in LTB4 production by pleural cells were observed, irrespective of the type of irritant used (isologous serum, dextran, carrageenan, microcrystals). In contrast, intrapleural injection of saline had no significant effect. In order to determine whether local inflammation may influence mediator release by phagocytic cells at remote sites, peritoneal cells were collected 3 or 72 after pleurisy induction. The production of LTB4, PGE2 and PGI2 was increased at 72 h. Mediator production by peritoneal macrophages was observed in both normal and inflamed rats. In conclusion, acute non-specific inflammation provoked increased arachidonic acid metabolite generation by phagocytes both locally and at a distance: this occurred more than 24 h after pleurisy resolution.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balter M. S., Toews G. B., Peters-Golden M. Different patterns of arachidonate metabolism in autologous human blood monocytes and alveolar macrophages. J Immunol. 1989 Jan 15;142(2):602–608. [PubMed] [Google Scholar]
- Bird J., Giroud J. P. The reactivity of neutrophils at the site of an acute inflammatory reaction as measured by chemiluminescence. Agents Actions. 1984 Oct;15(3-4):349–355. doi: 10.1007/BF01972370. [DOI] [PubMed] [Google Scholar]
- Bird J., Pelletier M., Tissot M., Giroud J. P. The modification of the oxidative metabolism of cells derived both locally and at distance from the site of an acute inflammatory reaction. J Leukoc Biol. 1985 Jan;37(1):109–120. doi: 10.1002/jlb.37.1.109. [DOI] [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Arachidonic acid metabolism in polymorphonuclear leukocytes: effects of ionophore A23187. Proc Natl Acad Sci U S A. 1979 May;76(5):2148–2152. doi: 10.1073/pnas.76.5.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braquet M., Lavaud P., Dormont D., Garay R., Ducousso R., Guilbaud J., Chignard M., Borgeat P., Braquet P. Leukocytic functions in burn-injured patients. Prostaglandins. 1985 May;29(5):747–764. doi: 10.1016/0090-6980(85)90135-2. [DOI] [PubMed] [Google Scholar]
- Bray M. A., Cunningham F. M., Ford-Hutchinson A. W., Smith M. J. Leukotriene B4: a mediator of vascular permeability. Br J Pharmacol. 1981 Mar;72(3):483–486. doi: 10.1111/j.1476-5381.1981.tb11000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florentin I., Pelletier M., Giroud J. P. Modification of thymo-dependent and thymo-independent antibody responses during non-specific acute inflammation. Int J Immunopharmacol. 1984;6(2):93–98. doi: 10.1016/0192-0561(84)90002-x. [DOI] [PubMed] [Google Scholar]
- Ford-Hutchinson A. W., Bray M. A., Doig M. V., Shipley M. E., Smith M. J. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980 Jul 17;286(5770):264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- Ford-Hutchinson A. W., Brunet G., Savard P., Charleson S. Leukotriene B4, polymorphonuclear leukocytes and inflammatory exudates in the rat. Prostaglandins. 1984 Jul;28(1):13–27. doi: 10.1016/0090-6980(84)90110-2. [DOI] [PubMed] [Google Scholar]
- Fridkin M., Najjar V. A. Tuftsin: its chemistry, biology, and clinical potential. Crit Rev Biochem Mol Biol. 1989;24(1):1–40. doi: 10.3109/10409238909082550. [DOI] [PubMed] [Google Scholar]
- Goldyne M. E., Burrish G. F., Poubelle P., Borgeat P. Arachidonic acid metabolism among human mononuclear leukocytes. Lipoxygenase-related pathways. J Biol Chem. 1984 Jul 25;259(14):8815–8819. [PubMed] [Google Scholar]
- Goodwin J. S., Ceuppens J. Regulation of the immune response by prostaglandins. J Clin Immunol. 1983 Oct;3(4):295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- Laviolette M., Carreau M., Coulombe R., Cloutier D., Dupont P., Rioux J., Braquet P., Borgeat P. Metabolism of arachidonic acid through the 5-lipoxygenase pathway in normal human peritoneal macrophages. J Immunol. 1988 Sep 15;141(6):2104–2109. [PubMed] [Google Scholar]
- Lefkowith J. B. Essential fatty acid deficiency inhibits the in vivo generation of leukotriene B4 and suppresses levels of resident and elicited leukocytes in acute inflammation. J Immunol. 1988 Jan 1;140(1):228–233. [PubMed] [Google Scholar]
- Newcombe D. S. Leukotrienes: regulation of biosynthesis, metabolism, and bioactivity. J Clin Pharmacol. 1988 Jun;28(6):530–549. doi: 10.1002/j.1552-4604.1988.tb03173.x. [DOI] [PubMed] [Google Scholar]
- Tissot M., Bonne C., Martin B., Solier M., Giroud J. P. Prostacyclin and thromboxanes in carrageenan-induced pleurisy in the rat. Agents Actions. 1984 Jan;14(1):76–81. doi: 10.1007/BF01966837. [DOI] [PubMed] [Google Scholar]
- Tissot M., D'Asniere M., Solier M., Giroud J. P., Engler R. Study of the evolution of acute phase reactants and of thromboxane and prostacyclin during calcium pyrophosphate-induced pleurisy in the rat. Agents Actions. 1984 Jan;14(1):82–87. doi: 10.1007/BF01966838. [DOI] [PubMed] [Google Scholar]
- Tissot M., Strzalko S., Thuret A., Giroud J. P. Prostanoid release by macrophages at a distance from an inflammatory site. Br J Exp Pathol. 1989 Oct;70(5):525–531. [PMC free article] [PubMed] [Google Scholar]
- Ueno A., Tanaka K., Katori M. Difference in the in vitro metabolism of leukotrienes in the exudates from allergic and nonallergic rat pleurisies. Prostaglandins. 1986 May;31(5):833–850. doi: 10.1016/0090-6980(86)90017-1. [DOI] [PubMed] [Google Scholar]
- White H. L., Faison L. D., Truax J. F., Selph J. L., Vinegar R. Arachidonate metabolic pathways in cells harvested from rat pleural cavity at various times after carrageenan administration. Prostaglandins Leukot Med. 1985 Oct;20(1):1–9. doi: 10.1016/0262-1746(85)90089-7. [DOI] [PubMed] [Google Scholar]


