Abstract
Increasing evidence supports involvement of inflammation and immunity in atherogenesis. We report here that CD40 ligand (CD40L), an immunoregulatory signaling molecule heretofore considered largely restricted to recently activated CD4+ T lymphocytes, is expressed by human vascular endothelial cells (EC), smooth muscle cells (SMC), and human macrophages in vitro, and is coexpressed with its receptor CD40 on all three cells types in human atherosclerotic lesions in situ. Cultured human vascular EC, SMC, and human macrophages all constitutively expressed CD40L mRNA as well as protein. Stimulation with interleukin 1β, tumor necrosis factor α, or interferon γ increased surface levels and de novo synthesis of CD40L on all three cell types. CD40L expressed on EC, SMC, and macrophages exhibited biological activity, as it induced B7.2 expression on B cells. Human vascular SMC also constitutively expressed CD40, the receptor for CD40L, and through CD40 signaling, human recombinant CD40L induced expression of proinflammatory cytokines in these cells, identifying SMC as a target for CD40L. Human atherosclerotic lesions (n = 8) showed expression of immunoreactive CD40L on EC, SMC, and macrophages, while normal arterial tissues (n = 5) contained no CD40L. In atheroma CD40L+ cells often also expressed CD40. These observations establish human vascular EC, SMC, and human macrophages as a novel source of CD40L, and point to T cell-independent CD40 signaling, and a broader function of this pathway in regulation of nonimmune cells, as illustrated here by potential autocrine and paracrine activation during atherogenesis.
CD40 ligand (CD40L), also referred as gp39, a cell surface molecule of 30–33 kDa thought restricted to activated CD4+ T cells, interacts with its receptor CD40 to mediate major immunoregulatory functions (1–5). Previous studies of the interactions between CD40L and CD40 have often focused on the role of these leukocyte surface proteins in T and B cell interactions (6, 7). In vivo, interrupting this signaling system by administration of anti-CD40L antibody limits experimental autoimmune diseases such as collagen-induced arthritis, lupus nephritis, and acute or chronic graft-versus-host disease (8–11). Stimulation with CD40L+ cells or purified human recombinant CD40L (rCD40L) induces secretion of various proinflammatory cytokines [e.g., interleukin 1 (IL-1), IL-8, tumor necrosis factor (TNF)] from monocytes (12), dendritic cells (13), fibroblasts (14), or epithelial cells (15), and also augments adhesion molecule expression on monocytes (12) and fibroblasts (14).
Activation of endothelial cells (EC) as well as smooth muscle cells (SMC) and macrophages participate importantly in atherogenesis because considerable evidence supports augmented expression of adhesion molecules and/or secretion of cytokines within human and experimental atheroma (16, 17). Indeed human EC express CD40 and ligation of this receptor activates leukocyte adhesion molecule expression by these cells (18–20). Increased levels of leukocyte adhesion molecules characterize EC and SMC in atherosclerotic lesions; therefore we tested the hypothesis that the CD40–CD40L dyad might participate in this disease. We report here the surprising finding that nonleukocytic cells involved in atherogenesis express functional CD40L, a molecule heretofore considered restricted to activated CD4+ T cells. Furthermore, in situ analysis of human atherosclerotic lesions revealed coexpression of CD40L and CD40 on vascular endothelium and SMC. These findings suggest a previously unsuspected role for CD40–CD40L interactions in this prevalent human disease.
MATERIALS AND METHODS
Reagents.
Human recombinant IL-1β, IL-6, IL-8, TNF-α, and interferon γ (IFN-γ) were obtained from Endogen (Cambridge, MA). Lipopolysaccharide and polymyxin B were purchased from Sigma. rCD40L was a kind gift from Glaxo (21). Anti-human CD40 mAb [fluorescein isothiocyanate (FITC) conjugated and unconjugated] and anti-human B7.2 mAb were obtained from PharMingen and Serotec. Anti-human CD40L mAbs (FITC conjugated and unconjugated) were purchased from Calbiochem and Genzyme. Mouse IgG1 mAb (FITC conjugated) was obtained from PharMingen. Polyclonal anti-CD40 and anti-CD40L antibodies were obtained from Santa Cruz Biotechnology.
Cell Isolation and Culture.
Human vascular EC and SMC were isolated from saphenous veins and cultured as described (22, 23). Culture medium (DMEM, M199, BioWhittaker) and FCS (Atlanta Biologicals) contained <40 pg lipopolysaccharide/ml as determined by chromogenic Limulus amoebocyte assay analysis (QLC-1000; BioWhittaker). EC and SMC were characterized by immunostaining with anti-von Willebrand factor and anti-SMC α-actin antibody (Dako), respectively. We found no evidence for the presence of CD4+ lymphocytes in these cultures by fluorescence-activated cell sorter (FACS) analysis. In some experiments, vascular SMC and EC were cultured 24 h before the experiment in medium lacking FCS: EC in 0.1% human serum albumin, and SMC in insulin/transferrin medium (24).
Monocytes were isolated from freshly prepared human peripheral blood mononuclear cells obtained from leukopacs of healthy donors (kindly provided by Steve K. Clinton, Dana–Farber Cancer Institute), by density gradient centrifugation (25), and subsequent adherence to plastic culture flasks (90 min, 37°C). Monocytes were harvested by scraping and plated at 5 × 105 cells/cm2 on 6- or 96-well plates (Nunc) in RPMI 1640 medium (BioWhittaker) containing 2% human serum (Sigma). The cells were cultured 10 days, and medium was changed after 5 days. Purity of macrophages derived from monocytes after 10 days was ≥98% as determined by FACS analysis (anti-human CD64 mAb, FITC conjugated; PharMingen). For specified studies, macrophages were cultured 24 h before the experiment in RPMI 1640 medium lacking serum.
The Epstein–Barr virus-infected B cell line (IB4) was kindly provided by Elliot Kieff (Brigham and Women’s Hospital, Boston). Freshly isolated CD4+ T cells were a gift from Andrew Lichtman (Brigham and Women’s Hospital).
Isolation of RNA and PCR.
Total RNA from EC, SMC, or macrophages was isolated according to the method of Chomczynski and Sacchi (26), and cDNA was prepared as described previously (22). PCR was performed for 35 cycles at 95°C (120 sec), 62°C (120 sec), and 72°C (180 sec, 2-sec prolongation per cycle) after hot start. The sequences of primers for human CD40 were as follows: antisense, 5′-GGGACCACAGACAACATCAG-3′ (complementary to nt 572–591) (27); and sense, 5′-TGCCAGCCAGGACAGAAACT-3′ (complementary to nt 168–187). Primer sequences for human CD40L were as follows: antisense, 5′-CGGAACTGTGGGTATTT-3′ (complementary to nt 667–683) (3); and sense, 5′-ACTTTTTGCTGTGTATC-3′ (complementary to nt 176–192). The primers were obtained from Integrated DNA Technologies (Coraville, IA). Aliquots of the PCR products were run on 1.3% agarose gels and visualized by UV transillumination.
Western Blotting and Immunoprecipitation Analysis.
Cell extracts (20 × 106 cells/ml) were separated by SDS/PAGE under reducing conditions and blotted to polyvinylidene difluoride membranes (Bio-Rad) using a semidry blotting apparatus (0.8 mA/cm2, 30 min; Bio-Rad). Blots were blocked and dilution of first and second mAb was made in 5% defatted dry milk/PBS/0.1% Tween. After 1 h of incubation with the respective primary antibody, blots were washed three times (PBS/0.1% Tween) and the secondary peroxidase-conjugated goat-anti-mouse antibody (Jackson ImmunoResearch) was added for another hour. Finally, after washing the blots, diaminobenzidine (1 mg/ml; Sigma) resuspended in substrate buffer (17 mM acetic acid/65 mM Na2HPO4/0.01% thimerosal/0.1% H2O2) was added for detection of the proteins. Immunoprecipitation analyses were performed as described (22). Samples were separated on SDS/PAGE and transferred to polyvinylidene difluoride membranes. Blots were dried and exposed to x-ray film for detection of immunoprecipitation.
Flow Cytometry.
Human SMC, EC, or macrophages harvested by scraping were incubated with the FITC-conjugated specific antibody (30 min, 4°C) and analyzed in a Becton Dickinson FACScan flow cytometer. Data were analyzed using cellquest software (Becton Dickinson). For each treatment the mean fluorescence intensity (MFI) value for the control stained population was subtracted from the MFI value of the positive-stained sample. MFI are the geometric mean intensities and refer to values normalized to the log scale. For human macrophage analysis, a gate was used which included only the CD64+ cells.
Cytokine Assays.
Release of cytokines from human vascular SMC, EC, and human macrophages was measured using ELISA kits, as suggested by the manufacturer (Endogen). Experiments were performed in the presence of polymyxin B (1 μg/ml). Antibody binding was detected by adding p-nitrophenyl phosphate (1.39 mg/ml; Sigma), and absorbance was measured at 405 nm in a Dynatech plate reader. The amount of cytokine detected was calculated from a standard curve prepared from the respective recombinant cytokine. Samples were assayed in triplicate.
Immunohistochemistry.
Surgical specimens of human carotid atheroma and aorta were obtained by protocols approved by the Human Investigation Review Committee at the Brigham and Women’s Hospital. Serial cryostat sections (5 μm) were cut, air dried onto microscope slides (Fisher Scientific), and fixed in acetone at −20°C for 5 min. Sections preincubated with PBS containing 0.3% hydrogen peroxidase activity were incubated (60 min) with primary or control (mouse myeloma protein MOPC-21; Sigma) antibody diluted in PBS supplemented with 5% appropriate serum. Finally, sections were incubated with the respective biotinylated secondary antibody (45 min; Vector Laboratories) followed by avidine-biotin-peroxidase complex (Vectastain ABC kit), and antibody binding was visualized with 3-amino-9-ethyl carbazole (Vector Laboratories). Cell types were characterized by immunofluorescence double staining with the respective cell selective antibody [anti-muscle actin mAb for SMC (Enzo Diagnostics), anti-CD31 mAb for EC (Dako), anti-CD68 mAb for macrophages (Dako), and anti-CD3 mAb for T lymphocyte (Dako)], using FITC (cell-specific antibody) and Texas-red (CD40L- or CD40-specific antibody) conjugated streptavidin. Counterstaining was performed with bisbenzamide (white/blue staining).
RESULTS
Expression of CD40L on Cultured Human EC, SMC, and Macrophages as Well as CD40 on SMC.
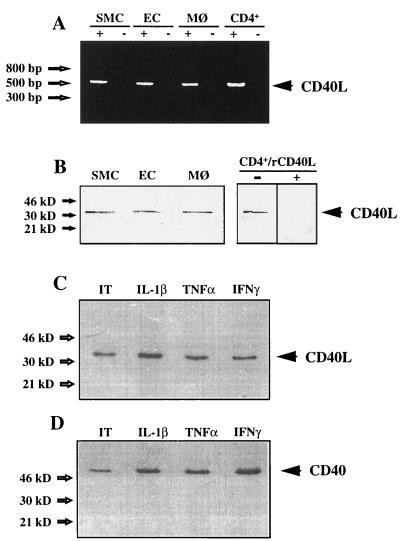
To investigate the expression of both CD40L and CD40 by cells involved in atherosclerosis, we analyzed mRNA and protein expression of these molecules in vitro. RT-PCR of total mRNA derived from cultured human SMC, EC, or macrophages performed with CD40L-specific primers showed products of the expected size (520 bp) (Fig. 1A). Genomic contamination was excluded by the absence of product following PCR of nonreverse transcribed mRNA preparations, and cDNA obtained from phorbol 12-myristate 13-acetate-stimulated CD4+ T cells was used as a positive control. Sequences of PCR products obtained from EC, SMC, and macrophages were identical to T cell-derived CD40L (data not shown). SMC also contained CD40 mRNA as detected by RT-PCR (data not shown). Human vascular EC, SMC, and macrophages expressed CD40L as a cell-associated protein with an apparent molecular mass of 33 kDa (Fig. 1B). The bands comigrated with the immunoreactive protein obtained from activated CD4+ T cells. Anti-CD40L antibody preincubated with human rCD40L no longer bound CD40L on the Western blot, demonstrating the specificity of the antibody (Fig. 1B). Besides EC and macrophages, vascular SMC expressed CD40 as a cell-associated 49-kDa protein. De novo synthesis of CD40L and CD40 was demonstrated by radioimmunoprecipitation analysis of unstimulated as well as IL-1β, TNF-α, or IFN-γ treated cells, as shown for SMC (Fig. 1 C and D). The protein expression was verified by using an independent antibody. These data showed that human vascular EC, SMC, and human macrophages constitutively expressed CD40L and CD40 mRNA, as well as protein in vitro under usual (serum-containing) culture conditions.
Figure 1.
Human vascular SMC, EC, and human macrophages express CD40L and CD40 in vitro. (A) PCR with reverse transcribed total RNA preparations (+) obtained from human vascular SMC, EC, or human macrophages (MØ) resulted in the detection of the CD40L–cDNA product of the expected size (520 bp). Genomic contamination was excluded by PCR of nonreverse transcribed mRNA preparations (−), and cDNA derived from phorbol 12-myristate 13-acetate (PMA)-stimulated (50 ng/ml, 24 h) CD4+ T cells (CD4+) was used as a positive control. (B) Human vascular SMC, EC, or human MØ express CD40L as a cell-associated 33-kDa protein. Cell preparations of unstimulated SMC, EC, and MØ were analyzed by Western blot. Preparations of PMA-stimulated (50 ng/ml, 24 h) CD4+ T cells (CD4+) were used as positive control. Preincubation of anti-CD40L antibody with human rCD40L (1 μg/ml) inhibited detection of CD40L in cell preparations, demonstrating the specificity of the antibodies. (C) De novo synthesis of CD40L is demonstrated by radioimmunoprecipitation analysis of unstimulated [insulin/transferrin (IT); ref. 24] as well as human recombinant IL-1β (10 ng/ml), TNF-α (50 ng/ml), or IFN-γ (1000 units/ml) treated (24 h) SMC, cultured under serum-free conditions. (D) De novo synthesis of CD40 on SMC is demonstrated by radioimmunoprecipitation analysis of unstimulated (IT) as well as human recombinant IL-1β (10 ng/ml), TNF-α (50 ng/ml), or IFN-γ (1000 units/ml) treated (24 h) cells, cultured under serum-free conditions. Results shown were reproduced in four independent experiments.
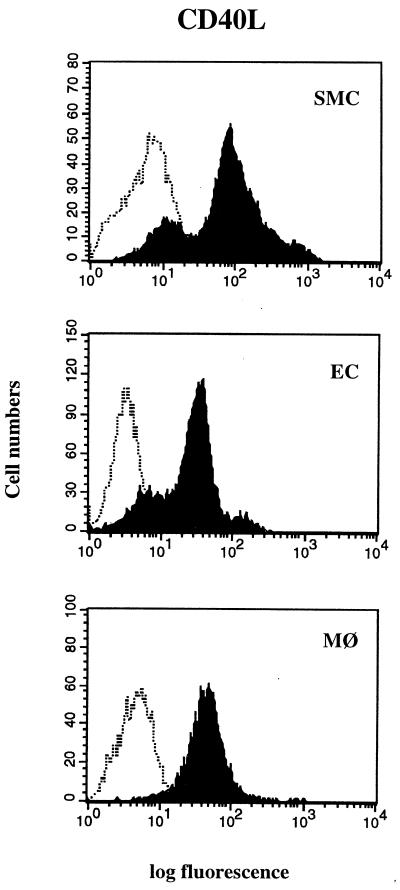
To investigate possible regulation of CD40L expression in vitro, we stimulated EC, SMC, and macrophages cultured in medium containing or lacking serum, with various cytokines considered relevant to atherogenesis. Cells maintained in serum-containing medium constitutively expressed CD40L on the cell surface as detected by FACS analysis (Fig. 2). However, in vivo, vascular cells do not encounter serum, a fluid that contains platelet-derived growth factor, transforming growth factor β, and other well known regulators of vascular functions. Cells cultured in defined medium under quiescent conditions showed increased CD40L when exposed to various cytokines (Table 1). CD40L expression on SMC was induced more potently by IL-1β than by TNF-α or IFN-γ. On EC and macrophages, each cytokine induced CD40L to an equivalent degree. On human vascular SMC, as previously described for EC and macrophages (18–20, 28), all three cytokines augmented CD40 expression (data not shown). Similar results were obtained with two independent antibodies for CD40L and CD40.
Figure 2.
FACS analysis of human vascular SMC, EC, and human macrophages (MØ) cultured in serum-containing medium, stained for CD40L (solid histograms), as well as isotype control (open histograms) are shown. Each panel is a histogram representing cell numbers (y axis) vs. log fluorescence intensity (x axis) for 30,000 viable cells. Results shown were reproduced in three independent experiments.
Table 1.
Regulation of CD40L expression on human vascular SMC, EC, or macrophages (MØ) by proinflammatory cytokines
| Cell type | CD40L mean fluorescence intensity
|
|||
|---|---|---|---|---|
| None | IL-1β | TNF-α | IFN-γ | |
| SMC | 38.3 | 128.1 | 72.4 | 62.2 |
| EC | 5.8 | 18.6 | 17.7 | 15.5 |
| MØ | 6.7 | 12.5 | 12.4 | 12.1 |
Cells cultured under quiescent conditions (serum-free media) were incubated 24 h without cytokine (None), or with 10 ng/ml IL-1β, 50 ng/ml TNF-α, or 1000 units/ml IFN-γ, and analyzed for CD40L expression by flow cytometry. Similar results were obtained from three different experiments.
CD40L and CD40 Function on Cultured Human EC, SMC, and Macrophages.
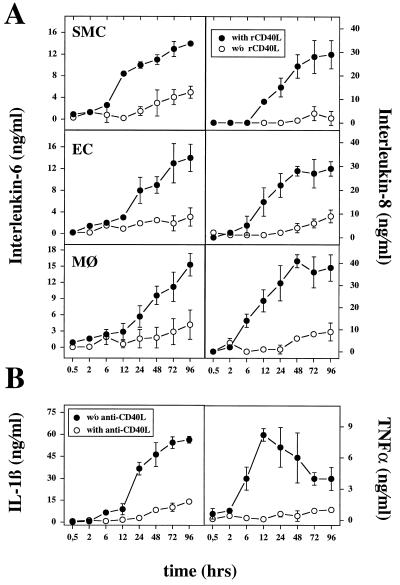
To test the potential functional significance of CD40–CD40L interactions on these cells, we examined the ability of purified human rCD40L to stimulate production of proinflammatory cytokines in SMC, EC, and macrophages. rCD40L induced the release of IL-6 and IL-8 by SMC, EC, and macrophages, as well as TNF-α and macrophages in a time-dependent fashion (Fig. 3). For all cell types, release of IL-6 and IL-8 required at least 6–12 h of stimulation with rCD40L. Maximal levels of IL-6 and IL-8 occurred after 24–48 h, reaching a plateau maintained for at least 48 h. TNF-α rose after 2–6 h in macrophages, and reached a maximum after 12 h. In contrast to IL-6 or IL-8, TNF-α declined over the next 84 h. In addition to these cytokines, we also detected IL-1β in the supernatants of rCD40L-stimulated macrophages with the time course similar to that shown for IL-6 or IL-8 (Fig. 3). Release of these cytokines depended on rCD40L concentration. Activation of all three cell types required at least 100 ng/ml rCD40L. Specificity of the rCD40L-induced activation was demonstrated by inhibition of the effects with an anti-CD40L antibody, as shown for IL-1β (Fig. 3B).
Figure 3.
Induction of proinflammatory cytokine expression in human vascular SMC, EC, or human macrophages by human rCD40L. (A) Supernatants of rCD40L-stimulated (5 μg/ml; •) or unstimulated (○) human vascular SMC, EC, or human macrophages (MØ) were analyzed for IL-6 and IL-8 by ELISA. (B) Macrophages were incubated with rCD40L (5 μg/ml) in the absence (•) or presence (○) of anti-CD40L mAb and analyzed for IL-1β and TNF-α release. Error bars represent SD. Human rCD40L significantly increased cytokine expression relative to control [without (w/o) rCD40L or with anti-CD40L, respectively] after 24 h (P ≤ 0.05, Student’s unpaired t test). Results shown were reproduced for each of the cytokines in three independent experiments.
To test the biological function of CD40L expressed on human vascular cells or macrophages, we monitored expression of the inducible surface molecule B7.2 on B cells (29). Coculture of EC, SMC, or macrophages with the B cell line IB4 increased the expression of B7.2 on the lymphocytes (Table 2). Addition of neutralizing antibody against CD40L abrogated this response. These results demonstrated the biological activity of CD40L and CD40 on human vascular EC, SMC, and human macrophages.
Table 2.
Regulation of B7.2 expression on human IB4 cells coincubated with human vascular SMC, EC, or human macrophages (MØ) by CD40–CD40L interaction
| IB4 cocultured cell type | B7.2 mean fluorescence intensity
|
|
|---|---|---|
| − | + | |
| SMC | 672.6 | 208.5 |
| EC | 482.5 | 199.5 |
| MØ | 456.7 | 232.1 |
| None | 195.8 | 178.5 |
IB4 cells (0.5 × 106/ml) were coincubated for 48 h with confluent cultures of human SMC, EC, or human MØ in serum-containing media, in the absence (−) or presence (+) of anti-CD40L mAb (10 μg/ml). Nonadherent cells were harvested and CD19+ cells were explored for B7.2 expression by flow cytometry analysis. IB4 cells alone were used as control (None).
Expression of CD40L and CD40 in Human Atherosclerotic Plaques.
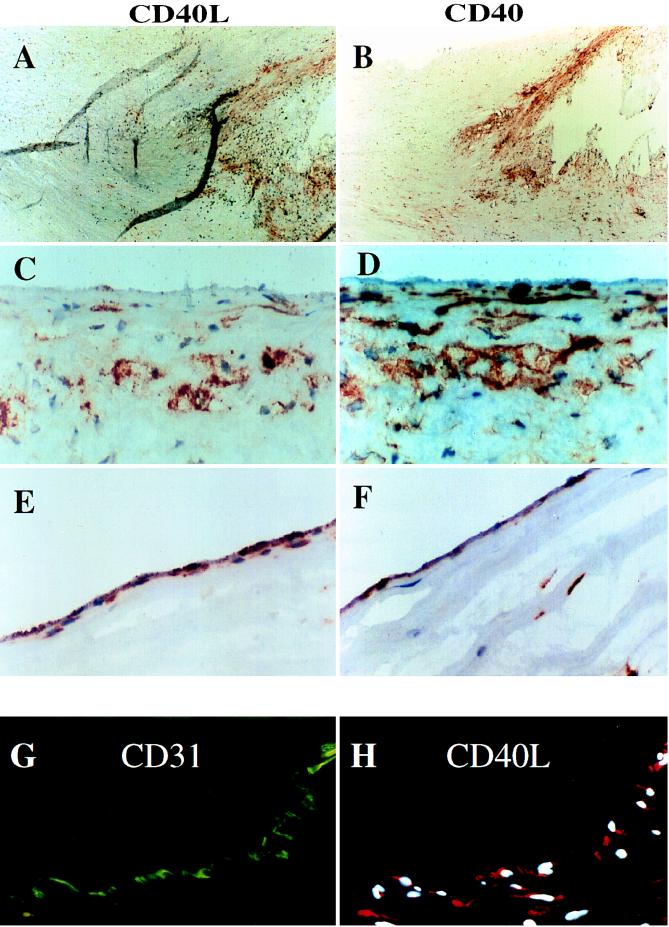
In view of the findings that cells involved in atherogenesis express biologically functional CD40L and CD40 protein in vitro, we investigated the expression of these molecules on EC, SMC, and macrophages in human atherosclerotic lesions. Immunohistochemical analysis showed little or no expression of CD40L, but basal CD40 expression by EC in nonatherosclerotic human arteries (n = 5, data not shown). However, human carotid atherosclerotic lesions (n = 8) showed immunoreactive CD40L and CD40, most prominently in the shoulder region of the plaque, the border between the lesion and the unaffected portion of the artery (Fig. 4 A and B). Higher magnification of these regions (Fig. 4 C–F), as well as immunofluorescence double staining (Fig. 4 G and H) with cell-selective antibodies colocalized CD40L and CD40 protein with EC, SMC, and macrophages of the plaque. EC of neointimal microvessels also stained intensely for both CD40L and CD40. Moreover, immunofluorescent double-staining revealed that EC, SMC, and macrophages coexpressed the receptor and its ligand (Fig. 5 B and C). T cells in atheroma also expressed CD40L (data not shown), another indication of activation of lesional T lymphocytes (30). Frozen sections of human saphenous veins (the source of our cultured SMC and EC) showed little expression of CD40L and CD40 on either EC and SMC (data not shown). Preincubation of the antibody with human rCD40L inhibited staining of the sections (data not shown). No immunoreactivity was observed in tissues stained with the control IgG1 antibody (mouse myeloma protein MOPC-21; data not shown).
Figure 4.
Expression of CD40L and CD40 in human vascular atherosclerotic lesions in situ. (A and B) Low power views (×40) of frozen sections of human carotid lesions show expression of CD40L and CD40 in the shoulder region of the plaque. (C–F) High power views (×400) of this region revealed specific staining for CD40L and CD40 on SMC and macrophages (C and D), as well as on EC of the luminal border (E and F). As demonstrated here for CD40L on EC (G and H), cell types were characterized by immunofluorescence-double staining as described. The lumen of the artery is at the top of each photomicrograph. Analysis of eight atheroma showed similar results.
Figure 5.
Coexpression of CD40L and CD40 in human vascular atherosclerotic lesions in situ. (A) Low power view (×40) of a human carotid atherosclerotic plaque, stained for macrophages (MØ) (CD68). The rectangle indicates the macrophage-rich region sampled in the high power views (×400, B and C) of an adjacent section to the one depicted in A, visualized with different emission filters. (B) Fluorescent staining for CD40L (red) and nuclear DNA (blue). (C) Immunofluorescent staining for CD40 (green) in the same section shown in B. The lumen of the artery is at the top of each photomicrograph. Analysis of four atheroma showed similar results.
DISCUSSION
Despite the increasing appreciation that atherogenesis involves participation of immune cells and inflammatory pathways, the mediators that evoke these responses remain incompletely defined. Much previous work in this area has focused on the roles of soluble mediators rather than surface-associated immunoregulatory molecules. A variety of cells constitutively express the cell surface molecule CD40, initially recognized as a B-cell antigen (6, 7). CD40 is a receptor for CD40L (gp39), a molecule originally described as a 33-kDa protein, transiently expressed after activation on the surface of CD4+ T (1–5), and other leukocytes (31, 32). The signals mediated by CD40 in response to its ligand regulate B-cell growth, differentiation, and death (6, 7, 33). Furthermore, CD40 ligation through CD40L augments tumoricidal activity and cytokine production in monocytes (28) and increases expression of leukocyte adhesion molecules on EC (18–20).
We report here three new findings supporting the involvement of CD40–CD40L interactions in atherosclerosis: (i) the unexpected observation that human endothelial cells and smooth muscle cells express CD40L, a molecule previously described only on activated leukocytes; (ii) the expression of the receptor CD40 on human vascular smooth muscle cells; and (iii) coexpression of CD40L and CD40 by these cell types in human atheroma in situ. The finding of CD40 on human SMC adds a new dimension to the vascular biology of this signaling pair, together with the recent reports of CD40 expression on human EC and macrophages (18–20, 28). In response to CD40L, all three cell types studied here increased expression of cytokines considered involved in atherogenesis (16, 17). Expression of IL-1β by vascular EC and SMC was not investigated because these cells do not release the cytokine (34).
We found abundant expression of CD40L and CD40 in human atherosclerotic lesions but not in uninvolved human arteries or veins. Our localization of CD40 on SMC, and especially of CD40L on EC, SMC, and macrophages establishes the in vivo relevance of our observations on cultured cells and suggests a novel signaling pathway for cellular activation during human atherogenesis. CD40L expressed on vascular wall cells as well as T cells within lesions could engage CD40 on EC, SMC, or macrophages in an autocrine or paracrine fashion, stimulating expression of cytokines, matrix-metalloproteinases, or leukocyte adhesion molecules, proteins present in human atheroma and of physiologic importance (16, 17, 35). This scenario suggests CD40–CD40L signaling among intrinsic vascular wall cells independent of lymphoid cells.
In addition to potential role of CD40L in nonimmune inflammation through modulation of cytokine and adhesion molecule expression, our data also have implications for the development of specific immune reactions within the arterial wall. CD40L could directly contribute to B cell activation, differentiation, and isotype switching. CD40L participates in T cell-mediated immunity by triggering the expression of essential costimulators, such as B7.1 and B7.2 molecules, on antigen-presenting cells, allowing for productive recognition of antigen (36). However, T cells encountering antigen in the context of major histocompatibility complex (MHC) class II without appropriate costimulation may develop tolerance rather than becoming activated. Vessel wall cells in atheromatous plaques express class II MHC molecules. In the atheromatous lesion, it is not known whether antigen–MHC class II complexes serve to activate or to tolerize T cells. The involvement of the CD40L system may decisively shift this balance toward activation. Thus the CD40 signaling pathway may play two distinct roles during atherogenesis. First, by regulating antigen-specific T cells responses to yield activation instead of tolerance. Second, the presence of functional CD40L on nonleukocytic cells associated with atherosclerotic lesion indicates a novel T cell-independent route of inflammatory activation, a now well recognized component of atherogenesis.
Acknowledgments
We thank Dr. Maria Muszynski, Curran Murphy, Eugenia Shvartz and Elissa Simon-Morrissey (Brigham & Women’s Hospital) for their skillful assistance, and Dr. Jorge Plutzky for helpful discussions. This work was supported in part by grants from the National Heart, Lung, and Blood Institute to P.L. and J.S.P. (HL-43364), from the Geneva University Hospital to F.M., and from the Deutsche Forschungsgemeinschaft to U.S. (Scho 614/1-1).
ABBREVIATIONS
- EC
endothelial cells
- SMC
smooth muscle cells
- CD40L
CD40 ligand
- rCD40L
recombinant CD40L
- IL
interleukin
- TNF
tumor necrosis factor
- IFN-γ
interferon γ
- FITC
fluorescein isothiocyanate
- FCS
fetal calf serum
- FACS
fluorescence-activated cell sorter
- RT
reverse transcription
References
- 1.Lederman S, Yellin M J, Krichevsky A, Belko J, Lee J J, Chess L. J Exp Med. 1992;175:1091–1101. doi: 10.1084/jem.175.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armitage R J, Fanslow W C, Strockbine L, Sato T A, Clifford K N, Macduff B M, Anderson D M, Gimpel S D, Davis-Smith T, Maliszewski C R, Clark E A, Smith C A, Grabstein K H, Cosman D, Spriggs M K. Nature (London) 1992;357:80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- 3.Graf D, Korthauer U, Mages H W, Senger G, Kroczek R A. Eur J Immunol. 1992;22:3191–3194. doi: 10.1002/eji.1830221226. [DOI] [PubMed] [Google Scholar]
- 4.Hollenbaugh D, Grosmaire L S, Kullas C D, Chalupny N J, Braesch-Andersen S, Noelle R J, Stamenkovic I, Ledbetter J A, Aruffo A. EMBO J. 1992;11:4313–4321. doi: 10.1002/j.1460-2075.1992.tb05530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noelle R J, Roy M, Shepherd D M, Stamenkovic I, Ledbetter J A, Aruffo A. Proc Natl Acad Sci USA. 1992;89:6550–6554. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foy T M, Aruffo A, Bajorath J, Buhlmann J E, Noelle R J. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 7.Laman J D, Claassen E, Noelle R J. Crit Rev Immunol. 1996;16:59–108. doi: 10.1615/critrevimmunol.v16.i1.40. [DOI] [PubMed] [Google Scholar]
- 8.Durie F H, Fava R A, Foy T M, Aruffo A, Ledbetter J A, Noelle R J. Science. 1993;261:1328–1330. doi: 10.1126/science.7689748. [DOI] [PubMed] [Google Scholar]
- 9.Mohan C, Shi Y, Laman J D, Datta S K. J Immunol. 1995;154:1470–1480. [PubMed] [Google Scholar]
- 10.Larsen C P, Elwood E T, Alexander D Z, Ritchie S C, Hendrix R, Tucker-Burden C, Rae Choe H, Aruffo A, Hollenbaugh D, Linsley P S, Winn K J, Pearson C. Nature (London) 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 11.Durie F A, Aruffo A, Ledbetter J, Crassi K M, Green W R, Fast L D, Noelle R J. J Clin Invest. 1994;94:1333–1338. doi: 10.1172/JCI117453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiener P A, Moran-Davis P, Rankin B M, Wahl A F, Aruffo A, Hollenbaugh D. J Immunol. 1995;155:4917–4925. [PubMed] [Google Scholar]
- 13.Caux C, Massacrier C, Banbervliet B, Dubois B, Van Kooten I, Durand C, Banchereau J. J Exp Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yellin M J, Winikiff S, Fortune S M, Baum D, Crow M K, Lederman S, Chess L. J Leukocyte Biol. 1995;58:209–216. doi: 10.1002/jlb.58.2.209. [DOI] [PubMed] [Google Scholar]
- 15.Galy A H, Spits H. J Immunol. 1992;149:775–782. [PubMed] [Google Scholar]
- 16.Libby P. Atherosclerosis (Berlin) 1990;21:79–89. [Google Scholar]
- 17.Libby P, Ross R. In: Atherosclerosis and Coronary Artery Disease. Fuster V, Ross R, Topol E, editors. Philadelphia: Lippincott–Raven; 1996. pp. 585–594. [Google Scholar]
- 18.Karmann K, Hughes C C W, Schechner J, Fanslow W C, Pober J S. Proc Natl Acad Sci USA. 1995;92:4342–4346. doi: 10.1073/pnas.92.10.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollenbaugh D, Mischel-Petty N, Edwards C P, Simons J C, Denfeld R W, Kiener P A, Aruffo A. J Exp Med. 1995;182:33–40. doi: 10.1084/jem.182.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yellin M J, Brett J, Baum D, Matsushima A, Szablocs M, Stern D, Chess L. J Exp Med. 1995;182:1857–1864. doi: 10.1084/jem.182.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazzei G J, Edgerton M D, Losberger C, Lecoanet-Henchoz S, Graber P, Durandy A, Gauchat J-F, Bernard A, Allet B, Bonnefoy J-Y. J Biol Chem. 1995;270:7025–7028. doi: 10.1074/jbc.270.13.7025. [DOI] [PubMed] [Google Scholar]
- 22.Libby P, Ordovàs J M, Auger K R, Robbins H, Birinyi L K, Dinarello C A. Am J Pathol. 1986;124:179–186. [PMC free article] [PubMed] [Google Scholar]
- 23.Ross R, Kariya B. In: Handbook of Physiology. Bohr D F, Somlyo A P, Sparks H Y, editors. Bethesda, MD: Am. Physiol. Soc.; 1980. Section 2, pp. 66–91. [Google Scholar]
- 24.Libby P, O’Brien K. J Cell Physiol. 1983;115:217–223. doi: 10.1002/jcp.1041150217. [DOI] [PubMed] [Google Scholar]
- 25.Böyum A. Scan J Clin Lab Invest. 1968;21:77–89. [PubMed] [Google Scholar]
- 26.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 27.Stamenkovic I, Clark E A, Seed B. EMBO J. 1989;8:1403–1410. doi: 10.1002/j.1460-2075.1989.tb03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alderson M R, Armitage R J, Tough T W, Strockbine L, Fanslow W C, Springs M K. J Exp Med. 1993;178:669–674. doi: 10.1084/jem.178.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buhlmann J E, Foy T M, Aruffo A, Crassi K M, Ledbetter J A, Green W R, Xu J C, Schultz L D, Roopesian D, Flavell R A, Fast L, Noelle R J, Durie F H. Immunity. 1995;2:645–653. doi: 10.1016/1074-7613(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 30.Stemme S, Holm J, Hansson G K. Arterioscler Thromb. 1996;12:206–211. doi: 10.1161/01.atv.12.2.206. [DOI] [PubMed] [Google Scholar]
- 31.Gauchat J-F, Henchoz S, Mazzei G, Aubry J-P, Brunner T, Blasey H, Life P, Talabot D, Flores-Romo L, Thompson J, Kishi K, Butterfield J, Dahinden C, Bonnefoy J-Y. Nature (London) 1993;365:340–343. doi: 10.1038/365340a0. [DOI] [PubMed] [Google Scholar]
- 32.Gauchat J-F, Henchoz S, Fattah D, Mazzei G, Aubry J-P, Jomotte T, Dash L, Page K, Solari R, Aldebert D, Capron M, Dahinden C, Bonnefoy J-Y. Eur J Immunol. 1995;25:863–867. doi: 10.1002/eji.1830250335. [DOI] [PubMed] [Google Scholar]
- 33.Hess S, Engelmann H. J Exp Med. 1996;183:159–167. doi: 10.1084/jem.183.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schönbeck U, Brandt E, Petersen F, Flad H-D, Loppnow H. J Immunol. 1995;154:2375–2383. [PubMed] [Google Scholar]
- 35.Malik N, Greenfield B W, Wahl A F, Kiener P A. J Immunol. 1996;156:3952–3960. [PubMed] [Google Scholar]
- 36.Yang Y, Wilson J M. Science. 1996;273:1862–1864. doi: 10.1126/science.273.5283.1862. [DOI] [PubMed] [Google Scholar]