Abstract
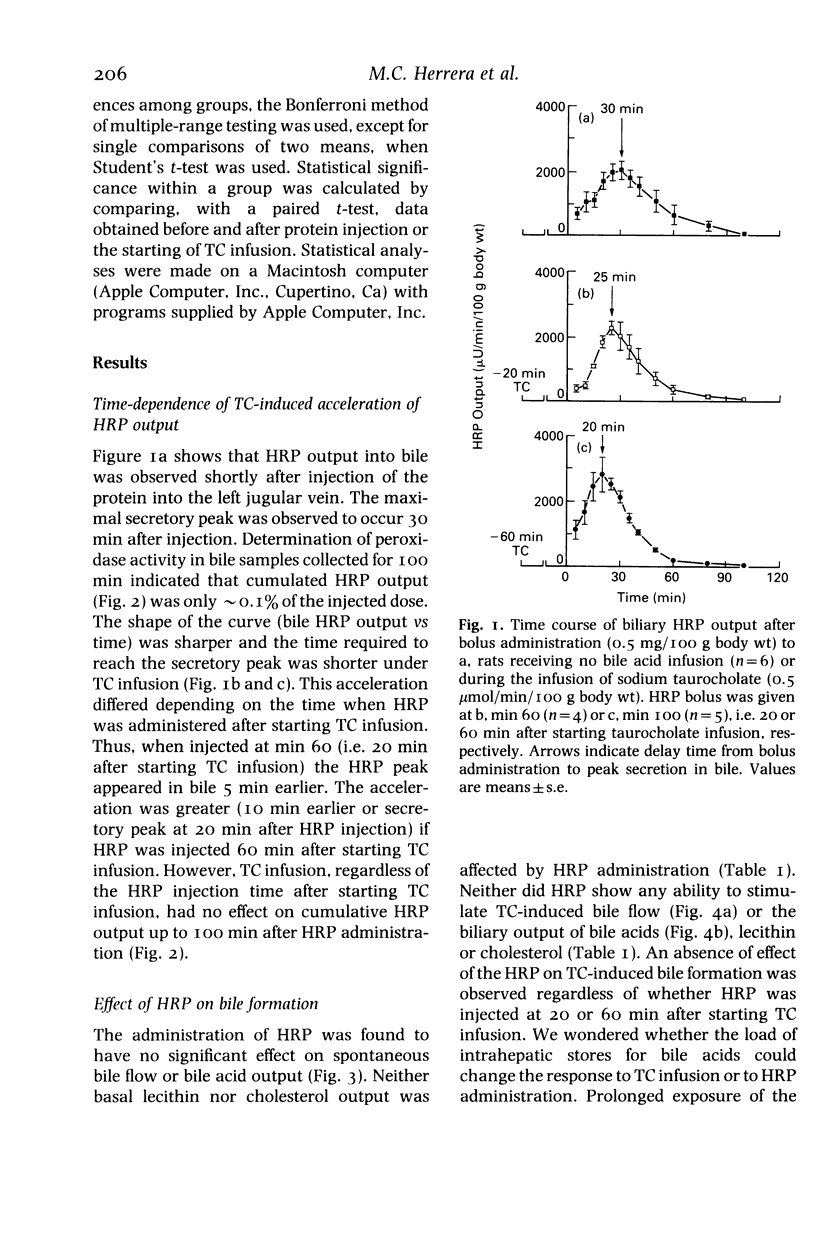
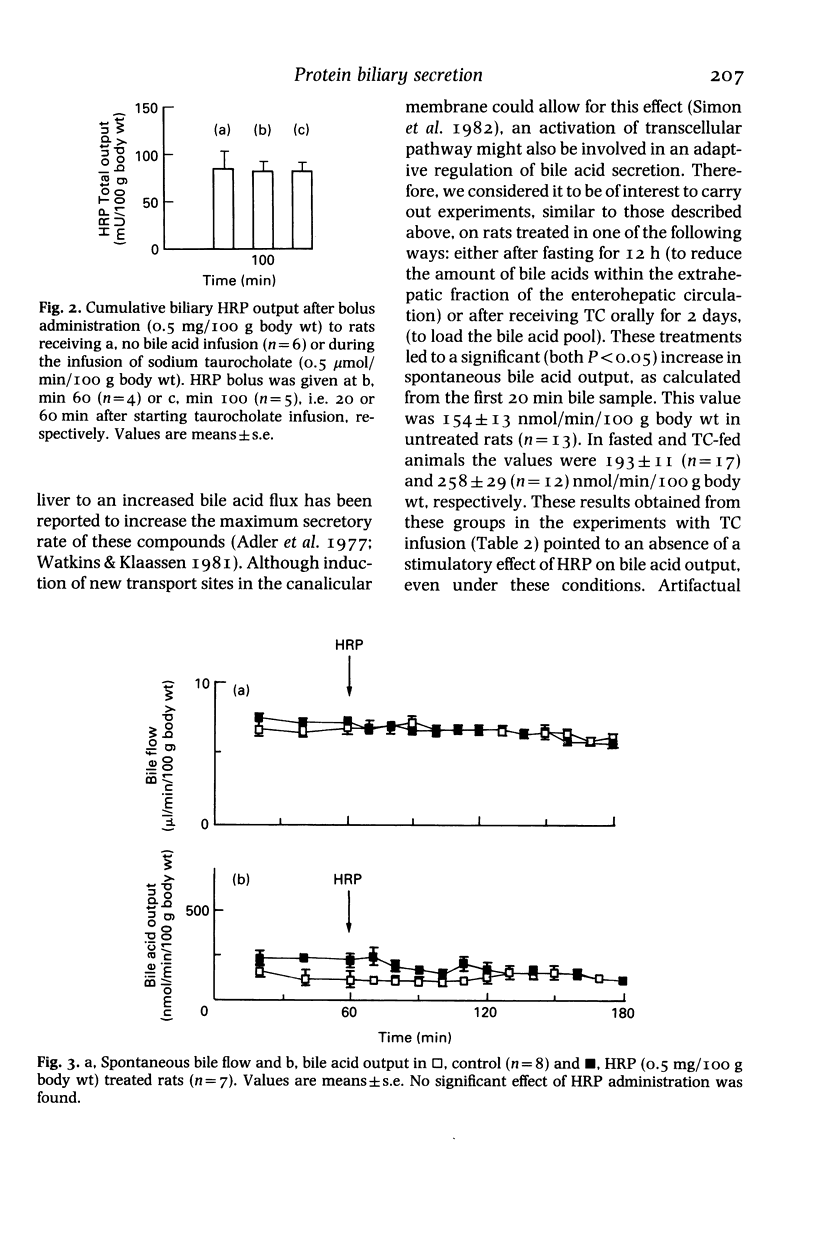
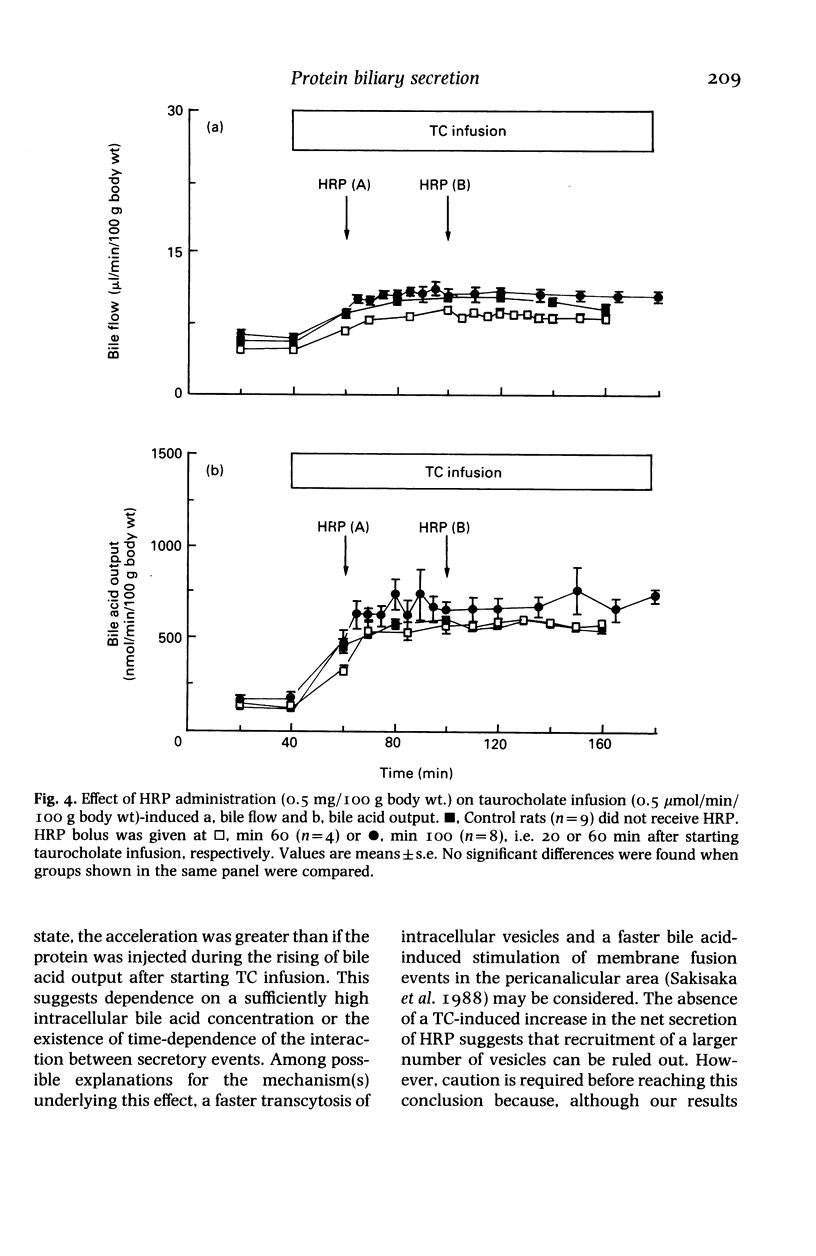
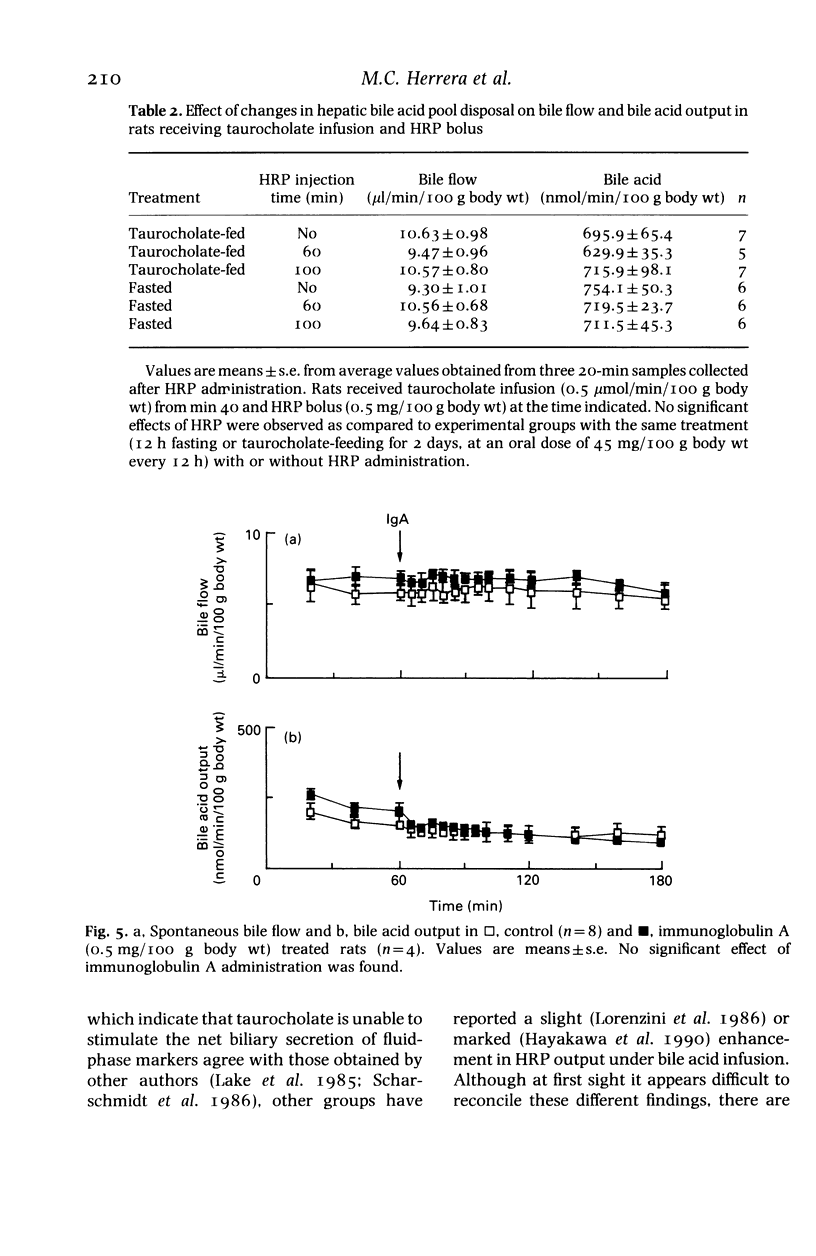
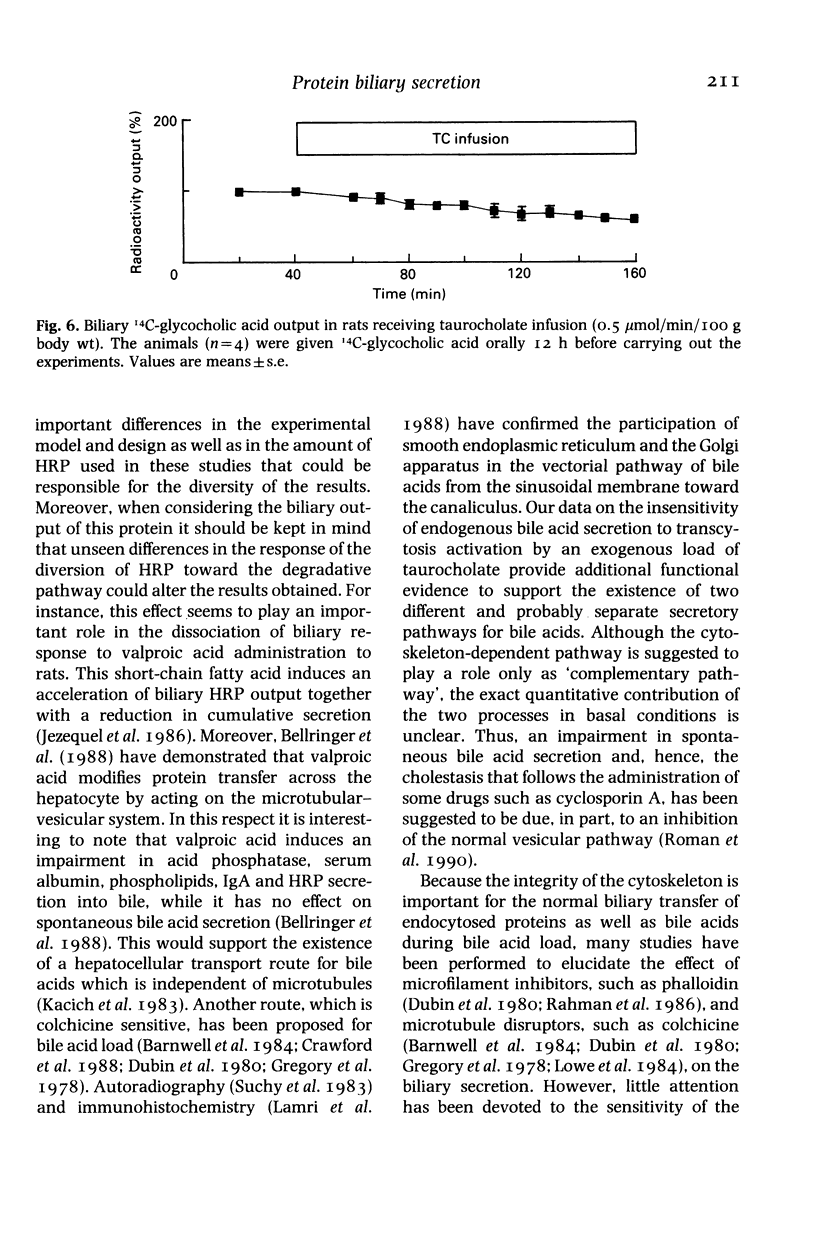
Experiments on the relationship between the hepatocellular transport of endogenous or exogenously loaded bile acids (sodium taurocholate, TC, 0.5 mumol/min/100 g body wt) and horseradish peroxidase (HRP) or immunoglobulin A (IgA) (0.5 mg/100 g body wt) were carried out on anaesthetized Wistar rats. The time course of HRP excretion into bile (acceleration in the secretory peak), but not the total amount of HRP output, was affected by TC infusion. Administration of HRP was found to have no stimulatory effect on either spontaneous or TC-induced bile flow, bile acid, lecithin or cholesterol output. Spontaneous bile acid output was increased (25 and 67%, respectively) in rats that were treated for 12-h fasting or by oral administration of TC (45 mg/100 g body wt, every 12 h, for 2 days). These manoeuvres did not change the inability of HRP and IgA to increase bile acid output. Exogenous TC load had no stimulatory effect on the hepatocellular transport of endogenous bile acid pool, that was labelled by a combination of fasting and oral administration of 14C-glycocholic acid 12 h before the experiments. Therefore, exogenous bile acid load-induced stimulation of transcytosis had no effect on endogenous bile acid output. Moreover, bile secretion of both endogenous and exogenously loaded bile acids is unaffected by the administration of proteins, irrespective of whether they are endocytosed by a receptor or nonreceptor mediated process.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler R. D., Wannagat F. J., Ockner R. K. Bile secretion in selective biliary obstruction. Adaptation of taurocholate transport maximum to increased secretory load in the rat. Gastroenterology. 1977 Jul;73(1):129–136. [PubMed] [Google Scholar]
- Barnwell S. G., Lowe P. J., Coleman R. The effects of colchicine on secretion into bile of bile salts, phospholipids, cholesterol and plasma membrane enzymes: bile salts are secreted unaccompanied by phospholipids and cholesterol. Biochem J. 1984 Jun 15;220(3):723–731. doi: 10.1042/bj2200723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton C. H., Nicholls J. S., Heaton K. W. Estimation of cholesterol in bile: assessment of an enzymatic method. Clin Chim Acta. 1980 Aug 4;105(2):225–230. doi: 10.1016/0009-8981(80)90464-7. [DOI] [PubMed] [Google Scholar]
- Coleman R. Biochemistry of bile secretion. Biochem J. 1987 Jun 1;244(2):249–261. doi: 10.1042/bj2440249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin M., Maurice M., Feldmann G., Erlinger S. Influence of colchicine and phalloidin on bile secretion and hepatic ultrastructure in the rat. Possible interaction between microtubules and microfilaments. Gastroenterology. 1980 Oct;79(4):646–654. [PubMed] [Google Scholar]
- Gebhardt R. Primary cultures of rat hepatocytes as a model system of canalicular development, biliary secretion, and intrahepatic cholestasis. III. Properties of the biliary transport of immunoglobulin A revealed by immunofluorescence. Gastroenterology. 1983 Jun;84(6):1462–1470. [PubMed] [Google Scholar]
- Hashieh I. A., Rémy L., Mathieu S., Gérolami A. The effects of monensin on the transport of horseradish peroxidase into intracellular lumina in cultured rat hepatocytes. Hepatology. 1989 Jul;10(1):61–65. doi: 10.1002/hep.1840100113. [DOI] [PubMed] [Google Scholar]
- Lowe P. J., Barnwell S. G., Coleman R. Rapid kinetic analysis of the bile-salt-dependent secretion of phospholipid, cholesterol and a plasma-membrane enzyme into bile. Biochem J. 1984 Sep 15;222(3):631–637. doi: 10.1042/bj2220631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman K., Hammond T. G., Lowe P. J., Barnwell S. G., Clark B., Coleman R. Control of biliary phospholipid secretion. Effect of continuous and discontinuous infusion of taurocholate on biliary phospholipid secretion. Biochem J. 1986 Mar 1;234(2):421–427. doi: 10.1042/bj2340421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renston R. H., Maloney D. G., Jones A. L., Hradek G. T., Wong K. Y., Goldfine I. D. Bile secretory apparatus: evidence for a vesicular transport mechanism for proteins in the rat, using horseradish peroxidase and [125I]insulin. Gastroenterology. 1980 Jun;78(6):1373–1388. [PubMed] [Google Scholar]
- Román I. D., Monte M. J., Gonzalez-Buitrago J. M., Esteller A., Jiménez R. Inhibition of hepatocytary vesicular transport by cyclosporin A in the rat: relationship with cholestasis and hyperbilirubinemia. Hepatology. 1990 Jul;12(1):83–91. doi: 10.1002/hep.1840120114. [DOI] [PubMed] [Google Scholar]
- Scharschmidt B. F., Lake J. R., Renner E. L., Licko V., Van Dyke R. W. Fluid phase endocytosis by cultured rat hepatocytes and perfused rat liver: implications for plasma membrane turnover and vesicular trafficking of fluid phase markers. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9488–9492. doi: 10.1073/pnas.83.24.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Suchy F. J., Balistreri W. F., Hung J., Miller P., Garfield S. A. Intracellular bile acid transport in rat liver as visualized by electron microscope autoradiography using a bile acid analogue. Am J Physiol. 1983 Nov;245(5 Pt 1):G681–G689. doi: 10.1152/ajpgi.1983.245.5.G681. [DOI] [PubMed] [Google Scholar]
- TALALAY P. Enzymic analysis of steroid hormones. Methods Biochem Anal. 1960;8:119–143. doi: 10.1002/9780470110249.ch3. [DOI] [PubMed] [Google Scholar]
- Takayama M., Itoh S., Nagasaki T., Tanimizu I. A new enzymatic method for determination of serum choline-containing phospholipids. Clin Chim Acta. 1977 Aug 15;79(1):93–98. doi: 10.1016/0009-8981(77)90465-x. [DOI] [PubMed] [Google Scholar]


