Abstract
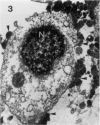
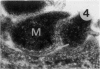
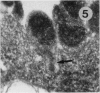
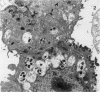
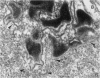
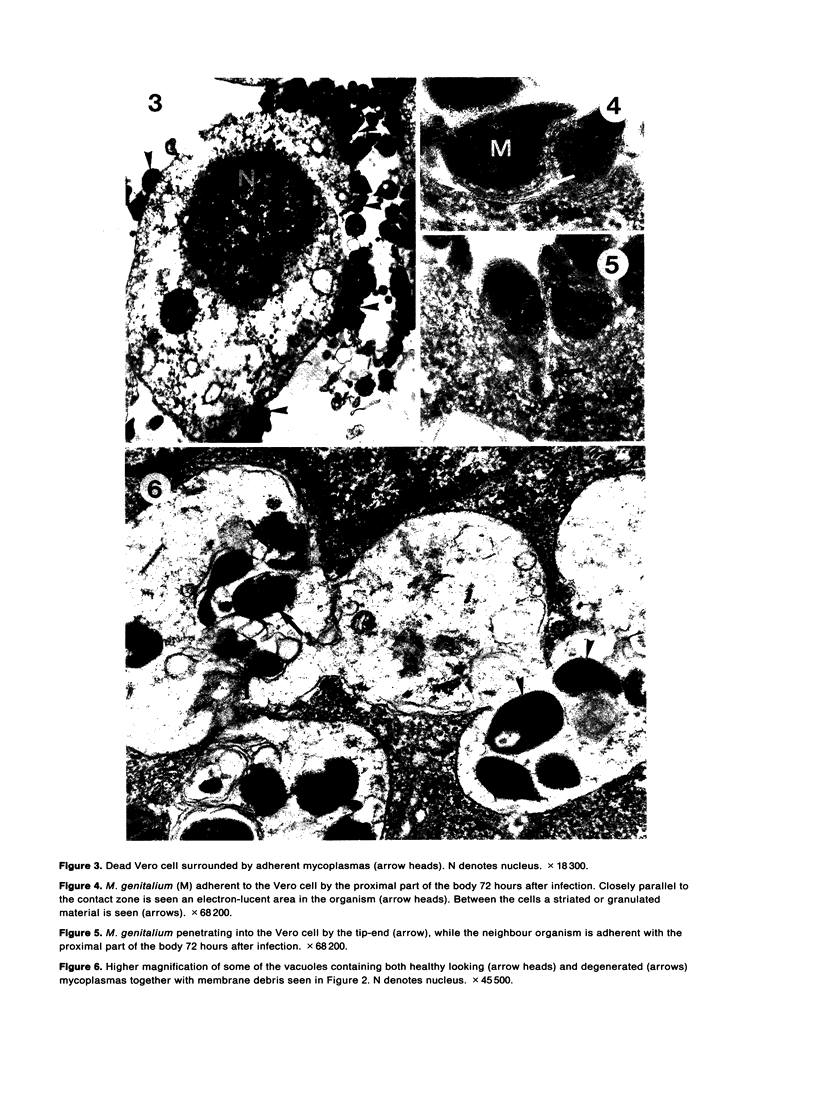
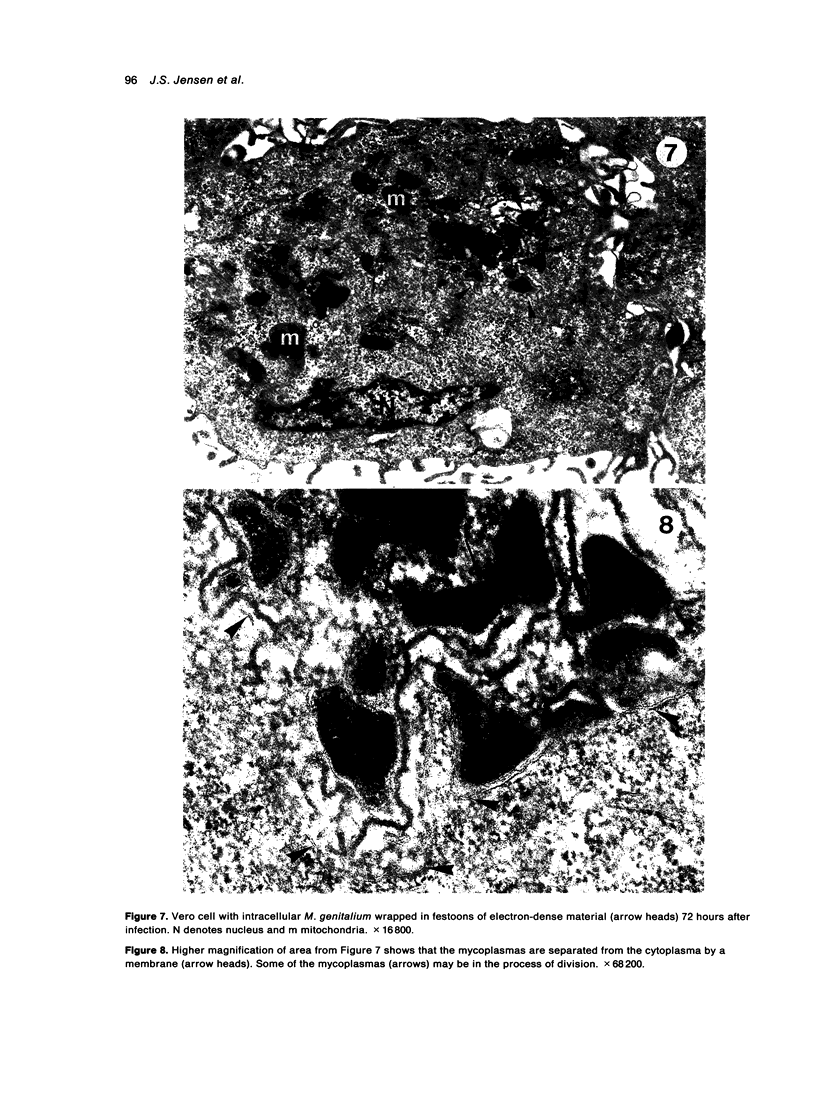
The original two strains of Mycoplasma genitalium were isolated from the human urogenital tract. No other strains have been isolated from this site since then. We have recently succeeded in propagating a third strain from a urogenital specimen from a patient with urethritis in Vero cell cultures. By electron microscopy mycoplasmas were demonstrated intracellularly in about 10% of the examined Vero cells. Various stages of penetration into the cells could be observed. The flask-shaped organisms seemed to penetrate into the cells by the tip-end which included a rodlike structure. The intracellular location of normal mycoplasmas were in membrane-bound vacuoles very close to the nucleus, occasionally together with a few disintegrated organisms. In a few cells additional material was entangling the mycoplasmas in the cytoplasmic vacuoles. The potential for intracellular survival of M. genitalium may help the organism to evade the defence mechanisms of the human body. This trait may be considered a pathogenic property which supports the presumption that M. genitalium has clinical importance.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen T. C., Stevens J. O., Florance E. R., Hampton R. O. Ultrastructure of Mycoplasma gallisepticum isolate 1056. J Ultrastruct Res. 1970 Nov;33(3):318–331. doi: 10.1016/s0022-5320(70)90025-0. [DOI] [PubMed] [Google Scholar]
- Baseman J. B., Cole R. M., Krause D. C., Leith D. K. Molecular basis for cytadsorption of Mycoplasma pneumoniae. J Bacteriol. 1982 Sep;151(3):1514–1522. doi: 10.1128/jb.151.3.1514-1522.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biberfeld G., Biberfeld P. Ultrastructural features of Mycoplasma pneumoniae. J Bacteriol. 1970 Jun;102(3):855–861. doi: 10.1128/jb.102.3.855-861.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom J., Mansa B., Wilk A. A study of Russell bodies in human monoclonal plasma cells by means of immunofluorescence and electron microscopy. Acta Pathol Microbiol Scand A. 1976 Jul;84(4):335–349. doi: 10.1111/j.1699-0463.1976.tb00124.x. [DOI] [PubMed] [Google Scholar]
- Brunner H., Krauss H., Schaar H., Schiefer H. G. Electron microscopic studies on the attachment of Mycoplasma pneumoniae to guinea pig erythrocytes. Infect Immun. 1979 Jun;24(3):906–911. doi: 10.1128/iai.24.3.906-911.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallo S. F., Chavoya A., Su C. J., Baseman J. B. DNA and protein sequence homologies between the adhesins of Mycoplasma genitalium and Mycoplasma pneumoniae. Infect Immun. 1989 Apr;57(4):1059–1065. doi: 10.1128/iai.57.4.1059-1065.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Taylor-Robinson D., Davies H. A., Dourmashkin R. R. Interaction of Mycoplasma pneumoniae with human lung fibroblasts: characterization of the in vitro model. Infect Immun. 1979 Jul;25(1):446–454. doi: 10.1128/iai.25.1.446-454.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner P. J., Gilroy C. B., Thomas B. J., Naidoo R. O., Taylor-Robinson D. Association of Mycoplasma genitalium with acute non-gonococcal urethritis. Lancet. 1993 Sep 4;342(8871):582–585. doi: 10.1016/0140-6736(93)91411-e. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Schaper U., Collier A. M., Clyde W. A., Jr, Horikawa M., Huang Y. S., Barile M. F. A Mycoplasma genitalium protein resembling the Mycoplasma pneumoniae attachment protein. Infect Immun. 1987 May;55(5):1126–1131. doi: 10.1128/iai.55.5.1126-1131.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J. S., Orsum R., Dohn B., Uldum S., Worm A. M., Lind K. Mycoplasma genitalium: a cause of male urethritis? Genitourin Med. 1993 Aug;69(4):265–269. doi: 10.1136/sti.69.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J. S., Uldum S. A., Søndergård-Andersen J., Vuust J., Lind K. Polymerase chain reaction for detection of Mycoplasma genitalium in clinical samples. J Clin Microbiol. 1991 Jan;29(1):46–50. doi: 10.1128/jcm.29.1.46-50.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind K., Kristensen G. B. Significance of antibodies to Mycoplasma genitalium in salpingitis. Eur J Clin Microbiol. 1987 Apr;6(2):205–207. doi: 10.1007/BF02018216. [DOI] [PubMed] [Google Scholar]
- Lind K., Lindhardt B. O., Schütten H. J., Blom J., Christiansen C. Serological cross-reactions between Mycoplasma genitalium and Mycoplasma pneumoniae. J Clin Microbiol. 1984 Dec;20(6):1036–1043. doi: 10.1128/jcm.20.6.1036-1043.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S. C., Dawson M. S., Wong D. M., Newton P. B., 3rd, Sonoda M. A., Engler W. F., Wang R. Y., Shih J. W., Alter H. J., Wear D. J. Identification of Mycoplasma incognitus infection in patients with AIDS: an immunohistochemical, in situ hybridization and ultrastructural study. Am J Trop Med Hyg. 1989 Nov;41(5):601–616. doi: 10.4269/ajtmh.1989.41.601. [DOI] [PubMed] [Google Scholar]
- Lo S. C., Hayes M. M., Tully J. G., Wang R. Y., Kotani H., Pierce P. F., Rose D. L., Shih J. W. Mycoplasma penetrans sp. nov., from the urogenital tract of patients with AIDS. Int J Syst Bacteriol. 1992 Jul;42(3):357–364. doi: 10.1099/00207713-42-3-357. [DOI] [PubMed] [Google Scholar]
- Loomes L. M., Uemura K., Childs R. A., Paulson J. C., Rogers G. N., Scudder P. R., Michalski J. C., Hounsell E. F., Taylor-Robinson D., Feizi T. Erythrocyte receptors for Mycoplasma pneumoniae are sialylated oligosaccharides of Ii antigen type. Nature. 1984 Feb 9;307(5951):560–563. doi: 10.1038/307560a0. [DOI] [PubMed] [Google Scholar]
- Møller B. R., Taylor-Robinson D., Furr P. M. Serological evidence implicating Mycoplasma genitalium in pelvic inflammatory disease. Lancet. 1984 May 19;1(8386):1102–1103. doi: 10.1016/s0140-6736(84)92511-x. [DOI] [PubMed] [Google Scholar]
- Palmer H. M., Gilroy C. B., Claydon E. J., Taylor-Robinson D. Detection of Mycoplasma genitalium in the genitourinary tract of women by the polymerase chain reaction. Int J STD AIDS. 1991 Jul-Aug;2(4):261–263. doi: 10.1177/095646249100200407. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Silverman D. J., Santucci L. A., Meyers N., Sekeyova Z. Penetration of host cells by Rickettsia rickettsii appears to be mediated by a phospholipase of rickettsial origin. Infect Immun. 1992 Jul;60(7):2733–2740. doi: 10.1128/iai.60.7.2733-2740.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D., Davies H. A., Sarathchandra P., Furr P. M. Intracellular location of mycoplasmas in cultured cells demonstrated by immunocytochemistry and electron microscopy. Int J Exp Pathol. 1991 Dec;72(6):705–714. [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D., Furr P. M., Hanna N. F. Microbiological and serological study of non-gonococcal urethritis with special reference to Mycoplasma genitalium. Genitourin Med. 1985 Oct;61(5):319–324. doi: 10.1136/sti.61.5.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully J. G., Taylor-Robinson D., Cole R. M., Rose D. L. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981 Jun 13;1(8233):1288–1291. doi: 10.1016/s0140-6736(81)92461-2. [DOI] [PubMed] [Google Scholar]
- Tully J. G., Whitcomb R. F., Clark H. F., Williamson D. L. Pathogenic mycoplasmas: cultivation and vertebrate pathogenicity of a new spiroplasma. Science. 1977 Mar 4;195(4281):892–894. doi: 10.1126/science.841314. [DOI] [PubMed] [Google Scholar]