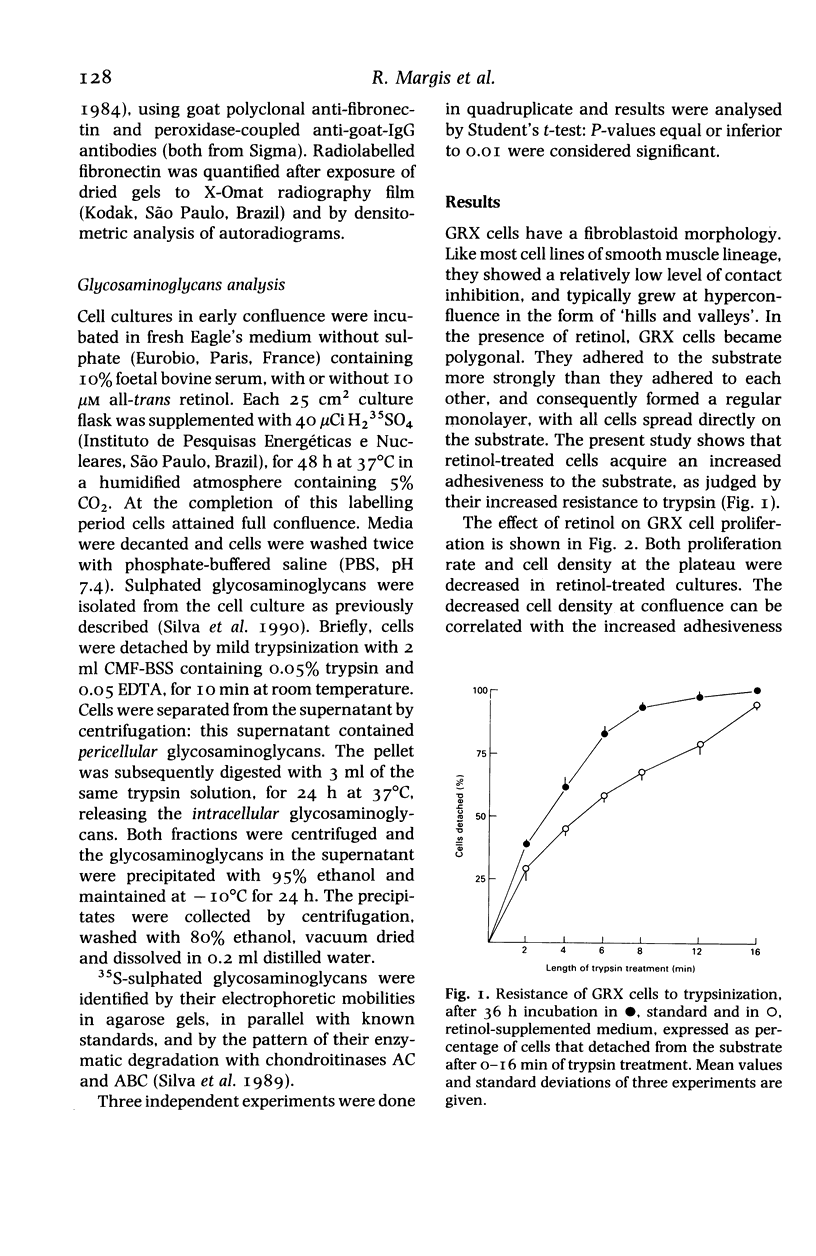
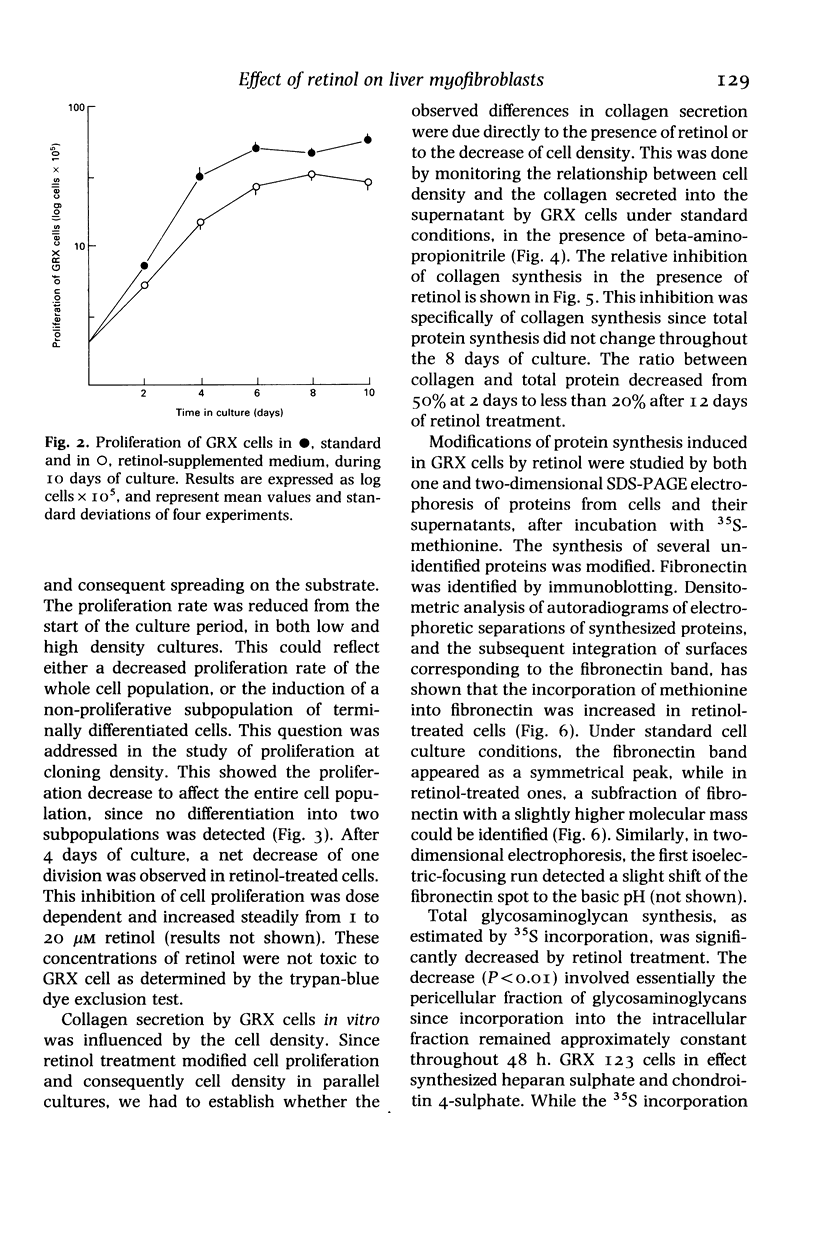
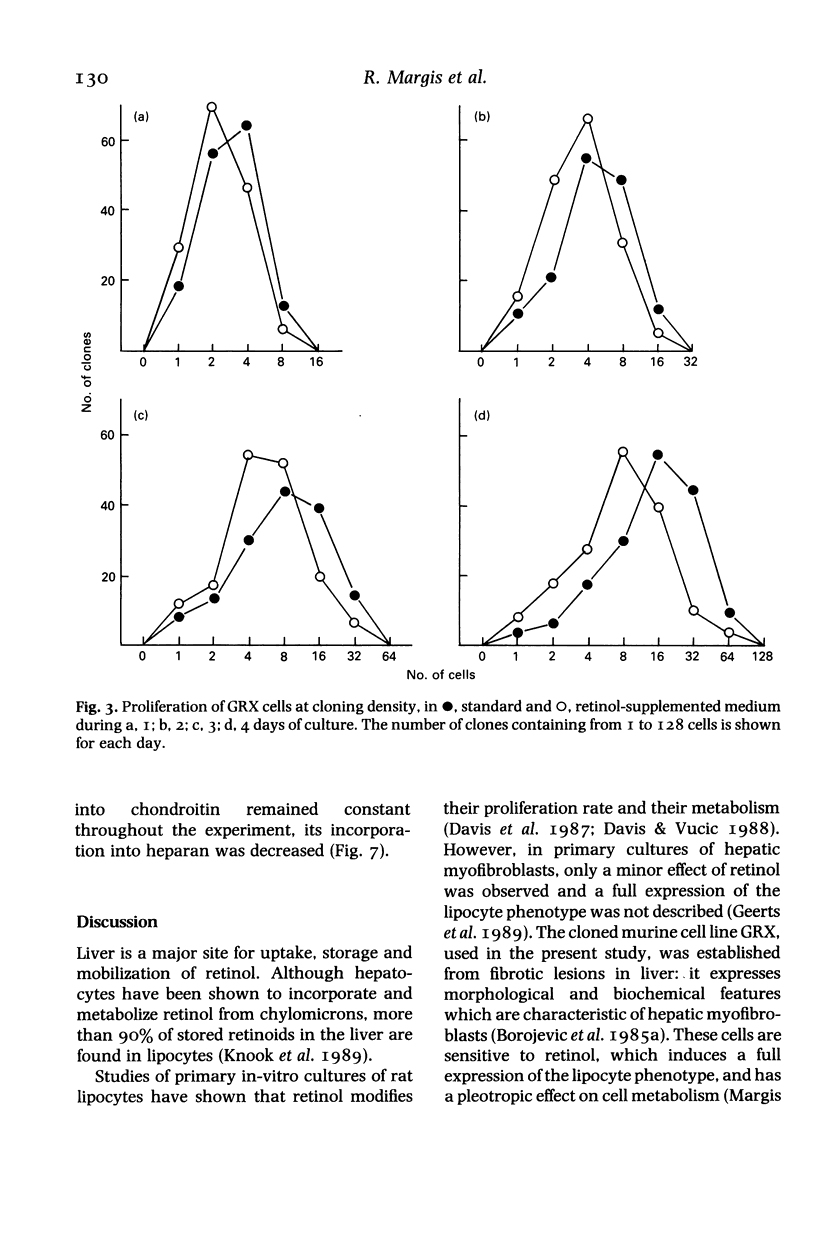
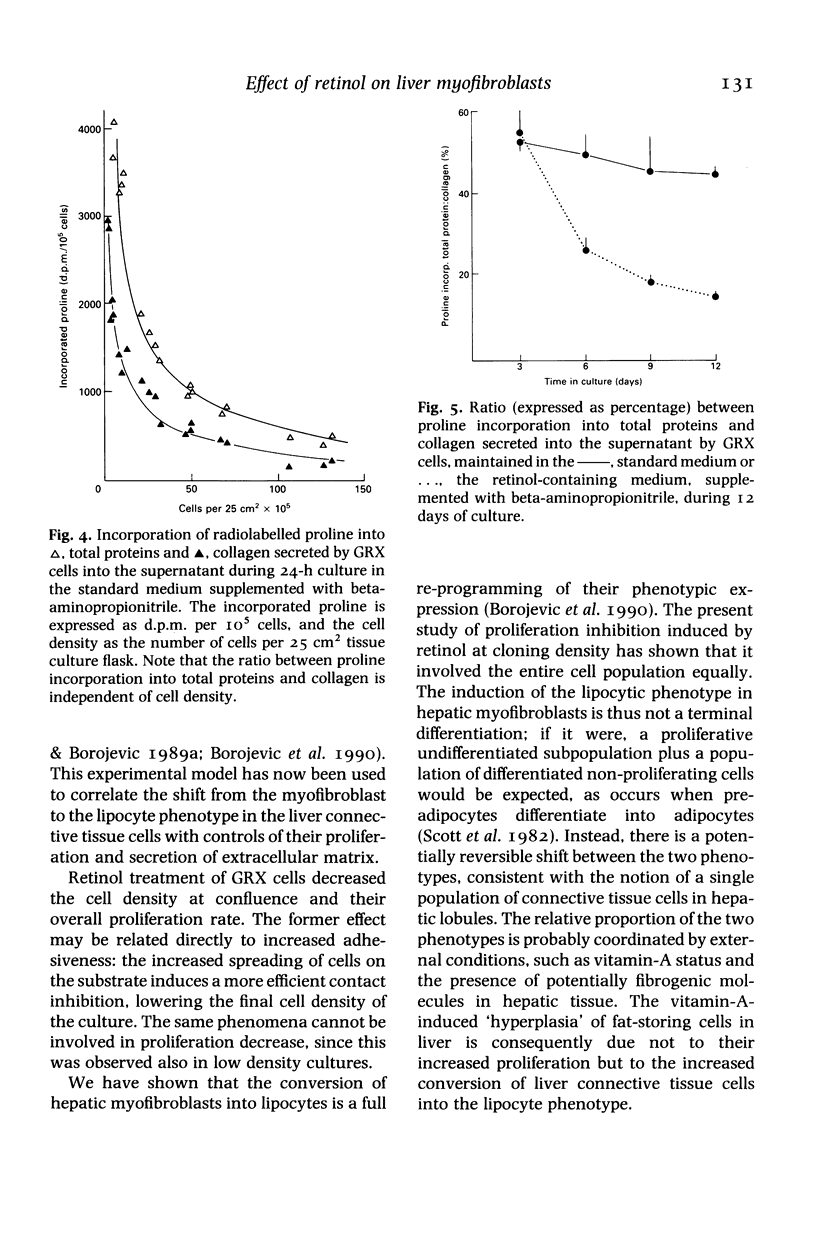
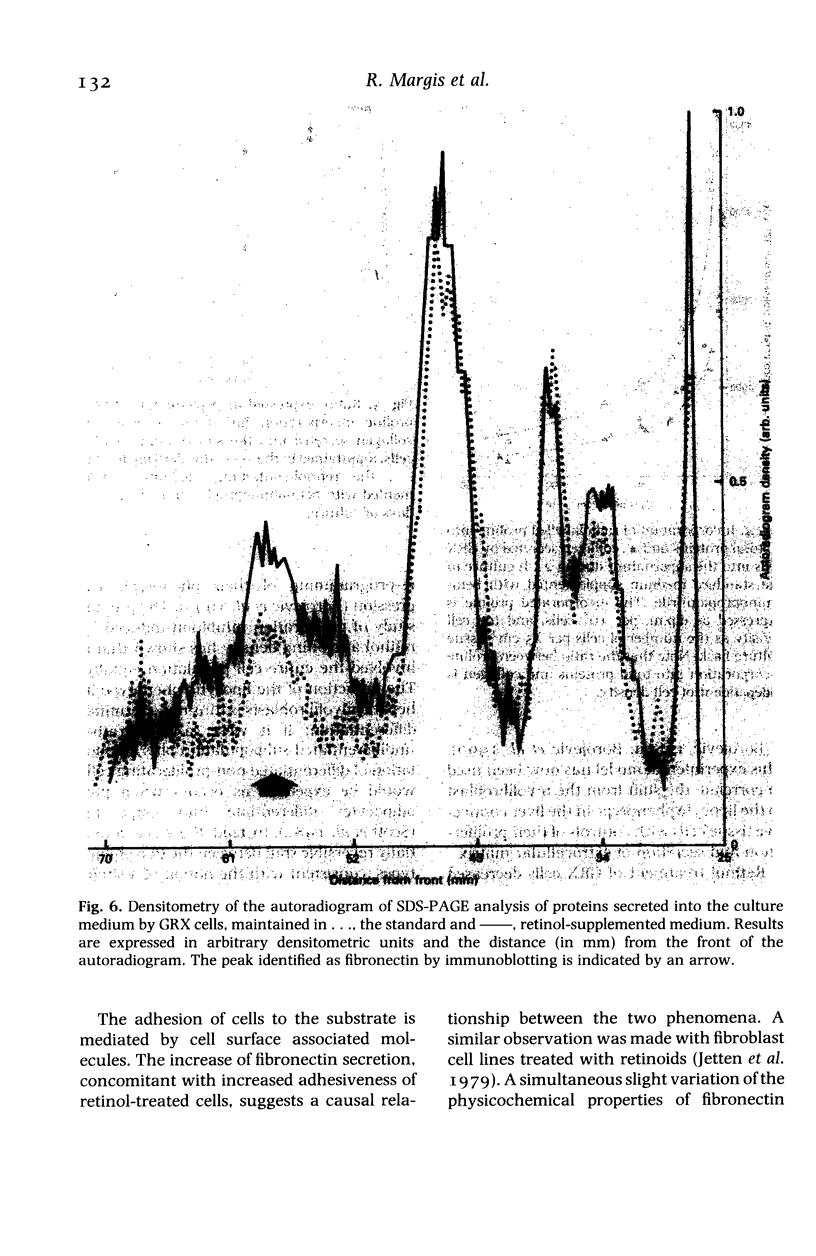
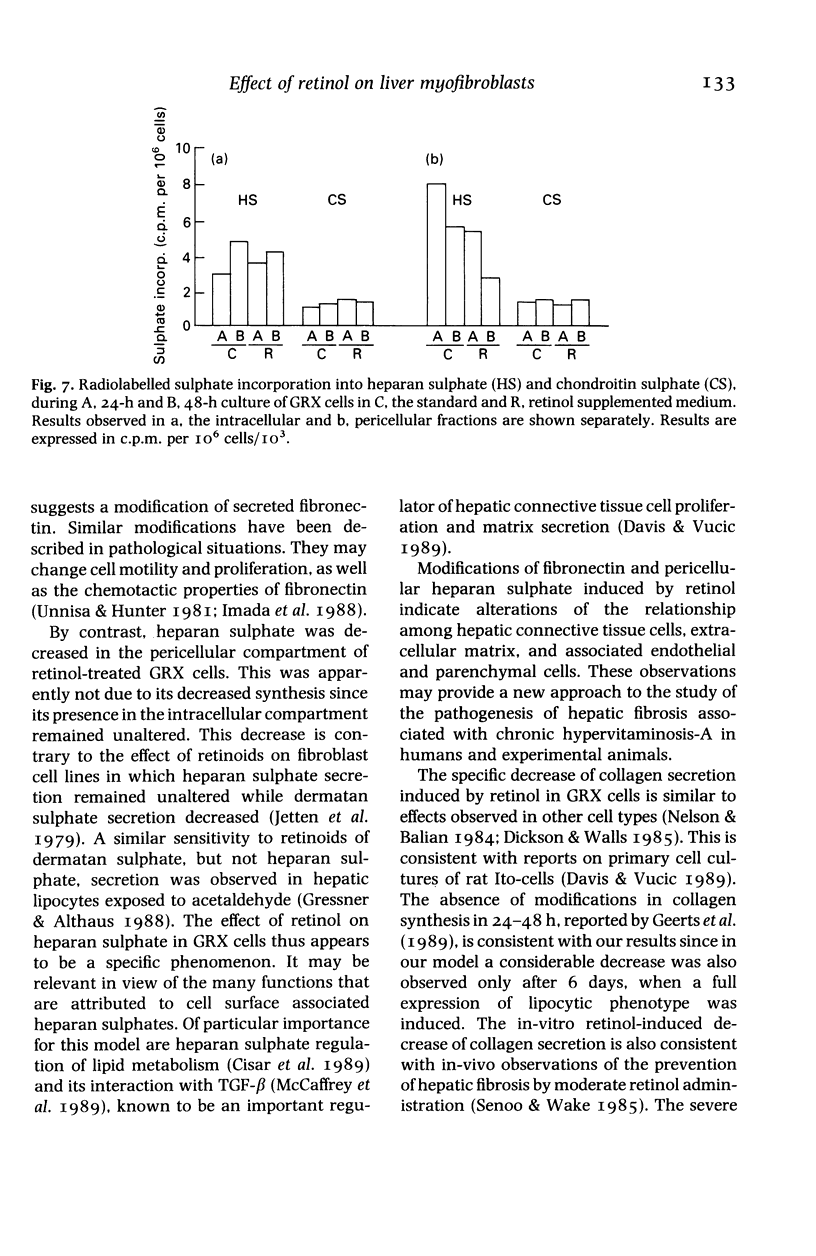
Abstract
We have studied the effect of retinol on an established murine cell line (GRX), representative of liver connective tissue cells. This cell line has myofibroblast characteristics; under retinol treatment it is induced into the lipocyte (Ito-cell) phenotype. Retinol decreased the proliferation rate in the entire cell population. It increased cell adherence to the substrate, which was correlated with the increased secretion of fibronectin. Collagen secretion was specifically decreased, whilst the total protein secretion remained stable. Heparan sulphate was decreased in the pericellular compartment, but other glycosaminoglycans were not affected by retinol treatment. Modulations of pericellular components induced by retinol may alter the relations among liver mesenchymal cells, and may be related to vitamin-A-induced modifications of the homoeostasis of hepatic connective tissue and hepatic fibrosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cisar L. A., Hoogewerf A. J., Cupp M., Rapport C. A., Bensadoun A. Secretion and degradation of lipoprotein lipase in cultured adipocytes. Binding of lipoprotein lipase to membrane heparan sulfate proteoglycans is necessary for degradation. J Biol Chem. 1989 Jan 25;264(3):1767–1774. [PubMed] [Google Scholar]
- Davis B. H., Vucic A. The effect of retinol on Ito cell proliferation in vitro. Hepatology. 1988 Jul-Aug;8(4):788–793. doi: 10.1002/hep.1840080416. [DOI] [PubMed] [Google Scholar]
- Friedman S. L., Roll F. J., Boyles J., Bissell D. M. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressner A. M., Haarmann R. Hyaluronic acid synthesis and secretion by rat liver fat storing cells (perisinusoidal lipocytes) in culture. Biochem Biophys Res Commun. 1988 Feb 29;151(1):222–229. doi: 10.1016/0006-291x(88)90582-7. [DOI] [PubMed] [Google Scholar]
- Imada S., Sugiyama Y., Imada M. Fibronectin phosphorylation by ecto-protein kinase. Exp Cell Res. 1988 Dec;179(2):554–564. doi: 10.1016/0014-4827(88)90293-5. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Jetten M. E., Shapiro S. S., Poon J. P. Characterization of the action of retinoids on mouse fibroblast cell lines. Exp Cell Res. 1979 Mar 15;119(2):289–299. doi: 10.1016/0014-4827(79)90356-2. [DOI] [PubMed] [Google Scholar]
- Kent G., Gay S., Inouye T., Bahu R., Minick O. T., Popper H. Vitamin A-containing lipocytes and formation of type III collagen in liver injury. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3719–3722. doi: 10.1073/pnas.73.10.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mak K. M., Leo M. A., Lieber C. S. Alcoholic liver injury in baboons: transformation of lipocytes to transitional cells. Gastroenterology. 1984 Jul;87(1):188–200. [PubMed] [Google Scholar]
- Margis R., Borojevic R. Retinoid-mediated induction of the fat-storing phenotype in a liver connective tissue cell line (GRX). Biochim Biophys Acta. 1989 Mar 28;1011(1):1–5. doi: 10.1016/0167-4889(89)90069-4. [DOI] [PubMed] [Google Scholar]
- Minato Y., Hasumura Y., Takeuchi J. The role of fat-storing cells in Disse space fibrogenesis in alcoholic liver disease. Hepatology. 1983 Jul-Aug;3(4):559–566. doi: 10.1002/hep.1840030414. [DOI] [PubMed] [Google Scholar]
- Monteiro A. N., Borojevic R. In vitro formation of fibrous septa by liver connective tissue cells. In Vitro Cell Dev Biol. 1987 Jan;23(1):10–14. doi: 10.1007/BF02623487. [DOI] [PubMed] [Google Scholar]
- Ramshaw J. A., Bateman J. F., Cole W. G. Precipitation of collagens by polyethylene glycols. Anal Biochem. 1984 Sep;141(2):361–365. doi: 10.1016/0003-2697(84)90056-3. [DOI] [PubMed] [Google Scholar]
- Scott R. E., Hoerl B. J., Wille J. J., Jr, Florine D. L., Krawisz B. R., Yun K. Coupling of proadipocyte growth arrest and differentiation. II. A cell cycle model for the physiological control of cell proliferation. J Cell Biol. 1982 Aug;94(2):400–405. doi: 10.1083/jcb.94.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoo H., Wake K. Suppression of experimental hepatic fibrosis by administration of vitamin A. Lab Invest. 1985 Feb;52(2):182–194. [PubMed] [Google Scholar]
- Silva L. C., Borojevic R., Mourão P. A. Identification of cells responsible for synthesis of sulphated glycosaminoglycans in schistosome-induced hepatic granulomas. Int J Exp Pathol. 1990 Dec;71(6):845–856. [PMC free article] [PubMed] [Google Scholar]
- da Silva L. C., Mourão P. A., Borojevic R. Patterns of sulfated glycosaminoglycan synthesis and accumulation in hepatic granulomas induced by schistosomal infection. Exp Mol Pathol. 1989 Jun;50(3):411–420. doi: 10.1016/0014-4800(89)90049-x. [DOI] [PubMed] [Google Scholar]