Abstract
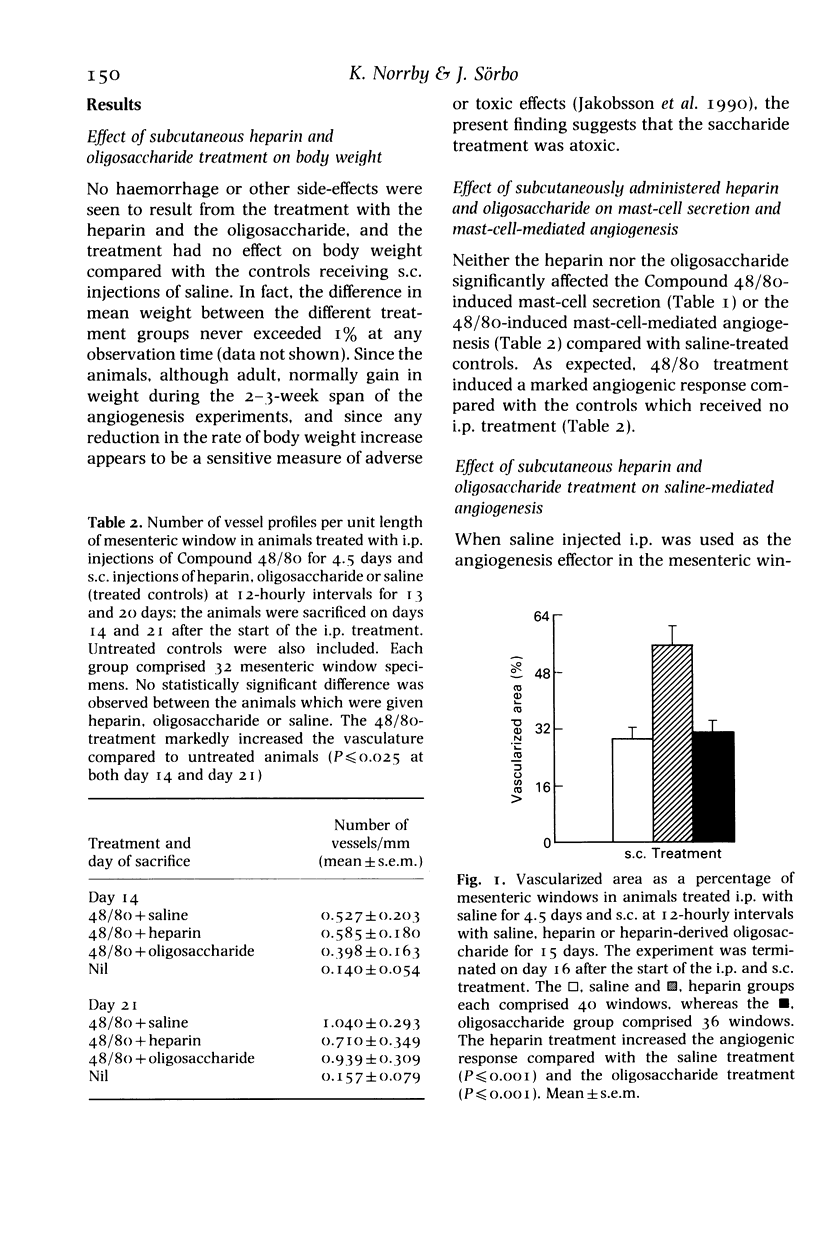
A systemically-administered standard sodium heparin, but not an oligosaccharide fraction derived from the heparin, significantly potentiated angiogenesis induced by saline in normal rats, as assessed by the quantitative mesenteric window angiogenesis assay. This is the first unambiguous evidence that any single specific mast-cell product can potentiate angiogenesis in normally vascularized mammalian tissue. Whether systemic treatment with a heparin-like substance may be useful for stimulating neoangiogenic formation of collaterals in situations of relative microvascular insufficiency, such as coronary collaterals in patients suffering from ischaemic heart disease, is briefly discussed.
Full text
PDF


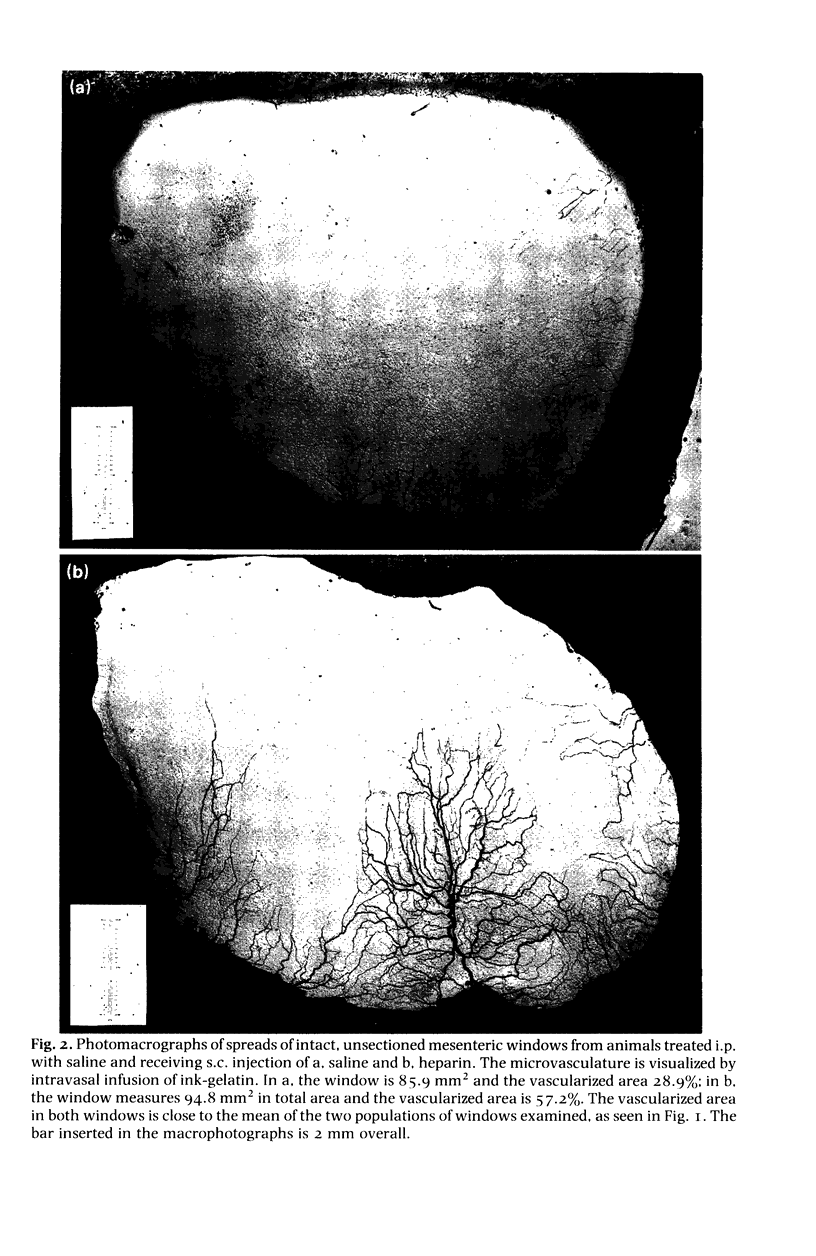




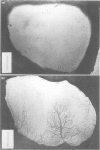
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barzu T., van Rijn J. L., Petitou M., Tobelem G., Caen J. P. Heparin degradation in the endothelial cells. Thromb Res. 1987 Sep 1;47(5):601–609. doi: 10.1016/0049-3848(87)90365-3. [DOI] [PubMed] [Google Scholar]
- Bashkin P., Doctrow S., Klagsbrun M., Svahn C. M., Folkman J., Vlodavsky I. Basic fibroblast growth factor binds to subendothelial extracellular matrix and is released by heparitinase and heparin-like molecules. Biochemistry. 1989 Feb 21;28(4):1737–1743. doi: 10.1021/bi00430a047. [DOI] [PubMed] [Google Scholar]
- Bârzu T., Lormeau J. C., Petitou M., Michelson S., Choay J. Heparin-derived oligosaccharides: affinity for acidic fibroblast growth factor and effect on its growth-promoting activity for human endothelial cells. J Cell Physiol. 1989 Sep;140(3):538–548. doi: 10.1002/jcp.1041400320. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Addonizio M. L., Rosenberg R., Karnovsky M. J. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981 Aug;90(2):372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Beeler D. L., Rosenberg R. D., Karnovsky M. J. Structural determinants of the capacity of heparin to inhibit the proliferation of vascular smooth muscle cells. J Cell Physiol. 1984 Sep;120(3):315–320. doi: 10.1002/jcp.1041200309. [DOI] [PubMed] [Google Scholar]
- Dryjski M., Mikat E., Bjornsson T. D. Inhibition of intimal hyperplasia after arterial injury by heparins and heparinoid. J Vasc Surg. 1988 Nov;8(5):623–633. doi: 10.1067/mva.1988.avs0080623. [DOI] [PubMed] [Google Scholar]
- Edelman E. R., Adams D. H., Karnovsky M. J. Effect of controlled adventitial heparin delivery on smooth muscle cell proliferation following endothelial injury. Proc Natl Acad Sci U S A. 1990 May;87(10):3773–3777. doi: 10.1073/pnas.87.10.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Weisz P. B., Joullié M. M., Li W. W., Ewing W. R. Control of angiogenesis with synthetic heparin substitutes. Science. 1989 Mar 17;243(4897):1490–1493. doi: 10.1126/science.2467380. [DOI] [PubMed] [Google Scholar]
- Fujita M., Sasayama S., Asanoi H., Nakajima H., Sakai O., Ohno A. Improvement of treadmill capacity and collateral circulation as a result of exercise with heparin pretreatment in patients with effort angina. Circulation. 1988 May;77(5):1022–1029. doi: 10.1161/01.cir.77.5.1022. [DOI] [PubMed] [Google Scholar]
- Galloway A. C., Pelletier R., D'Amore P. A. Do ischemic hearts stimulate endothelial cell growth? Surgery. 1984 Aug;96(2):435–439. [PubMed] [Google Scholar]
- Hoover R. L., Rosenberg R., Haering W., Karnovsky M. J. Inhibition of rat arterial smooth muscle cell proliferation by heparin. II. In vitro studies. Circ Res. 1980 Oct;47(4):578–583. doi: 10.1161/01.res.47.4.578. [DOI] [PubMed] [Google Scholar]
- Jakobsson A., Sörbo J., Norrby K. Protamine and mast-cell-mediated angiogenesis in the rat. J Exp Pathol (Oxford) 1990 Apr;71(2):209–217. [PMC free article] [PubMed] [Google Scholar]
- Kumar S., West D., Shahabuddin S., Arnold F., Haboubi N., Reid H., Carr T. Angiogenesis factor from human myocardial infarcts. Lancet. 1983 Aug 13;2(8346):364–368. doi: 10.1016/s0140-6736(83)90343-4. [DOI] [PubMed] [Google Scholar]
- Norrby K., Jakobsson A., Sörbo J. Age-dependent mast-cell-mediated angiogenesis. APMIS Suppl. 1988;2:251–261. [PubMed] [Google Scholar]
- Rosengart T. K., Johnson W. V., Friesel R., Clark R., Maciag T. Heparin protects heparin-binding growth factor-I from proteolytic inactivation in vitro. Biochem Biophys Res Commun. 1988 Apr 15;152(1):432–440. doi: 10.1016/s0006-291x(88)80732-0. [DOI] [PubMed] [Google Scholar]
- Vigny M., Ollier-Hartmann M. P., Lavigne M., Fayein N., Jeanny J. C., Laurent M., Courtois Y. Specific binding of basic fibroblast growth factor to basement membrane-like structures and to purified heparan sulfate proteoglycan of the EHS tumor. J Cell Physiol. 1988 Nov;137(2):321–328. doi: 10.1002/jcp.1041370216. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I., Folkman J., Sullivan R., Fridman R., Ishai-Michaeli R., Sasse J., Klagsbrun M. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2292–2296. doi: 10.1073/pnas.84.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]