Abstract
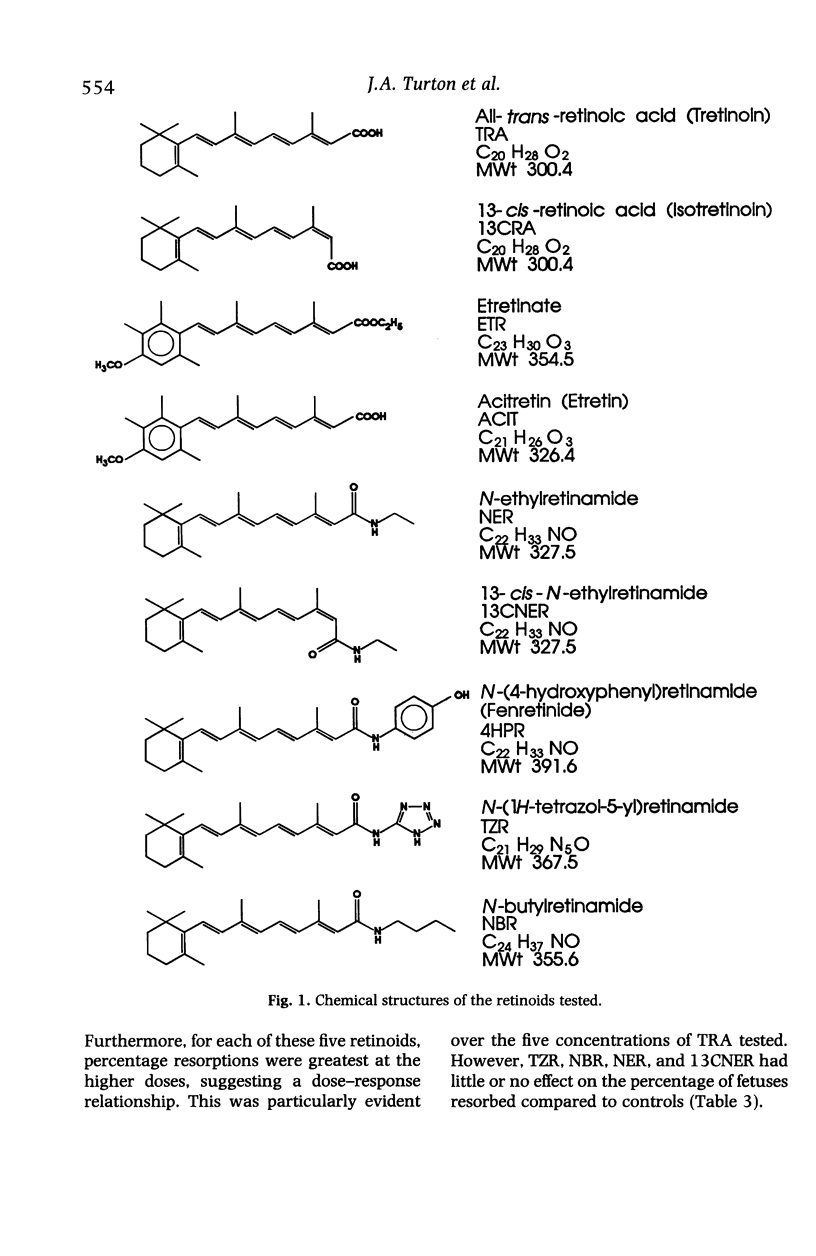
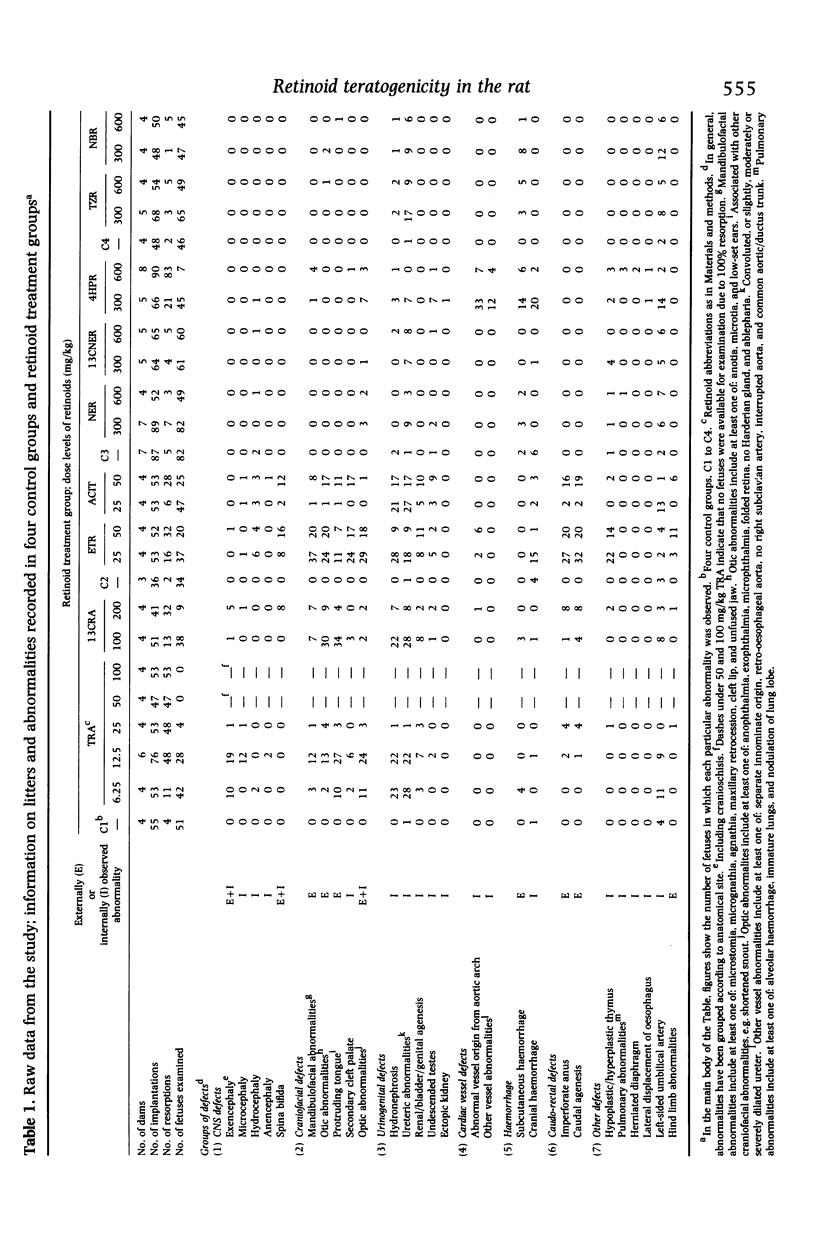
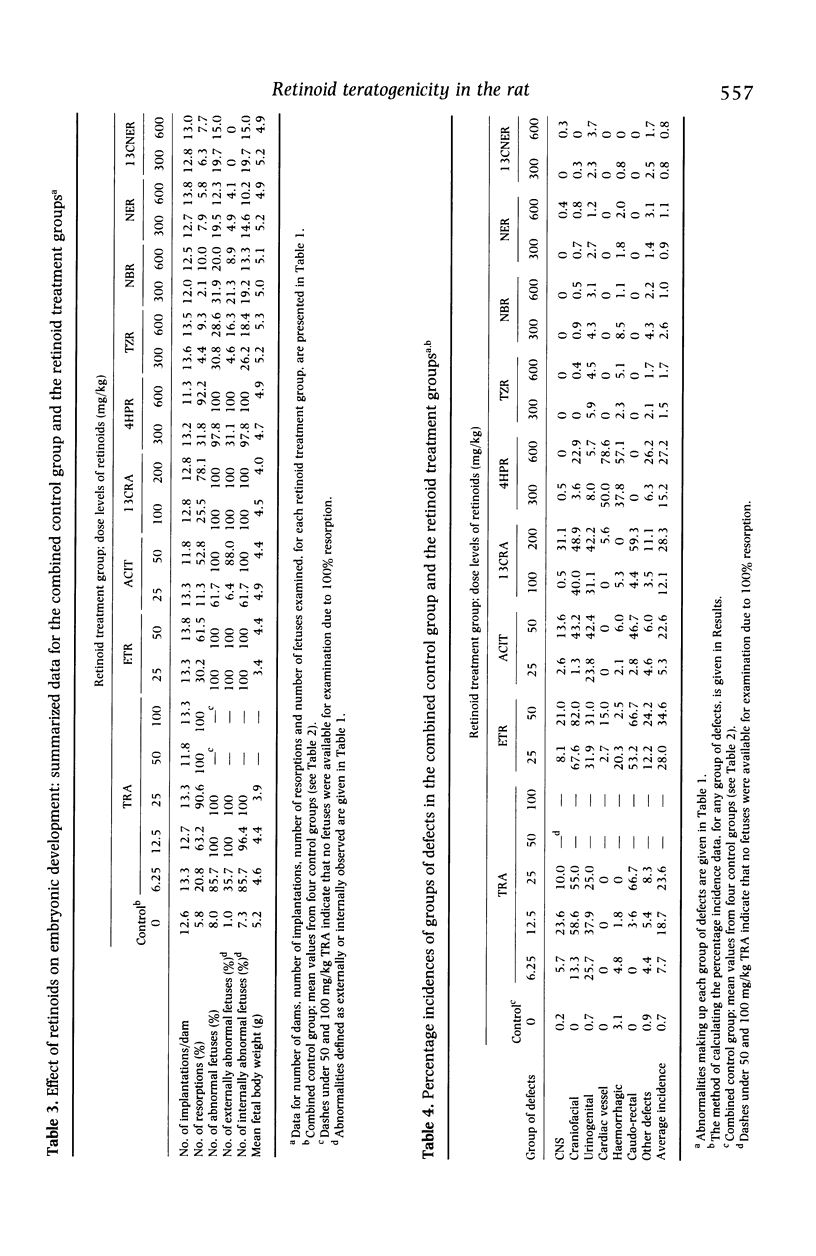
he comparative teratogenicity of nine retinoids in Wistar rats was investigated. The compounds studied and dose levels tested (mg/kg) were: all-trans-retinoic acid (TRA), 6.25, 12.5, 25, 50, 100; etretinate (ETR), 25, 50; acitretin (ACIT), 25, 50; 13-cis-retinoic acid (13CRA), 100, 200; and five retinamides, each at 300 and 600 mg/kg, N-(4-hydroxyphenyl)-retinamide (4HPR); N-tetrazol-5-ylretinamide (TZR); N-butylretinamide (NBR); N-ethylretinamide (NER); 13-cis-N-ethylretinamide (13CNER). Retinoids were administered by oral intubation on days 10 and 11 post coitum (p.c.). Dams were killed on day 22 p.c. and examinations carried out to assess teratogenic potential. TRA, ETR, ACIT, 13CRA and 4HPR increased the incidence of resorptions. The incidence of abnormal fetuses, irrespective of the specific abnormalities induced, was markedly increased (50-100%) by TRA, ETR, ACIT, 13CRA and 4HPR, whereas TZR and NBR caused moderate increases (20-50%), and NER and 13CNER induced mild increases (10-20%). The incidences of CNS, craniofacial and urinogenital defects were generally high with TRA, ETR, ACIT and 13CRA. Cardiac vessel defects were markedly increased by 4HPR. Using a number of criteria, a generalized ranking order of the toxicity of the compounds was drawn up: TRA > ETR > ACIT > 13CRA > 4HPR > TZR identical to NBR > NER identical to 13CNER. The ranked order of relative in-vivo teratogenicity for the nine retinoids is compared with a previously reported in-vitro assessment of the compounds using a rat whole embryo culture technique.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benke P. J. The isotretinoin teratogen syndrome. JAMA. 1984 Jun 22;251(24):3267–3269. [PubMed] [Google Scholar]
- Bollag W. Vitamin A and retinoids: from nutrition to pharmacotherapy in dermatology and oncology. Lancet. 1983 Apr 16;1(8329):860–863. doi: 10.1016/s0140-6736(83)91394-6. [DOI] [PubMed] [Google Scholar]
- Cicurel L., Schmid B. P. Postimplantation embryo culture for the assessment of the teratogenic potential and potency of compounds. Experientia. 1988 Oct 15;44(10):833–840. doi: 10.1007/BF01941180. [DOI] [PubMed] [Google Scholar]
- Fantel A. G. Culture of whole rodent embryos in teratogen screening. Teratog Carcinog Mutagen. 1982;2(3-4):231–242. doi: 10.1002/1520-6866(1990)2:3/4<231::aid-tcm1770020305>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Flint O. P., Orton T. C. An in vitro assay for teratogens with cultures of rat embryo midbrain and limb bud cells. Toxicol Appl Pharmacol. 1984 Nov;76(2):383–395. doi: 10.1016/0041-008x(84)90020-6. [DOI] [PubMed] [Google Scholar]
- Geelen J. A. Hypervitaminosis A induced teratogenesis. CRC Crit Rev Toxicol. 1979 Nov;6(4):351–375. doi: 10.3109/10408447909043651. [DOI] [PubMed] [Google Scholar]
- Gollnick H. New indications and new retinoids. Dermatologica. 1987;175 (Suppl 1):182–195. doi: 10.1159/000248882. [DOI] [PubMed] [Google Scholar]
- Goulding E. H., Jetten A. M., Abbott B. D., Pratt R. M. Teratogenicity of benzoic acid derivatives of retinoic acid in cultured mouse embryos. Reprod Toxicol. 1988;2(2):91–98. doi: 10.1016/0890-6238(88)90003-2. [DOI] [PubMed] [Google Scholar]
- Goulding E. H., Pratt R. M. Isotretinoin teratogenicity in mouse whole embryo culture. J Craniofac Genet Dev Biol. 1986;6(2):99–112. [PubMed] [Google Scholar]
- Hassell J. R., Horigan E. A. Chondrogenesis: a model developmental system for measuring teratogenic potential of compounds. Teratog Carcinog Mutagen. 1982;2(3-4):325–331. doi: 10.1002/1520-6866(1990)2:3/4<325::aid-tcm1770020314>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Kimmel G. L., Smith K., Kochhar D. M., Pratt R. M. Overview of In Vitro Teratogenicity Testing: aspects of validation and application to screening. Teratog Carcinog Mutagen. 1982;2(3-4):221–229. doi: 10.1002/1520-6866(1990)2:3/4<221::aid-tcm1770020304>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Kistler A., Hummler H. Teratogenesis and reproductive safety evaluation of the retinoid etretin (Ro 10-1670). Arch Toxicol. 1985 Oct;58(1):50–56. doi: 10.1007/BF00292617. [DOI] [PubMed] [Google Scholar]
- Kistler A. Limb bud cell cultures for estimating the teratogenic potential of compounds. Validation of the test system with retinoids. Arch Toxicol. 1987 Aug;60(6):403–414. doi: 10.1007/BF00302382. [DOI] [PubMed] [Google Scholar]
- Klug S., Creech Kraft J., Wildi E., Merker H. J., Persaud T. V., Nau H., Neubert D. Influence of 13-cis and all-trans retinoic acid on rat embryonic development in vitro: correlation with isomerisation and drug transfer to the embryo. Arch Toxicol. 1989;63(3):185–192. doi: 10.1007/BF00316367. [DOI] [PubMed] [Google Scholar]
- Klug S., Lewandowski C., Neubert D. Modification and standardization of the culture of early postimplantation embryos for toxicological studies. Arch Toxicol. 1985 Dec;58(2):84–88. doi: 10.1007/BF00348314. [DOI] [PubMed] [Google Scholar]
- Kochhar D. M. Embryonic limb bud organ culture in assessment of teratogenicity of environmental agents. Teratog Carcinog Mutagen. 1982;2(3-4):303–312. doi: 10.1002/1520-6866(1990)2:3/4<303::aid-tcm1770020311>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Kochhar D. M. In vitro testing of teratogenic agents using mammalian embryos. Teratog Carcinog Mutagen. 1980;1(1):63–74. doi: 10.1002/tcm.1770010107. [DOI] [PubMed] [Google Scholar]
- Kochhar D. M., Penner J. D. Developmental effects of isotretinoin and 4-oxo-isotretinoin: the role of metabolism in teratogenicity. Teratology. 1987 Aug;36(1):67–75. doi: 10.1002/tera.1420360110. [DOI] [PubMed] [Google Scholar]
- Kochhar D. M., Penner J. D., Tellone C. I. Comparative teratogenic activities of two retinoids: effects on palate and limb development. Teratog Carcinog Mutagen. 1984;4(4):377–387. doi: 10.1002/tcm.1770040407. [DOI] [PubMed] [Google Scholar]
- Kochhar D. M. The use of in vitro procedures in teratology. Teratology. 1975 Jun;11(3):273–287. doi: 10.1002/tera.1420110307. [DOI] [PubMed] [Google Scholar]
- Kraft J. C., Kochhar D. M., Scott W. J., Nau H. Low teratogenicity of 13-cis-retinoic acid (isotretinoin) in the mouse corresponds to low embryo concentrations during organogenesis: comparison to the all-trans isomer. Toxicol Appl Pharmacol. 1987 Mar 15;87(3):474–482. doi: 10.1016/0041-008x(87)90253-5. [DOI] [PubMed] [Google Scholar]
- Lammer E. J., Chen D. T., Hoar R. M., Agnish N. D., Benke P. J., Braun J. T., Curry C. J., Fernhoff P. M., Grix A. W., Jr, Lott I. T. Retinoic acid embryopathy. N Engl J Med. 1985 Oct 3;313(14):837–841. doi: 10.1056/NEJM198510033131401. [DOI] [PubMed] [Google Scholar]
- Neubert D. Toxicity studies with cellular models of differentiation. Xenobiotica. 1985 Aug-Sep;15(8-9):649–660. doi: 10.3109/00498258509047423. [DOI] [PubMed] [Google Scholar]
- Orfanos C. E., Ehlert R., Gollnick H. The retinoids. A review of their clinical pharmacology and therapeutic use. Drugs. 1987 Oct;34(4):459–503. doi: 10.2165/00003495-198734040-00003. [DOI] [PubMed] [Google Scholar]
- Reiners J., Löfberg B., Kraft J. C., Kochhar D. M., Nau H. Transplacental pharmacokinetics of teratogenic doses of etretinate and other aromatic retinoids in mice. Reprod Toxicol. 1988;2(1):19–29. doi: 10.1016/s0890-6238(88)80005-4. [DOI] [PubMed] [Google Scholar]
- Renault J. Y., Melcion C., Cordier A. Limb bud cell culture for in vitro teratogen screening: validation of an improved assessment method using 51 compounds. Teratog Carcinog Mutagen. 1989;9(2):83–96. doi: 10.1002/tcm.1770090204. [DOI] [PubMed] [Google Scholar]
- Rosa F. W., Wilk A. L., Kelsey F. O. Teratogen update: vitamin A congeners. Teratology. 1986 Jun;33(3):355–364. doi: 10.1002/tera.1420330315. [DOI] [PubMed] [Google Scholar]
- Sadler T. W., Horton W. E., Warner C. W. Whole embryo culture: a screening technique for teratogens? Teratog Carcinog Mutagen. 1982;2(3-4):243–253. doi: 10.1002/1520-6866(1990)2:3/4<243::aid-tcm1770020306>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Shenefelt R. E. Morphogenesis of malformations in hamsters caused by retinoic acid: relation to dose and stage at treatment. Teratology. 1972 Feb;5(1):103–118. doi: 10.1002/tera.1420050115. [DOI] [PubMed] [Google Scholar]
- Silverman A. K., Ellis C. N., Voorhees J. J. Hypervitaminosis A syndrome: a paradigm of retinoid side effects. J Am Acad Dermatol. 1987 May;16(5 Pt 1):1027–1039. doi: 10.1016/s0190-9622(87)70133-9. [DOI] [PubMed] [Google Scholar]
- Teelmann K. Retinoids: toxicology and teratogenicity to date. Pharmacol Ther. 1989;40(1):29–43. doi: 10.1016/0163-7258(89)90072-7. [DOI] [PubMed] [Google Scholar]
- Turton J. A., Hicks R. M., Gwynne J., Hunt R., Hawkey C. M. Retinoid toxicity. Ciba Found Symp. 1985;113:220–251. doi: 10.1002/9780470720943.ch13. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Pratt R. M. Effects of retinoic acid on embryonic development of mice in culture. Experientia. 1991 May 15;47(5):493–497. doi: 10.1007/BF01959953. [DOI] [PubMed] [Google Scholar]
- Wilk A. L., Greenberg J. H., Horigan E. A., Pratt R. M., Martin G. R. Detection of teratogenic compounds using differentiating embryonic cells in culture. In Vitro. 1980 Apr;16(4):269–276. doi: 10.1007/BF02618331. [DOI] [PubMed] [Google Scholar]
- Willhite C. C., Dawson M. I., Williams K. J. Structure-activity relationships of retinoids in developmental toxicology. I. Studies on the nature of the polar terminus of the vitamin A molecule. Toxicol Appl Pharmacol. 1984 Jul;74(3):397–410. doi: 10.1016/0041-008x(84)90293-x. [DOI] [PubMed] [Google Scholar]
- Willhite C. C., Shealy Y. F. Amelioration of embryotoxicity by structural modification of the terminal group of cancer chemopreventive retinoids. J Natl Cancer Inst. 1984 Mar;72(3):689–695. [PubMed] [Google Scholar]
- Yob E. H., Pochi P. E. Side effects and long-term toxicity of synthetic retinoids. Arch Dermatol. 1987 Oct;123(10):1375–1378. [PubMed] [Google Scholar]
- Zimmermann B., Tsambaos D., Stürje H. Teratogenicity of arotinoid ethyl ester (RO 13-6298) in mice. Teratog Carcinog Mutagen. 1985;5(6):415–431. doi: 10.1002/tcm.1770050605. [DOI] [PubMed] [Google Scholar]



