Abstract
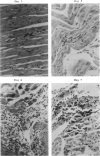
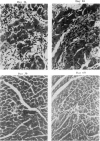
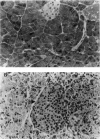
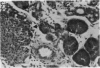
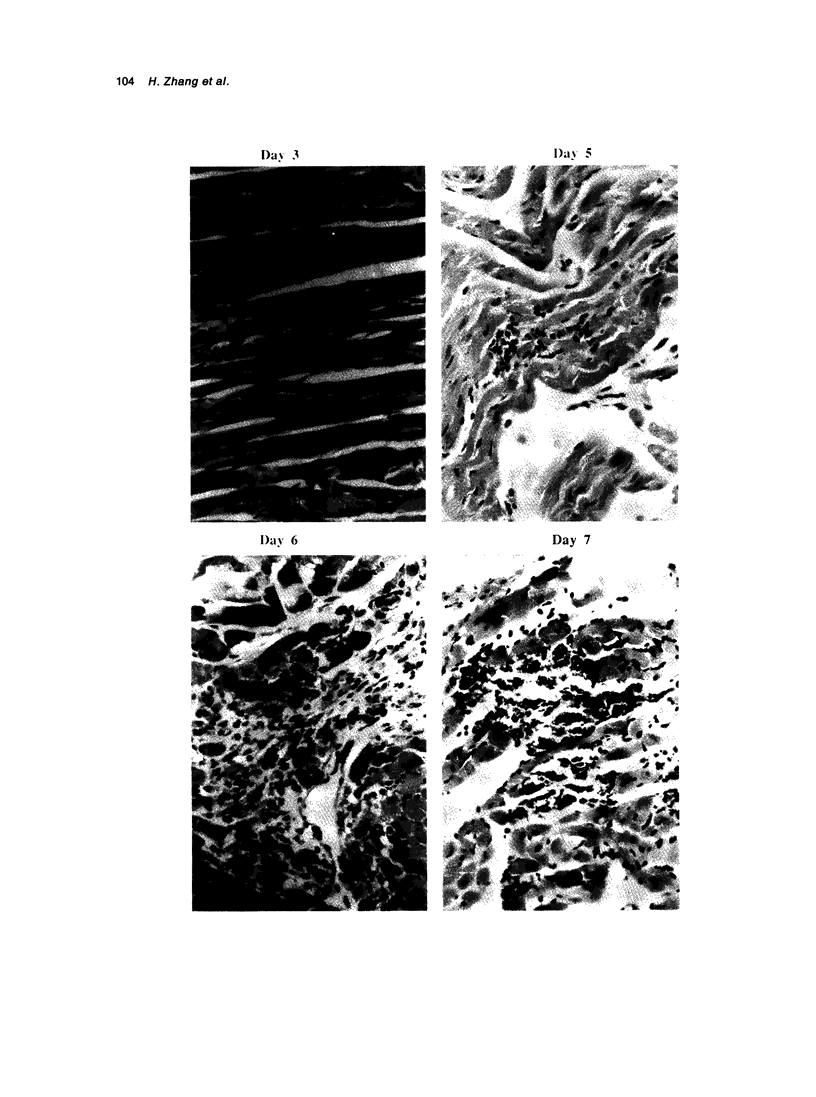
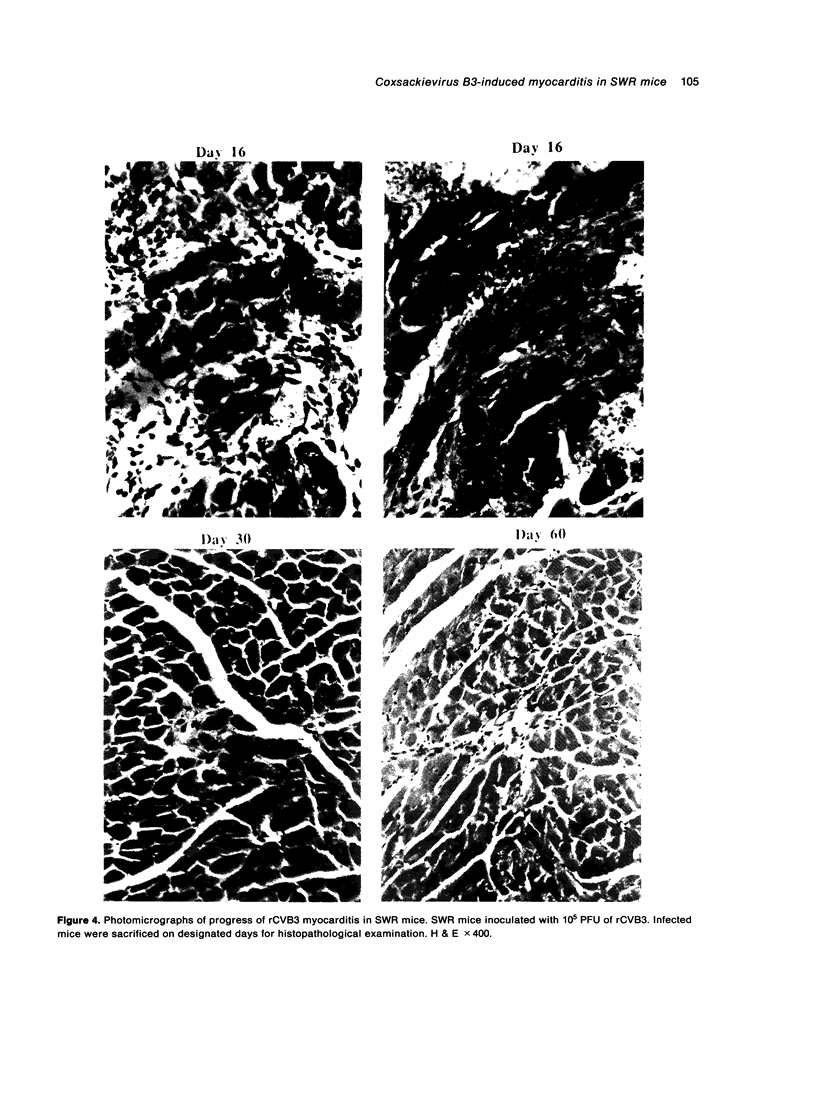
A transfection-reactivated Coxsackievirus B3 (rCVB3), from a full-length cDNA clone of Nancy strain, has previously been shown to be as cardiovirulent as the wild-type virus. Myocarditis induced by this genetically defined virus was compared in SWR mice with the traditional Balb/c model. SWR mice inoculated with rCVB3 developed more severe myocarditis but less severe pancreatitis than Balb/c mice. In contrast to the poor general health and frequent mortality of Balb/c mice following CVB3 infection, the body weight of SWR mice was not affected by CVB3 inoculation and no mortality occurred at titres of 10(2)-10(7) plaque forming units (PFU). Typical myocarditis developed in SWR mice 7 days post infection. Myocarditic foci consisting of necrotic myocardial fibres and mononuclear cell infiltrates resolved by day 30, similar to that observed in Balb/c. However, SWR mice were more sensitive to rCVB3-induced myocarditis than were Balb/c mice: mild myocarditis was induced (4/4) by as low as 10(2) PFU of the virus (ID50 < 10(1.5) PFU), and more severe myocarditis was seen at higher PFU of virus in a dose-dependent manner. The SWR model was tested with attenuated variants derived from cardiovirulent rCVB3. The ID50 for myocarditis was 10(7) PFU for a large plaque-size attenuant and 10(6) PFU for a minute plaque-size attenuant, indicating loss of cardiovirulence by a factor of more than 10(4)-10(5). rCVB3-induced SWR mouse is a sensitive and reliable model for myocarditis. It is useful in assessing the cardiovirulence of different CVB3 variants and evaluating the efficacies of anti-viral therapies. It will allow follow-up study after high dose infection with cardiovirulent rCVB3.
Full text
PDF

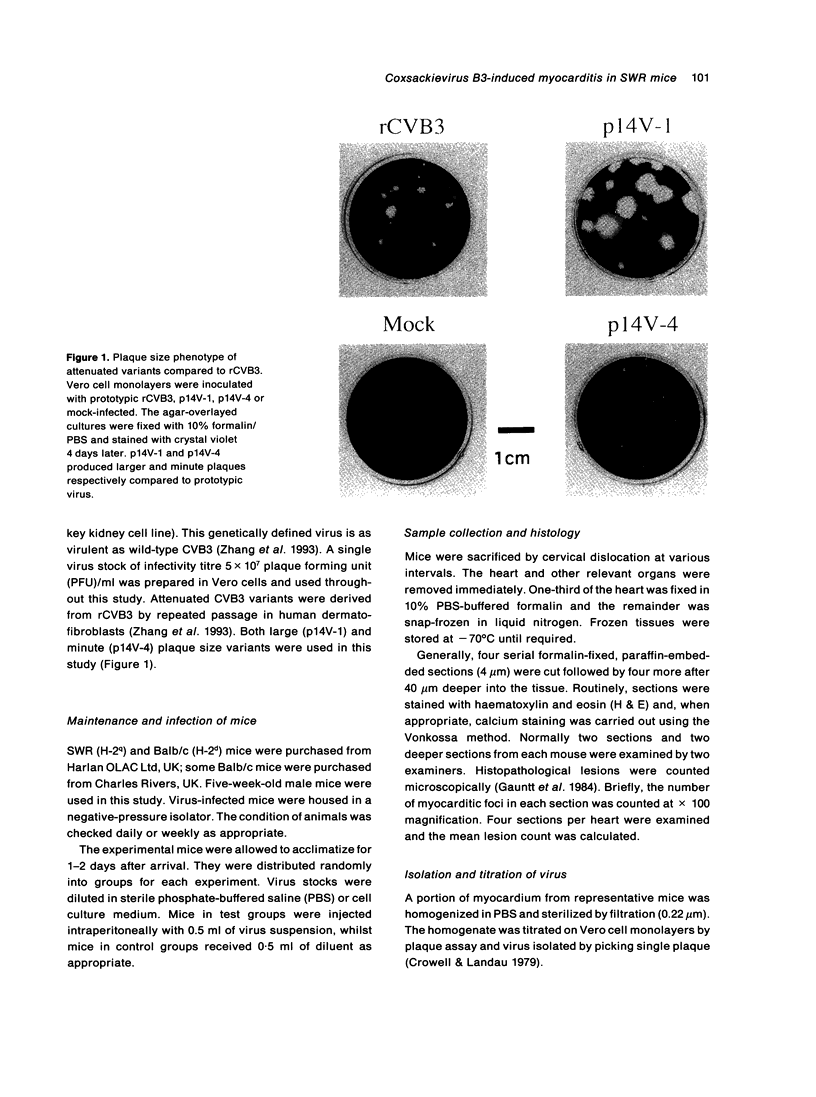
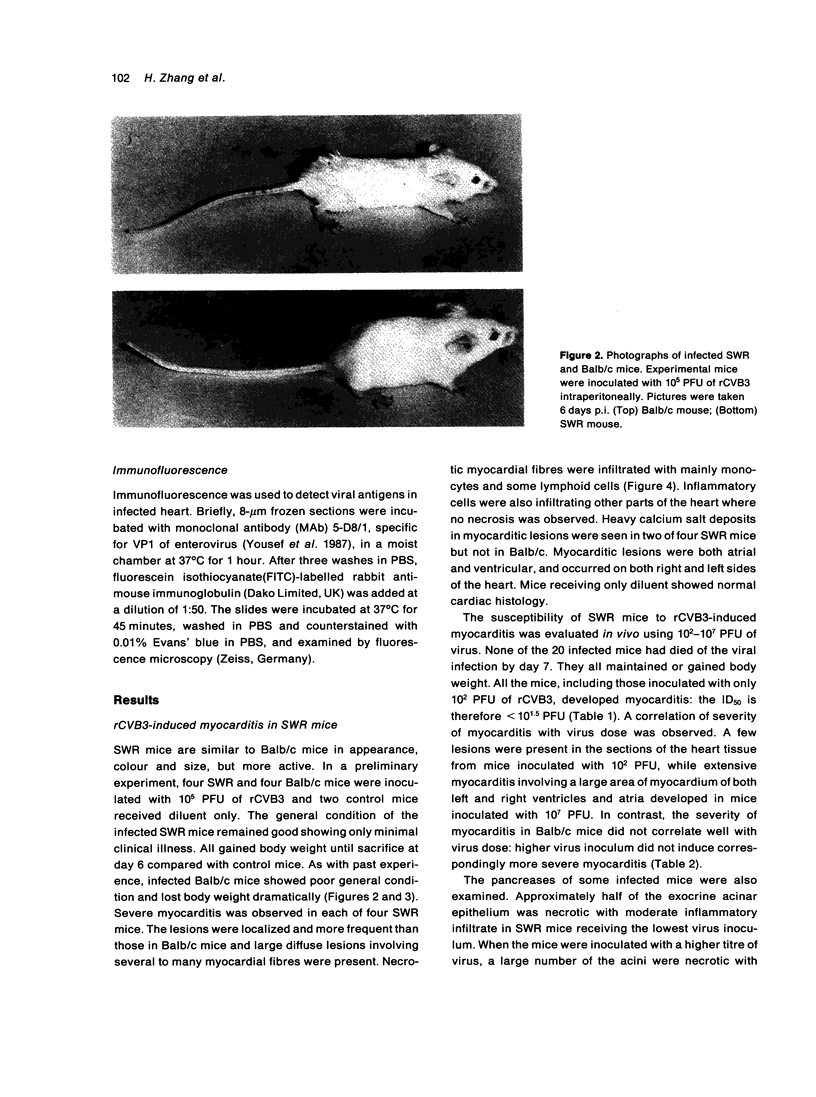
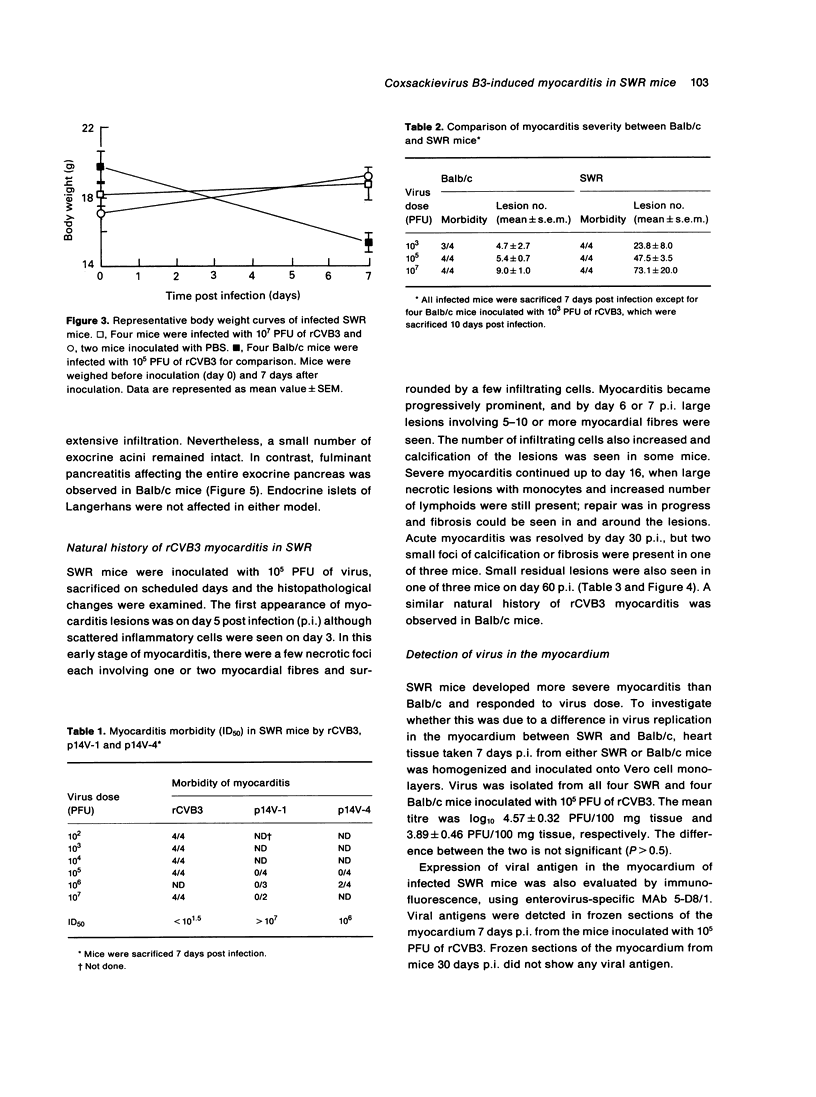



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowles N. E., Richardson P. J., Olsen E. G., Archard L. C. Detection of Coxsackie-B-virus-specific RNA sequences in myocardial biopsy samples from patients with myocarditis and dilated cardiomyopathy. Lancet. 1986 May 17;1(8490):1120–1123. doi: 10.1016/s0140-6736(86)91837-4. [DOI] [PubMed] [Google Scholar]
- Bowles N. E., Rose M. L., Taylor P., Banner N. R., Morgan-Capner P., Cunningham L., Archard L. C., Yacoub M. H. End-stage dilated cardiomyopathy. Persistence of enterovirus RNA in myocardium at cardiac transplantation and lack of immune response. Circulation. 1989 Nov;80(5):1128–1136. doi: 10.1161/01.cir.80.5.1128. [DOI] [PubMed] [Google Scholar]
- Cao Y., Schnurr D. P., Schmidt N. J. Differing cardiotropic and myocarditic properties of group B type 4 coxsackievirus strains. Arch Virol. 1984;80(2-3):119–130. doi: 10.1007/BF01310653. [DOI] [PubMed] [Google Scholar]
- Fohlman J., Friman G., Ilbäck N. G., Akesson A., Huber S. A qualitative and quantitative method for in situ characterization of the inflammatory response in experimental myocarditis. APMIS. 1990 Jun;98(6):559–567. doi: 10.1111/j.1699-0463.1990.tb01071.x. [DOI] [PubMed] [Google Scholar]
- Gauntt C. J., Gomez P. T., Duffey P. S., Grant J. A., Trent D. W., Witherspoon S. M., Paque R. E. Characterization and myocarditic capabilities of coxsackievirus B3 variants in selected mouse strains. J Virol. 1984 Nov;52(2):598–605. doi: 10.1128/jvi.52.2.598-605.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
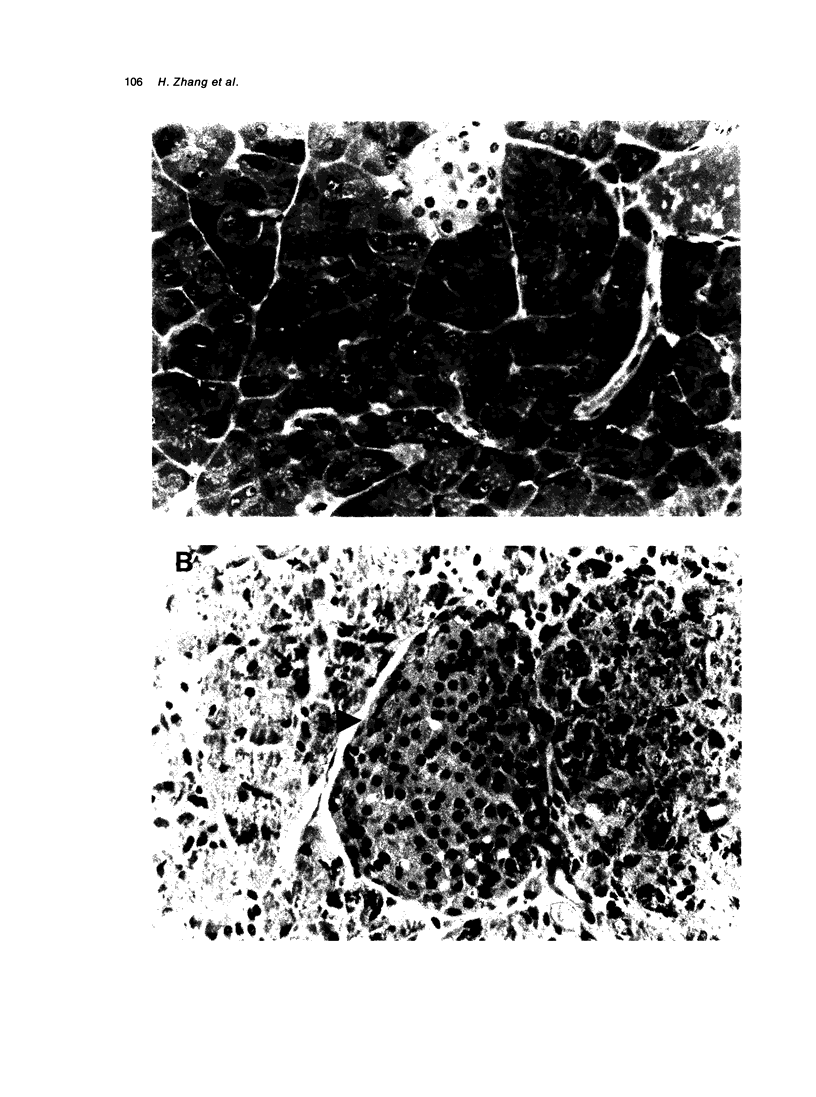
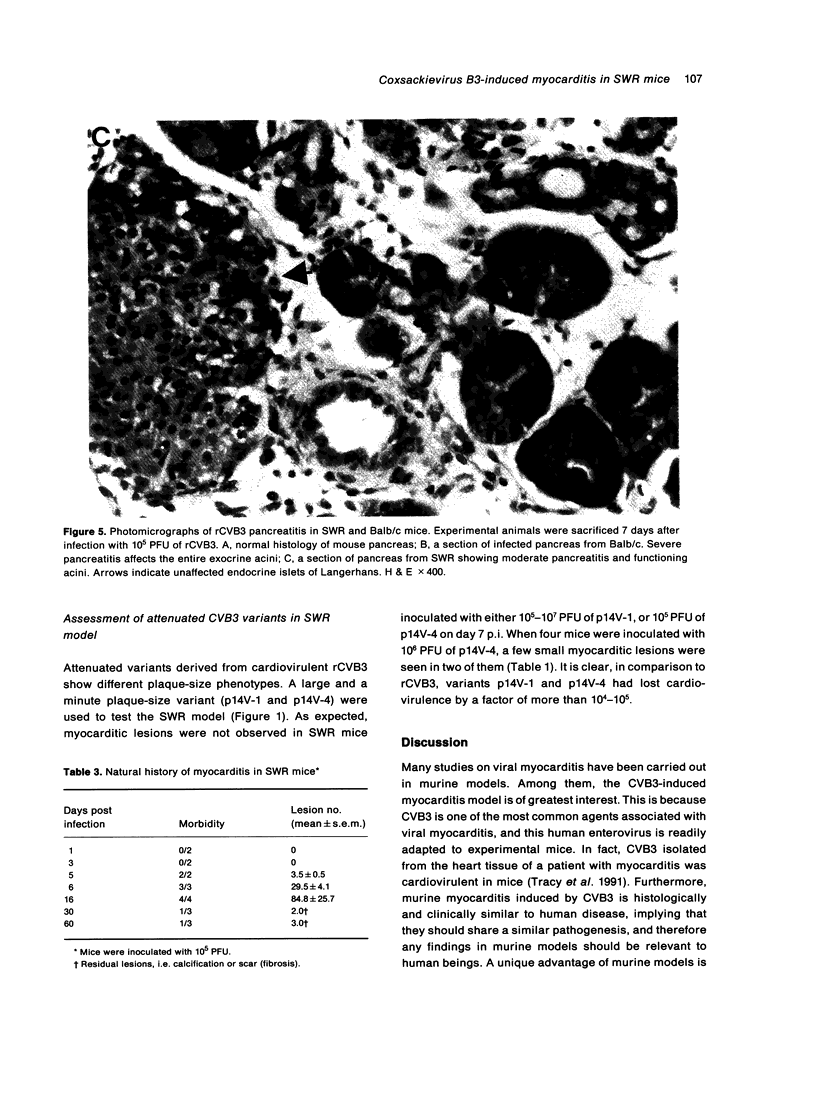
- Gómez R. M., Lascano E. F., Berría M. I. Murine acinar pancreatitis preceding necrotizing myocarditis after Coxsackievirus B3 inoculation. J Med Virol. 1991 Oct;35(2):71–75. doi: 10.1002/jmv.1890350202. [DOI] [PubMed] [Google Scholar]
- Hoshino T., Kawai C., Tokuda M. Experimental coxsackie B viral myocarditis in cynomolgus monkeys. Jpn Circ J. 1983 Jan;47(1):59–66. doi: 10.1253/jcj.47.59. [DOI] [PubMed] [Google Scholar]
- Huber S. A., Lodge P. A. Coxsackievirus B-3 myocarditis. Identification of different pathogenic mechanisms in DBA/2 and Balb/c mice. Am J Pathol. 1986 Feb;122(2):284–291. [PMC free article] [PubMed] [Google Scholar]
- Kandolf R., Hofschneider P. H. Molecular cloning of the genome of a cardiotropic Coxsackie B3 virus: full-length reverse-transcribed recombinant cDNA generates infectious virus in mammalian cells. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4818–4822. doi: 10.1073/pnas.82.14.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingel K., Hohenadl C., Canu A., Albrecht M., Seemann M., Mall G., Kandolf R. Ongoing enterovirus-induced myocarditis is associated with persistent heart muscle infection: quantitative analysis of virus replication, tissue damage, and inflammation. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):314–318. doi: 10.1073/pnas.89.1.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump W. M., Bergmann I., Müller B. C., Ameis D., Kandolf R. Complete nucleotide sequence of infectious Coxsackievirus B3 cDNA: two initial 5' uridine residues are regained during plus-strand RNA synthesis. J Virol. 1990 Apr;64(4):1573–1583. doi: 10.1128/jvi.64.4.1573-1583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide H., Kitaura Y., Deguchi H., Ukimura A., Kawamura K., Hirai K. Genomic detection of enteroviruses in the myocardium--studies on animal hearts with coxsackievirus B3 myocarditis and endomyocardial biopsies from patients with myocarditis and dilated cardiomyopathy. Jpn Circ J. 1992 Oct;56(10):1081–1093. doi: 10.1253/jcj.56.1081. [DOI] [PubMed] [Google Scholar]
- Kyu B., Matsumori A., Sato Y., Okada I., Chapman N. M., Tracy S. Cardiac persistence of cardioviral RNA detected by polymerase chain reaction in a murine model of dilated cardiomyopathy. Circulation. 1992 Aug;86(2):522–530. doi: 10.1161/01.cir.86.2.522. [DOI] [PubMed] [Google Scholar]
- Lawson C. M., O'Donoghue H., Bartholomaeus W. N., Reed W. D. Genetic control of mouse cytomegalovirus-induced myocarditis. Immunology. 1990 Jan;69(1):20–26. [PMC free article] [PubMed] [Google Scholar]
- Lerner A. M., Wilson F. M. Virus myocardiopathy. Prog Med Virol. 1973;15:63–91. [PubMed] [Google Scholar]
- Liljeqvist J. A., Bergström T., Holmström S., Samuelson A., Yousef G. E., Waagstein F., Jeansson S. Failure to demonstrate enterovirus aetiology in Swedish patients with dilated cardiomyopathy. J Med Virol. 1993 Jan;39(1):6–10. doi: 10.1002/jmv.1890390103. [DOI] [PubMed] [Google Scholar]
- Lyden D. C., Olszewski J., Feran M., Job L. P., Huber S. A. Coxsackievirus B-3-induced myocarditis. Effect of sex steroids on viremia and infectivity of cardiocytes. Am J Pathol. 1987 Mar;126(3):432–438. [PMC free article] [PubMed] [Google Scholar]
- Lyden D., Olszewski J., Huber S. Variation in susceptibility of Balb/c mice to coxsackievirus group B type 3-induced myocarditis with age. Cell Immunol. 1987 Apr 1;105(2):332–339. doi: 10.1016/0008-8749(87)90081-5. [DOI] [PubMed] [Google Scholar]
- Lynch C. J. The so-called Swiss mouse. Lab Anim Care. 1969 Apr;19(2):214–220. [PubMed] [Google Scholar]
- Matsumori A. Lessons from animal experiments in myocarditis. Herz. 1992 Apr;17(2):107–111. [PubMed] [Google Scholar]
- Petitjean J., Kopecka H., Freymuth F., Langlard J. M., Scanu P., Galateau F., Bouhour J. B., Ferrière M., Charbonneau P., Komajda M. Detection of enteroviruses in endomyocardial biopsy by molecular approach. J Med Virol. 1992 May;37(1):76–82. doi: 10.1002/jmv.1890370114. [DOI] [PubMed] [Google Scholar]
- RABIN E. R., HASSAN S. A., JENSON A. B., MELNICK J. L. COXSACKIE VIRUS B3 MYOCARDITIS IN MICE. AN ELECTRON MICROSCOPIC, IMMUNOFLUORESCENT AND VIRUS-ASSAY STUDY. Am J Pathol. 1964 May;44:775–797. [PMC free article] [PubMed] [Google Scholar]
- Sherry B., Schoen F. J., Wenske E., Fields B. N. Derivation and characterization of an efficiently myocarditic reovirus variant. J Virol. 1989 Nov;63(11):4840–4849. doi: 10.1128/jvi.63.11.4840-4849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy S., Chapman N. M., Tu Z. Coxsackievirus B3 from an infectious cDNA copy of the genome is cardiovirulent in mice. Arch Virol. 1992;122(3-4):399–409. doi: 10.1007/BF01317202. [DOI] [PubMed] [Google Scholar]
- Trousdale M. D., Paque R. E., Nealon T., Gauntt C. J. Assessment of coxsackievirus B3 ts mutants for induction of myocarditis in a murine model. Infect Immun. 1979 Feb;23(2):486–495. doi: 10.1128/iai.23.2.486-495.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuorinen T., Kallajoki M., Hyypiä T., Vainionpä R. Coxsackievirus B3-induced acute pancreatitis: analysis of histopathological and viral parameters in a mouse model. Br J Exp Pathol. 1989 Aug;70(4):395–403. [PMC free article] [PubMed] [Google Scholar]
- Wee L., Liu P., Penn L., Butany J. W., McLaughlin P. R., Sole M. J., Liew C. C. Persistence of viral genome into late stages of murine myocarditis detected by polymerase chain reaction. Circulation. 1992 Nov;86(5):1605–1614. doi: 10.1161/01.cir.86.5.1605. [DOI] [PubMed] [Google Scholar]
- Wolfgram L. J., Beisel K. W., Herskowitz A., Rose N. R. Variations in the susceptibility to Coxsackievirus B3-induced myocarditis among different strains of mice. J Immunol. 1986 Mar 1;136(5):1846–1852. [PubMed] [Google Scholar]
- Woodruff J. F., Kilbourne E. D. The influence of quantitated post-weaning undernutrition on coxsackievirus B3 infection of adult mice. I. Viral persistence and increased severity of lesions. J Infect Dis. 1970 Feb;121(2):137–163. doi: 10.1093/infdis/121.2.137. [DOI] [PubMed] [Google Scholar]
- Woodruff J. F. Viral myocarditis. A review. Am J Pathol. 1980 Nov;101(2):425–484. [PMC free article] [PubMed] [Google Scholar]
- Woodruff J. F., Woodruff J. J. Involvement of T lymphocytes in the pathogenesis of coxsackie virus B3 heart disease. J Immunol. 1974 Dec;113(6):1726–1734. [PubMed] [Google Scholar]
- Yousef G. E., Brown I. N., Mowbray J. F. Derivation and biochemical characterization of an enterovirus group-specific monoclonal antibody. Intervirology. 1987;28(3):163–170. doi: 10.1159/000150012. [DOI] [PubMed] [Google Scholar]
- Zhang H. Y., Yousef G. E., Cunningham L., Blake N. W., OuYang X., Bayston T. A., Kandolf R., Archard L. C. Attenuation of a reactivated cardiovirulent coxsackievirus B3: The 5'-nontranslated region does not contain major attenuation determinants. J Med Virol. 1993 Oct;41(2):129–137. doi: 10.1002/jmv.1890410208. [DOI] [PubMed] [Google Scholar]
- Zoll G. J., Melchers W. J., Kopecka H., Jambroes G., van der Poel H. J., Galama J. M. General primer-mediated polymerase chain reaction for detection of enteroviruses: application for diagnostic routine and persistent infections. J Clin Microbiol. 1992 Jan;30(1):160–165. doi: 10.1128/jcm.30.1.160-165.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]