Abstract
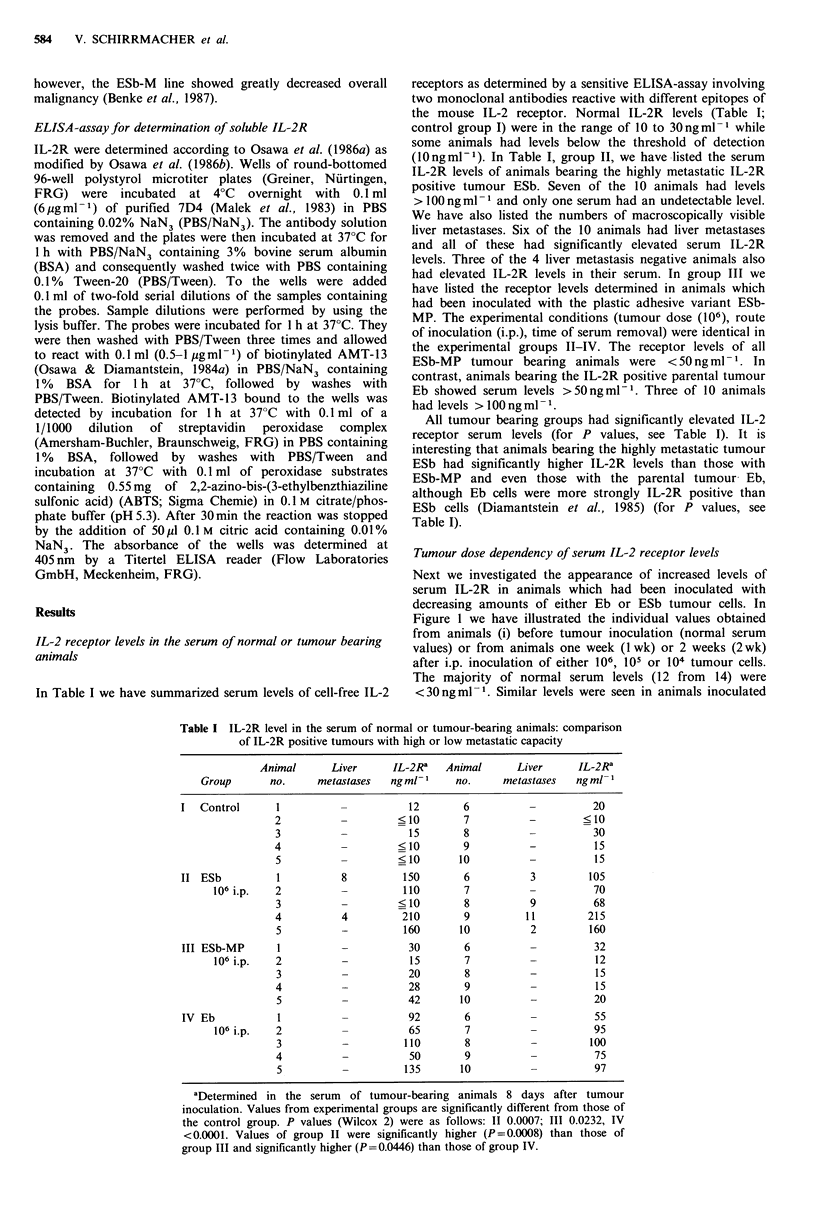
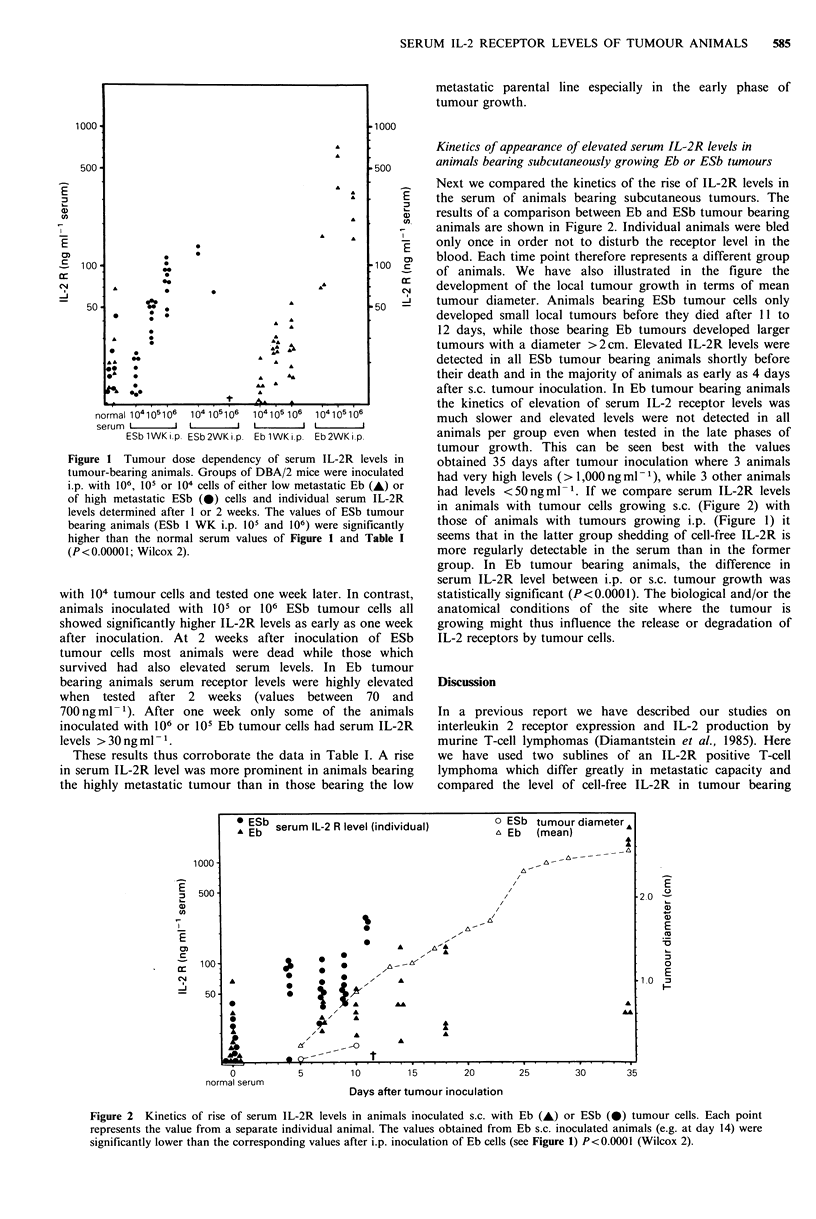
Serum levels of cell-free interleukin-2 receptors were elevated above normal in mice bearing the IL-2R positive T-cell lymphoma Eb or its highly metastatic variant ESb. Although ESb cells expressed less IL-2R molecules than Eb cells on their cell surface, serum receptor levels were raised more quickly in ESb than in Eb tumour bearing animals. Elevated IL-2R serum levels were a sensitive tumour marker in animals bearing the aggressive variant ESb but not in animals bearing the low metastatic line Eb. Peritoneal ascites tumour-bearing animals had higher serum IL-2R levels than corresponding animals with subcutaneously growing tumours. Thus, serum IL-2R levels in tumour-bearing animals were dependent on the tumour line and influenced by the site and mode of tumour growth.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altevogt P., Kurnick J. T., Kimura A. K., Bosslet K., Schirrmacher V. Different expression of Lyt differentiation antigens and cell surface glycoproteins by a murine T lymphoma line and its highly metastatic variant. Eur J Immunol. 1982 Apr;12(4):300–307. doi: 10.1002/eji.1830120409. [DOI] [PubMed] [Google Scholar]
- Barz D., Goppelt M., Szamel M., Schirrmacher V., Resch K. Characterization of cellular and extracellular plasma membrane vesicles from a non-metastasizing lymphoma (Eb) and its metastasizing variant (ESb). Biochim Biophys Acta. 1985 Mar 28;814(1):77–84. doi: 10.1016/0005-2736(85)90421-3. [DOI] [PubMed] [Google Scholar]
- Diamantstein T., Osawa H., Graf L., Schirrmacher V. Studies on interleukin 2 receptor expression and IL-2 production by murine T cell lymphomas. Br J Cancer. 1985 Jan;51(1):23–30. doi: 10.1038/bjc.1985.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamantstein T., Osawa H. The interleukin-2 receptor, its physiology and a new approach to a selective immunosuppressive therapy by anti-interleukin-2 receptor monoclonal antibodies. Immunol Rev. 1986 Aug;92:5–27. doi: 10.1111/j.1600-065x.1986.tb01491.x. [DOI] [PubMed] [Google Scholar]
- Fogel M., Altevogt P., Schirrmacher V. Metastatic potential severely altered by changes in tumor cell adhesiveness and cell-surface sialylation. J Exp Med. 1983 Jan 1;157(1):371–376. doi: 10.1084/jem.157.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther J., Haas W., Von Boehmer H. Suppression of T cell responses through competition for T cell growth factor (interleukin 2). Eur J Immunol. 1982 Mar;12(3):247–249. doi: 10.1002/eji.1830120315. [DOI] [PubMed] [Google Scholar]
- Kramer M. D., Robinson P., Vlodavsky I., Barz D., Friberger P., Fuks Z., Schirrmacher V. Characterization of an extracellular matrix-degrading protease derived from a highly metastatic tumor cell line. Eur J Cancer Clin Oncol. 1985 Mar;21(3):307–316. doi: 10.1016/0277-5379(85)90130-0. [DOI] [PubMed] [Google Scholar]
- Larizza L., Schirrmacher V., Graf L., Pflüger E., Peres-Martinez M., Stöhr M. Suggestive evidence that the highly metastatic variant ESb of the T-cell lymphoma Eb is derived from spontaneous fusion with a host macrophage. Int J Cancer. 1984 Nov 15;34(5):699–707. doi: 10.1002/ijc.2910340518. [DOI] [PubMed] [Google Scholar]
- Malek T. R., Ashwell J. D., Germain R. N., Shevach E. M., Miller J. The murine interleukin-2 receptor: biochemical structure and regulation of expression. Immunol Rev. 1986 Aug;92:81–101. doi: 10.1111/j.1600-065x.1986.tb01495.x. [DOI] [PubMed] [Google Scholar]
- Malek T. R., Robb R. J., Shevach E. M. Identification and initial characterization of a rat monoclonal antibody reactive with the murine interleukin 2 receptor-ligand complex. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5694–5698. doi: 10.1073/pnas.80.18.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama K., Levery S. B., Schirrmacher V., Hakomori S. Qualitative differences in position of sialylation and surface expression of glycolipids between murine lymphomas with low metastatic (Eb) and high metastatic (ESb) potentials and isolation of a novel disialoganglioside (GD1 alpha) from Eb cells. Cancer Res. 1986 Mar;46(3):1395–1402. [PubMed] [Google Scholar]
- Nicolas J. F., Reano A., Kaiserlian D., Thivolet J. Epithelial cell heterogeneity in the guinea pig thymus: immunohistochemical characterization of four thymic epithelial subsets defined by monoclonal anti-keratin antibodies. Eur J Immunol. 1986 Apr;16(4):457–464. doi: 10.1002/eji.1830160424. [DOI] [PubMed] [Google Scholar]
- Osawa H., Josimovic-Alasevic O., Diamantstein T. Enzyme-linked immunosorbent assay of mouse interleukin-2 receptors. J Immunol Methods. 1986 Aug 21;92(1):109–115. doi: 10.1016/0022-1759(86)90510-7. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Munck A., Smith K. A. T cell growth factor receptors. Quantitation, specificity, and biological relevance. J Exp Med. 1981 Nov 1;154(5):1455–1474. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirrmacher V., Barz D. Characterization of cellular and extracellular plasma membrane vesicles from a low metastatic lymphoma (Eb) and its high metastatic variant (ESb): inhibitory capacity in cell-cell interaction systems. Biochim Biophys Acta. 1986 Aug 21;860(2):236–242. doi: 10.1016/0005-2736(86)90519-5. [DOI] [PubMed] [Google Scholar]
- Schirrmacher V., Bosslet K., Shantz G., Clauer K., Hübsch D. Tumor metastases and cell-mediated immunity in a model system in DBA/2 mice. IV. Antigenic differences between a metastasizing variant and the parental tumor line revealed by cytotoxic T lymphocytes. Int J Cancer. 1979 Feb;23(2):245–252. doi: 10.1002/ijc.2910230216. [DOI] [PubMed] [Google Scholar]
- Schirrmacher V., Fogel M., Russmann E., Bosslet K., Altevogt P., Beck L. Antigenic variation in cancer metastasis: immune escape versus immune control. Cancer Metastasis Rev. 1982;1(3):241–274. doi: 10.1007/BF00046830. [DOI] [PubMed] [Google Scholar]
- Schirrmacher V., Jacobs W. Tumor metastases and cell-mediated immunity in a model system in DBA/2 mice. VIII. Expression and shedding of Fc gamma receptors on metastatic tumor cell variants. J Supramol Struct. 1979;11(1):105–111. doi: 10.1002/jss.400110111. [DOI] [PubMed] [Google Scholar]
- Schirrmacher V., Shantz G., Clauer K., Komitowski D., Zimmermann H. P., Lohmann-Matthes M. L. Tumor metastases and cell-mediated immunity in a model system in DBA/2 mice. I. Tumor invasiveness in vitro and metastasis formation in vivo. Int J Cancer. 1979 Feb;23(2):233–244. doi: 10.1002/ijc.2910230215. [DOI] [PubMed] [Google Scholar]
- Schwartz R., Schirrmacher V., Mühlradt P. F. Glycoconjugates of murine tumor lines with different metastatic capacities. I. Differences in fucose utilization and in glycoprotein patterns. Int J Cancer. 1984 Apr 15;33(4):503–509. doi: 10.1002/ijc.2910330414. [DOI] [PubMed] [Google Scholar]
- Shimizu A., Kondo S., Sabe H., Ishida N., Honjo T. Structure and function of the interleukin 2 receptor: affinity conversion model. Immunol Rev. 1986 Aug;92:103–120. doi: 10.1111/j.1600-065x.1986.tb01496.x. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Waldmann T. A., Greene W. C., Sarin P. S., Saxinger C., Blayney D. W., Blattner W. A., Goldman C. K., Bongiovanni K., Sharrow S., Depper J. M. Functional and phenotypic comparison of human T cell leukemia/lymphoma virus positive adult T cell leukemia with human T cell leukemia/lymphoma virus negative Sézary leukemia, and their distinction using anti-Tac. Monoclonal antibody identifying the human receptor for T cell growth factor. J Clin Invest. 1984 Jun;73(6):1711–1718. doi: 10.1172/JCI111379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A. The structure, function, and expression of interleukin-2 receptors on normal and malignant lymphocytes. Science. 1986 May 9;232(4751):727–732. doi: 10.1126/science.3008337. [DOI] [PubMed] [Google Scholar]
- Waller C. A., Braun M., Schirrmacher V. Quantitative analysis of cancer invasion in vitro: comparison of two new assays and of tumour sublines with different metastatic capacity. Clin Exp Metastasis. 1986 Apr-Jun;4(2):73–89. doi: 10.1007/BF00119075. [DOI] [PubMed] [Google Scholar]
- Yodoi J., Uchiyama T. IL-2 receptor dysfunction and adult T-cell leukemia. Immunol Rev. 1986 Aug;92:135–156. doi: 10.1111/j.1600-065x.1986.tb01498.x. [DOI] [PubMed] [Google Scholar]
- Yodoi J., Uchiyama T., Maeda M. T-cell growth factor receptor in adult T-cell leukemia. Blood. 1983 Aug;62(2):509–511. [PubMed] [Google Scholar]


