Abstract
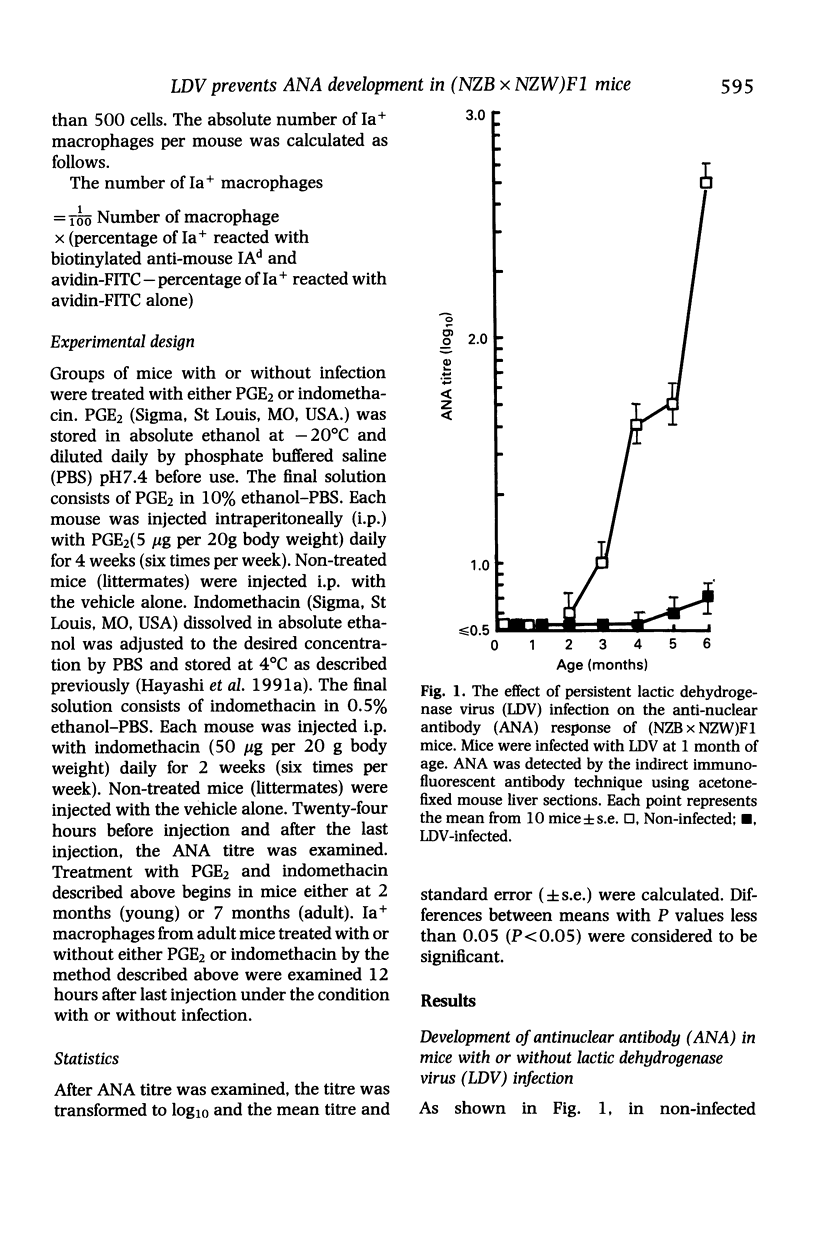
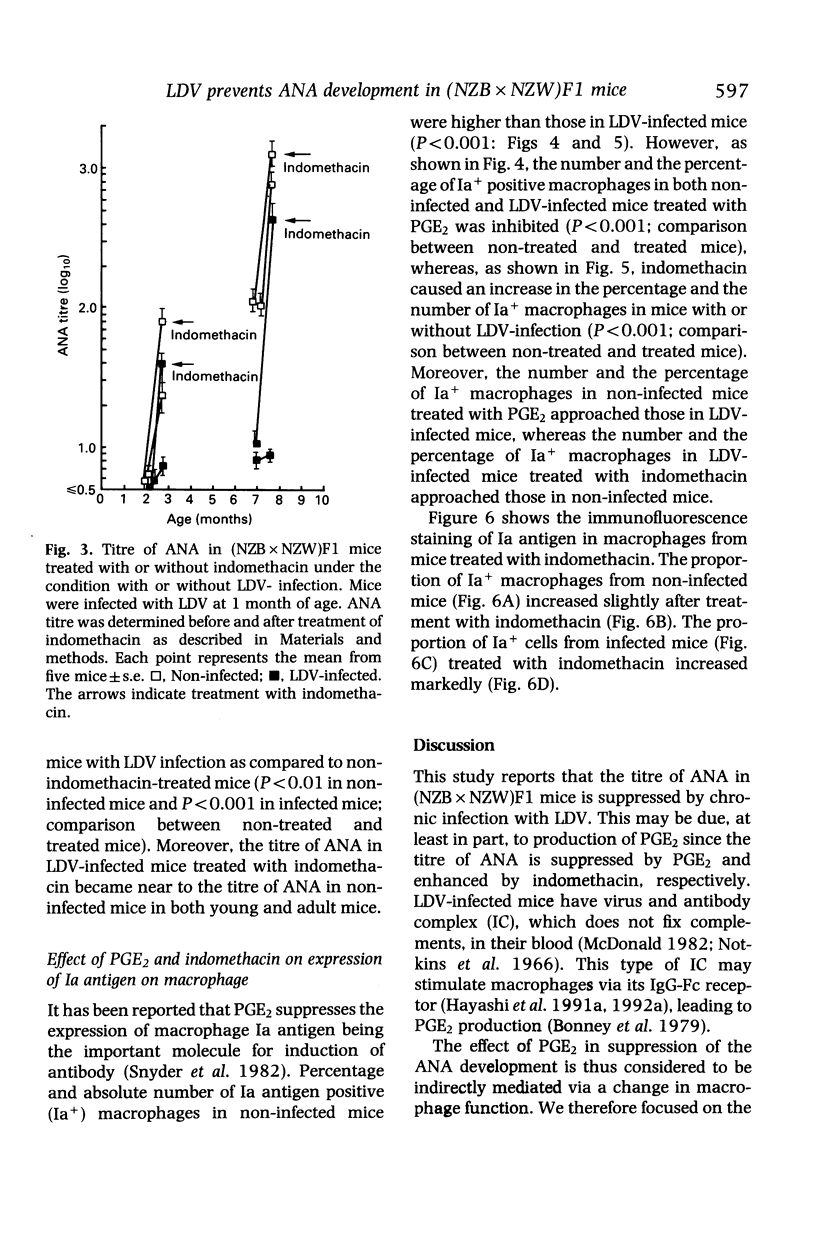
Persistent lactic dehydrogenase virus (LDV) infection prevents the development of antinuclear antibody (ANA) in (NZB x NZW)F1 mice. To assess the suppressive mechanisms, we focused on the role of the E series of prostaglandin(PGE), since previously we have shown enhanced production of PGE by macrophages from chronically LDV-infected mice. Treatment with PGE2 suppressed ANA titres more markedly in non-infected mice than in LDV-infected mice. Indomethacin enhanced ANA titres more markedly in LDV-infected mice than in non-infected mice. The number of Ia antigen positive(Ia+) macrophages was less in LDV-infected mice than in uninfected mice. The number of Ia+ macrophages was decreased in non-infected mice by PGE2 treatment and increased in LDV-infected mice by indomethacin treatment. These results suggest that the low ANA production in LDV-infected (NZB x NZW)F1 mice may be related to the decreased number of Ia+ macrophages and that one of the factors responsible for suppression of Ia+ macrophages may be the enhanced PGE2 production in the LDV-infected mice.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman N. E., Watling D. L., McDevitt H. O. Treatment of (NZB x NZW)F1 disease with anti-I-A monoclonal antibodies. J Exp Med. 1983 Oct 1;158(4):1350–1355. doi: 10.1084/jem.158.4.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonney R. J., Naruns P., Davies P., Humes J. L. Antigen-antibody complexes stimulate the synthesis and release of prostaglandins by mouse peritoneal macrophages. Prostaglandins. 1979 Oct;18(4):605–616. doi: 10.1016/0090-6980(79)90027-3. [DOI] [PubMed] [Google Scholar]
- Donnelly R. P., Levine J., Hartwell D. Q., Frendl G., Fenton M. J., Beller D. I. Aberrant regulation of IL-1 expression in macrophages from young autoimmune-prone mice. J Immunol. 1990 Nov 15;145(10):3231–3239. [PubMed] [Google Scholar]
- Hartung H. P., Schäfer B., Heininger K., Toyka K. V. Suppression of experimental autoimmune neuritis by the oxygen radical scavengers superoxide dismutase and catalase. Ann Neurol. 1988 May;23(5):453–460. doi: 10.1002/ana.410230505. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Ozaki M., Ami Y., Onodera T., Yamamoto H. Increased superoxide anion release by peritoneal macrophages in mice with a chronic infection of lactic dehydrogenase virus. J Comp Pathol. 1992 Jan;106(1):93–98. doi: 10.1016/0021-9975(92)90073-4. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Ozaki M., Mori I., Saito M., Itoh T., Yamamoto H. Enhanced clearance of lactic dehydrogenase-5 in severe combined immunodeficiency (SCID) mice: effect of lactic dehydrogenase virus on enzyme clearance. Int J Exp Pathol. 1992 Apr;73(2):173–181. [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Salata K., Kingman A., Notkins A. L. Regulation of enzyme levels in the blood. Influence of environmental and genetic factors on enzyme clearance. Am J Pathol. 1988 Sep;132(3):503–511. [PMC free article] [PubMed] [Google Scholar]
- Heremans H., Billiau A., Coutelier J. P., De Somer P. The inhibition of endotoxin-induced local inflammation by LDH virus or LDH virus-infected tumors is mediated by interferon. Proc Soc Exp Biol Med. 1987 May;185(1):6–15. doi: 10.3181/00379727-185-42509. [DOI] [PubMed] [Google Scholar]
- Hirano T., Taga T., Yasukawa K., Nakajima K., Nakano N., Takatsuki F., Shimizu M., Murashima A., Tsunasawa S., Sakiyama F. Human B-cell differentiation factor defined by an anti-peptide antibody and its possible role in autoantibody production. Proc Natl Acad Sci U S A. 1987 Jan;84(1):228–231. doi: 10.1073/pnas.84.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada T., Mims C. A. Infection of mice with lactic dehydrogenase virus prevents development of experimental allergic encephalomyelitis. J Neuroimmunol. 1986 Mar;11(1):53–56. doi: 10.1016/0165-5728(86)90074-3. [DOI] [PubMed] [Google Scholar]
- Isakov N., Feldman M., Segal S. Acute infection of mice with lactic dehydrogenase virus (LDV) impairs the antigen-presenting capacity of their macrophages. Cell Immunol. 1982 Jan 15;66(2):317–332. doi: 10.1016/0008-8749(82)90182-4. [DOI] [PubMed] [Google Scholar]
- Jacob C. O., McDevitt H. O. Tumour necrosis factor-alpha in murine autoimmune 'lupus' nephritis. Nature. 1988 Jan 28;331(6154):356–358. doi: 10.1038/331356a0. [DOI] [PubMed] [Google Scholar]
- Kelley V. E., Winkelstein A., Izui S. Effect of prostaglandin E on immune complex nephritis in NZB/W mice. Lab Invest. 1979 Dec;41(6):531–537. [PubMed] [Google Scholar]
- Kowalchyk K., Plagemann P. G. Cell surface receptors for lactate dehydrogenase-elevating virus on subpopulation of macrophages. Virus Res. 1985 Apr;2(3):211–229. doi: 10.1016/0168-1702(85)90010-3. [DOI] [PubMed] [Google Scholar]
- McDonald T. L. Isolation of Clq-binding virus-antibody immune complexes from lactic dehydrogenase virus (LDV)-infected mice. Immunology. 1982 Feb;45(2):365–370. [PMC free article] [PubMed] [Google Scholar]
- NOTKINS A. L. LACTIC DEHYDROGENASE VIRUS. Bacteriol Rev. 1965 Jun;29:143–160. doi: 10.1128/br.29.2.143-160.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notkins A. L., Mahar S., Scheele C., Goffman J. Infectious virus-antibody complex in the blood of chronically infected mice. J Exp Med. 1966 Jul 1;124(1):81–97. doi: 10.1084/jem.124.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Inhibition of antibodies to nuclear antigen and to DNA in New Zealand mice infected with lactate dehydrogenase virus. Science. 1972 Feb 18;175(4023):784–786. doi: 10.1126/science.175.4023.784. [DOI] [PubMed] [Google Scholar]
- Rokutan K., Hosokawa T., Nakamura K., Koyama K., Aoike A., Kawai K. Increased superoxide anion production and glutathione peroxidase activity in peritoneal macrophages from autoimmune-prone MRL/Mp-Ipr/lpr mice. Int Arch Allergy Appl Immunol. 1988;87(2):113–119. doi: 10.1159/000234660. [DOI] [PubMed] [Google Scholar]
- Satoh J., Seino H., Shintani S., Tanaka S., Ohteki T., Masuda T., Nobunaga T., Toyota T. Inhibition of type 1 diabetes in BB rats with recombinant human tumor necrosis factor-alpha. J Immunol. 1990 Sep 1;145(5):1395–1399. [PubMed] [Google Scholar]
- Snyder D. S., Beller D. I., Unanue E. R. Prostaglandins modulate macrophage Ia expression. Nature. 1982 Sep 9;299(5879):163–165. doi: 10.1038/299163a0. [DOI] [PubMed] [Google Scholar]
- Snyder D. S., Unanue E. R. Corticosteroids inhibit murine macrophage Ia expression and interleukin 1 production. J Immunol. 1982 Nov;129(5):1803–1805. [PubMed] [Google Scholar]
- Stenson W. F., Parker C. W. Prostaglandins, macrophages, and immunity. J Immunol. 1980 Jul;125(1):1–5. [PubMed] [Google Scholar]
- Wilson C. A., Jacobs C., Baker P., Baskin D. G., Dower S., Lernmark A., Toivola B., Vertrees S., Wilson D. IL-1 beta modulation of spontaneous autoimmune diabetes and thyroiditis in the BB rat. J Immunol. 1990 May 15;144(10):3784–3788. [PubMed] [Google Scholar]
- Wofsy D., Seaman W. E. Successful treatment of autoimmunity in NZB/NZW F1 mice with monoclonal antibody to L3T4. J Exp Med. 1985 Feb 1;161(2):378–391. doi: 10.1084/jem.161.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurier R. B., Damjanov I., Sayadoff D. M., Rothfield N. F. Prostaglandin E1 treatment of NZB/NZW F1 hybrid mice. II. Prevention of glomerulonephritis. Arthritis Rheum. 1977 Nov-Dec;20(8):1449–1456. doi: 10.1002/art.1780200802. [DOI] [PubMed] [Google Scholar]



