Abstract
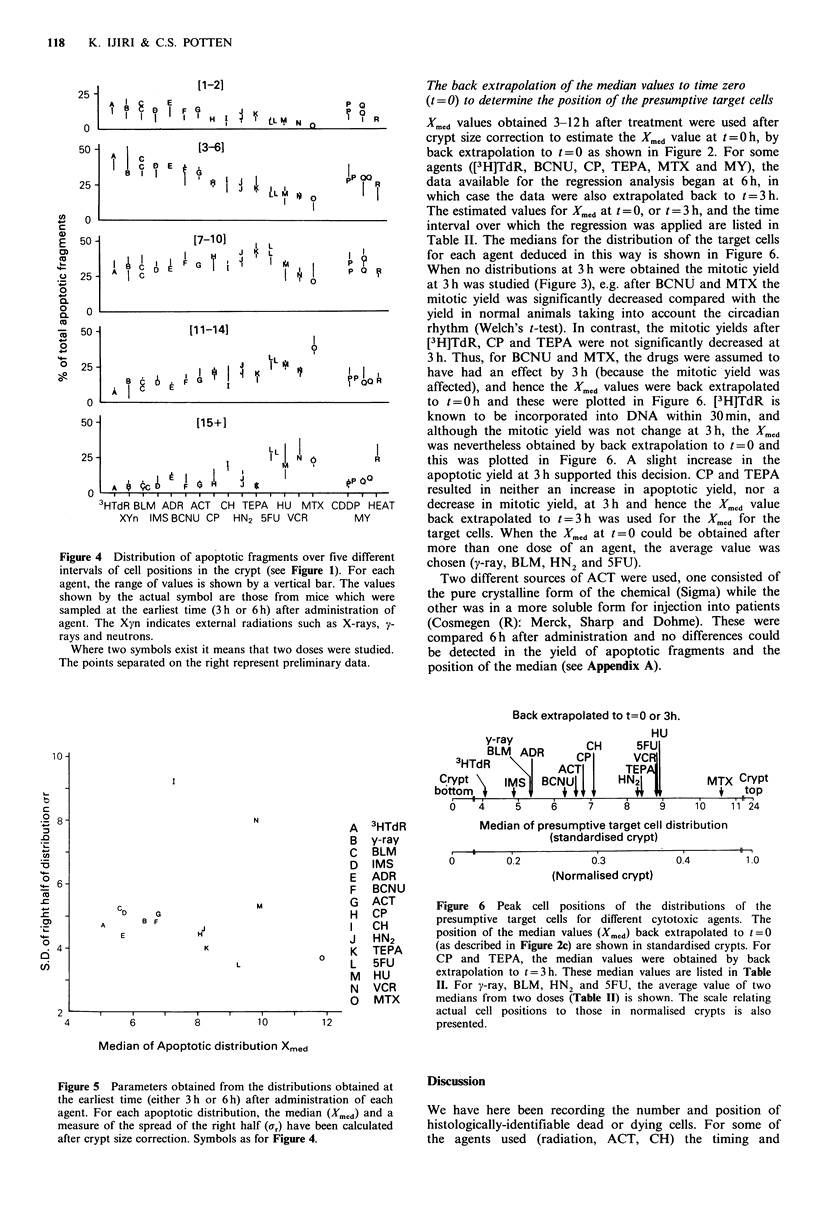
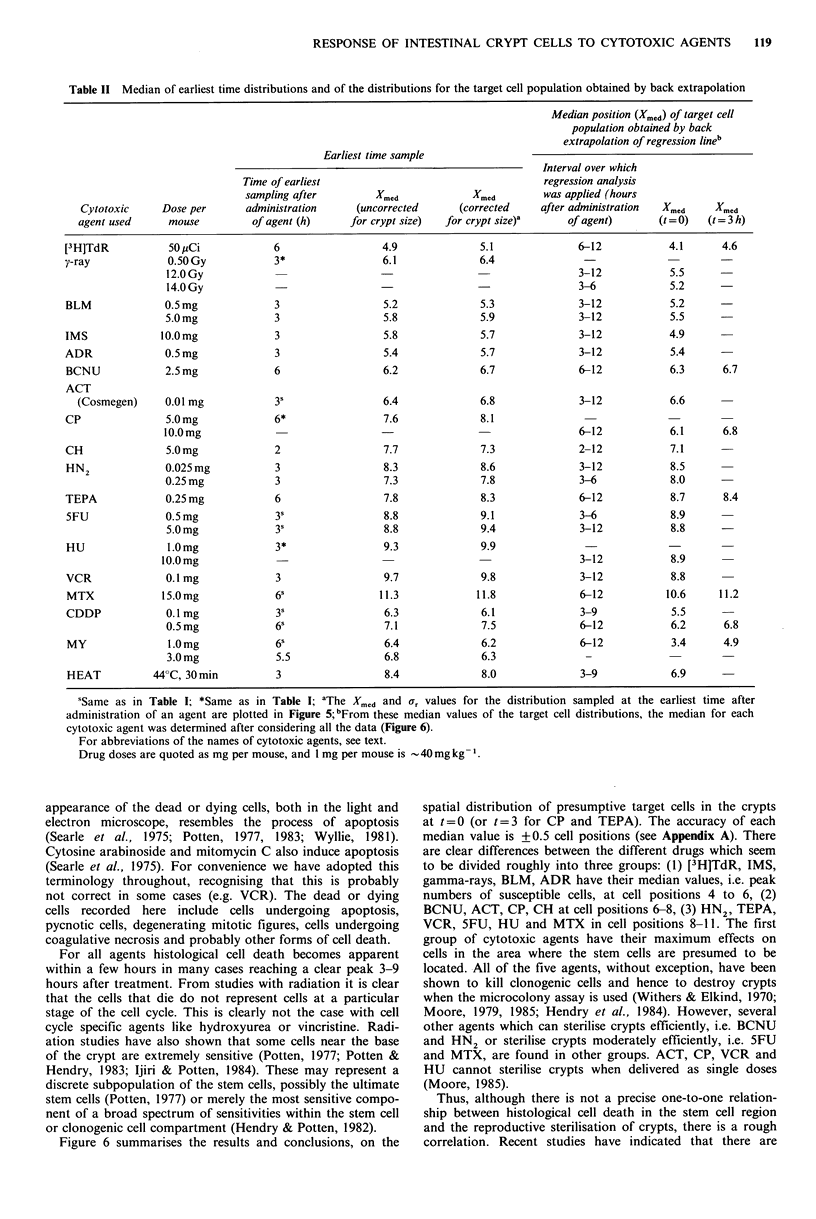
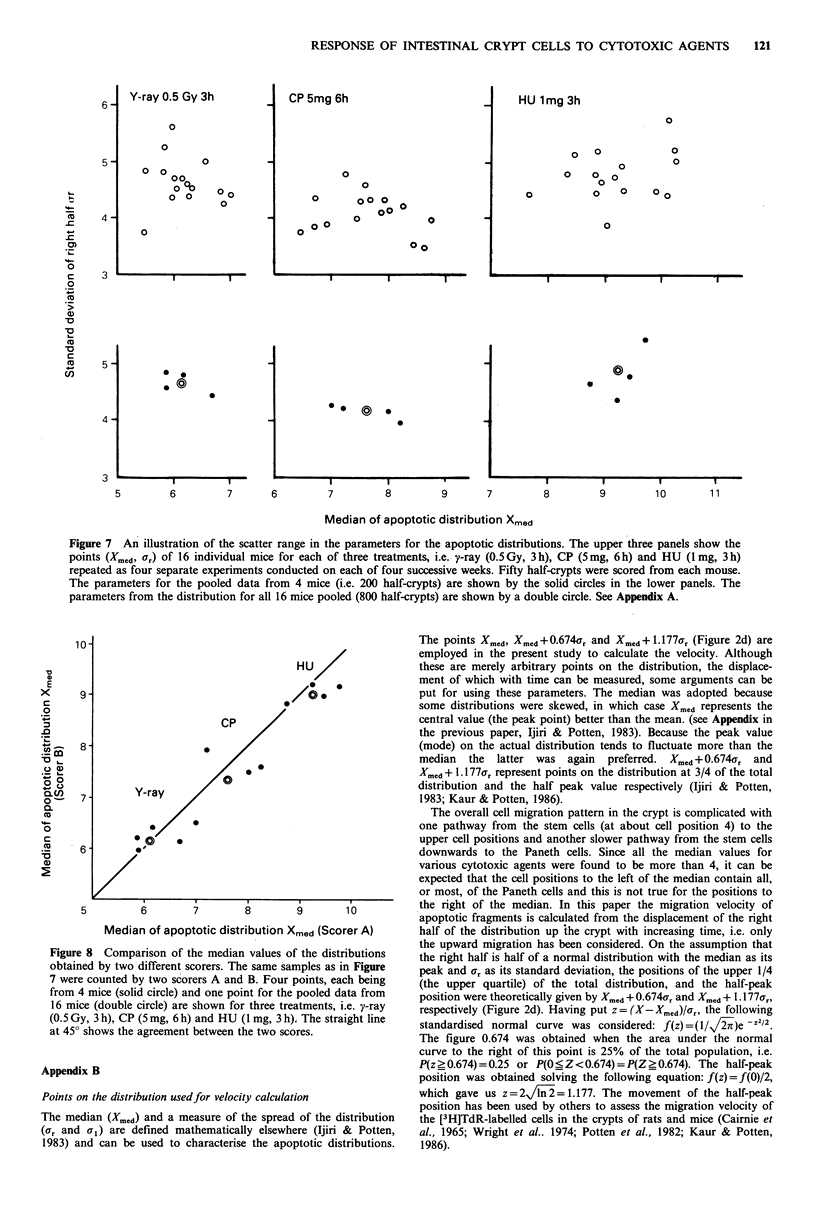
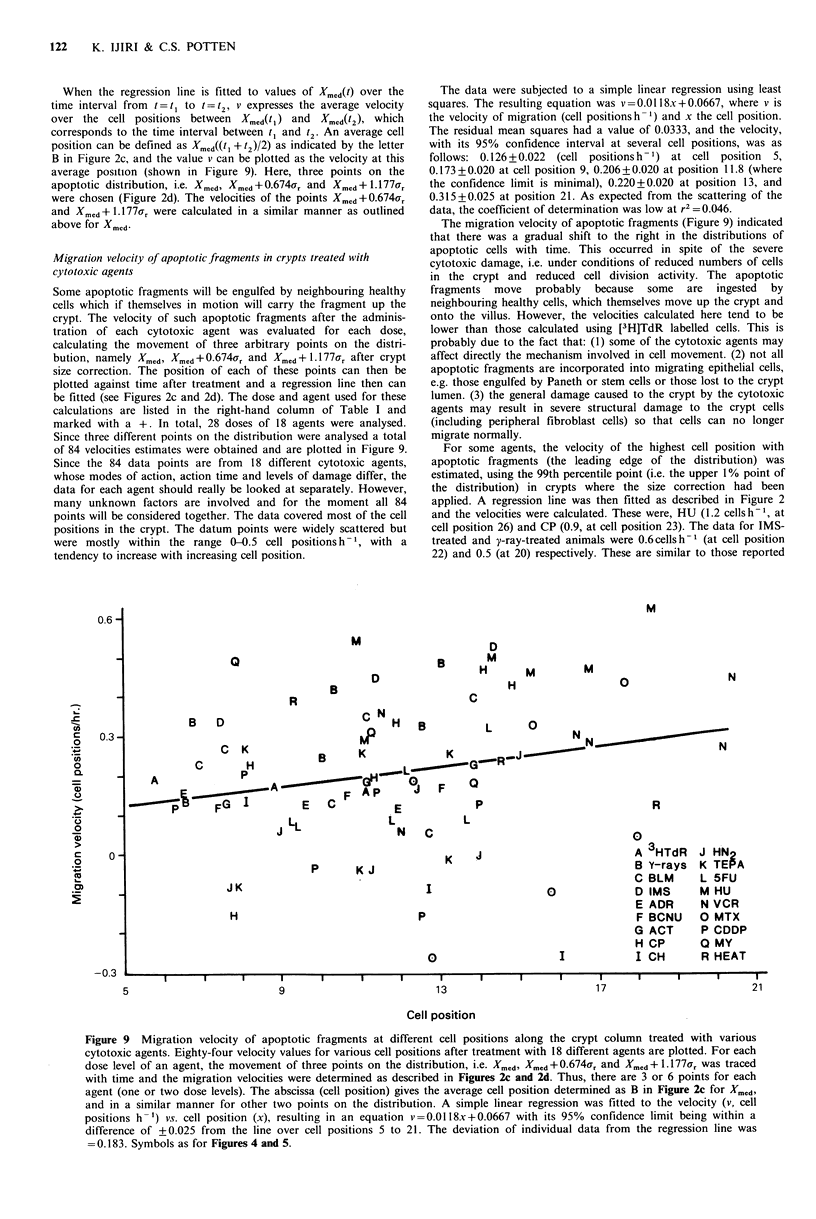
Adult male mice were treated with one or two different doses of each of 18 different cytotoxic agents. They were sampled at various times (3-12h) thereafter, and the spatial distributions of cell death in the small intestinal crypts were studied. Dead or dying cells or cells carrying dead cell fragments were examined histologically, and all of these were recorded (for convenience as apoptotic fragments), relative to the cell position in the crypt. Thus, distributions of apoptotic fragments against cell position were determined. A regression analysis of the data obtained at different times after administration of each agent was undertaken and the position of the median of the spatial distribution of presumptive target cells was deduced for each cytotoxic agent. The accuracy of this median value was determined to be +/- 0.5 cell positions. From these median values, the different cytotoxic agents could be divided roughly into three groups: [3H]thymidine, isopropyl-methane-sulphonate, gamma-rays, bleomycin and adriamycin all have their median values (susceptible cells) at cell positions 4 to 6; bischlorethylnitrosourea, actinomycin D, cyclophosphamide and cycloheximide at cell positions 6-8; mechlorethamine, triethylenethiophosphoramide, vincristine, 5-fluorouracil, hydroxyurea and methotrexate at cell positions 8-11. The position of these medians was considered in relation to the killing of clonogenic cells. Preliminary studies on the distributions of dead cells after myleran, cis-platinum and heat (hyperthermia) were also reported. There is a general tendency for antibiotics and radiation to attack the lower cell positions in the crypt. Alkylating agents on the other hand have a somewhat broad spectrum of action. Antimetabolites and a microtubule dissociating agent act on higher cell positions. No difference could be detected between two different forms (sources) of actinomycin D. The changes in the yields of apoptotic and mitotic cells with time and the migration velocities of cells in the crypts carrying apoptotic fragments after exposure to cytotoxics are also presented.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cairnie A. B., Lamerton L. F., Steel G. G. Cell proliferation studies in the intestinal epithelium of the rat. I. Determination of the kinetic parameters. Exp Cell Res. 1965 Sep;39(2):528–538. doi: 10.1016/0014-4827(65)90055-8. [DOI] [PubMed] [Google Scholar]
- Camplejohn R. S., Schultze B., Maurer W. An in vivo double labelling study of the subsequent fate of cells arrested in metaphase by vincristine in the JB-1 mouse ascites tumour. Cell Tissue Kinet. 1980 May;13(3):239–250. doi: 10.1111/j.1365-2184.1980.tb00463.x. [DOI] [PubMed] [Google Scholar]
- Cheng H., Leblond C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am J Anat. 1974 Dec;141(4):461–479. doi: 10.1002/aja.1001410403. [DOI] [PubMed] [Google Scholar]
- Hendry J. H., Moore J. V., Potten C. S. The proliferative status of microcolony-forming cells in mouse small intestine. Cell Tissue Kinet. 1984 Jan;17(1):41–47. doi: 10.1111/j.1365-2184.1984.tb00566.x. [DOI] [PubMed] [Google Scholar]
- Hendry J. H., Potten C. S. Intestinal cell radiosensitivity: a comparison for cell death assayed by apoptosis or by a loss of clonogenicity. Int J Radiat Biol Relat Stud Phys Chem Med. 1982 Dec;42(6):621–628. doi: 10.1080/09553008214551601. [DOI] [PubMed] [Google Scholar]
- Hume S. P., Marigold J. C., Field S. B. The effect of local hyperthermia on the small intestine of the mouse. Br J Radiol. 1979 Aug;52(620):657–662. doi: 10.1259/0007-1285-52-620-657. [DOI] [PubMed] [Google Scholar]
- Ijiri K., Potten C. S. Response of intestinal cells of differing topographical and hierarchical status to ten cytotoxic drugs and five sources of radiation. Br J Cancer. 1983 Feb;47(2):175–185. doi: 10.1038/bjc.1983.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijiri K., Potten C. S. The re-establishment of hypersensitive cells in the crypts of irradiated mouse intestine. Int J Radiat Biol Relat Stud Phys Chem Med. 1984 Nov;46(5):609–623. doi: 10.1080/09553008414551801. [DOI] [PubMed] [Google Scholar]
- Kaur P., Potten C. S. Cell migration velocities in the crypts of the small intestine after cytotoxic insult are not dependent on mitotic activity. Cell Tissue Kinet. 1986 Nov;19(6):601–610. doi: 10.1111/j.1365-2184.1986.tb00761.x. [DOI] [PubMed] [Google Scholar]
- Meistrich M. L., Finch M., da Cunha M. F., Hacker U., Au W. W. Damaging effects of fourteen chemotherapeutic drugs on mouse testis cells. Cancer Res. 1982 Jan;42(1):122–131. [PubMed] [Google Scholar]
- Moore J. V. Ablation of murine jejunal crypts by alkylating agents. Br J Cancer. 1979 Feb;39(2):175–181. doi: 10.1038/bjc.1979.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. V. Clonogenic response of cells of murine intestinal crypts to 12 cytotoxic drugs. Cancer Chemother Pharmacol. 1985;15(1):11–15. doi: 10.1007/BF00257286. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Al-Barwari S. E., Hume W. J., Searle J. Circadian rhythms of presumptive stem cells in three different epithelia of the mouse. Cell Tissue Kinet. 1977 Nov;10(6):557–568. doi: 10.1111/j.1365-2184.1977.tb00312.x. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Chwalinski S., Swindell R., Palmer M. The spatial organization of the hierarchical proliferative cells of the crypts of the small intestine into clusters of 'synchronized' cells. Cell Tissue Kinet. 1982 Jul;15(4):351–370. doi: 10.1111/j.1365-2184.1982.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Potten C. S. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature. 1977 Oct 6;269(5628):518–521. doi: 10.1038/269518a0. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Hendry J. H. Differential regeneration of intestinal proliferative cells and cryptogenic cells after irradiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1975 May;27(5):413–424. doi: 10.1080/09553007514550411. [DOI] [PubMed] [Google Scholar]
- Searle J., Lawson T. A., Abbott P. J., Harmon B., Kerr J. F. An electron-microscope study of the mode of cell death induced by cancer-chemotherapeutic agents in populations of proliferating normal and neoplastic cells. J Pathol. 1975 Jul;116(3):129–138. doi: 10.1002/path.1711160302. [DOI] [PubMed] [Google Scholar]
- Sigdestad C. P., Bauman J., Lesher S. W. Diurnal fluctuations in the number of cells in mitosis and DNA synthesis in the jejunum of the mouse. Exp Cell Res. 1969 Nov;58(1):159–162. doi: 10.1016/0014-4827(69)90126-8. [DOI] [PubMed] [Google Scholar]
- Withers H. R., Elkind M. M. Microcolony survival assay for cells of mouse intestinal mucosa exposed to radiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;17(3):261–267. doi: 10.1080/09553007014550291. [DOI] [PubMed] [Google Scholar]
- Wright N., Watson A., Morley A., Appleton D., Marks J., Douglas A. The measurement of cell production rates in the crypts of Lieberkuhn. An experimental and clinical study. Virchows Arch A Pathol Anat Histol. 1974;364(4):311–323. doi: 10.1007/BF00432729. [DOI] [PubMed] [Google Scholar]


