Abstract
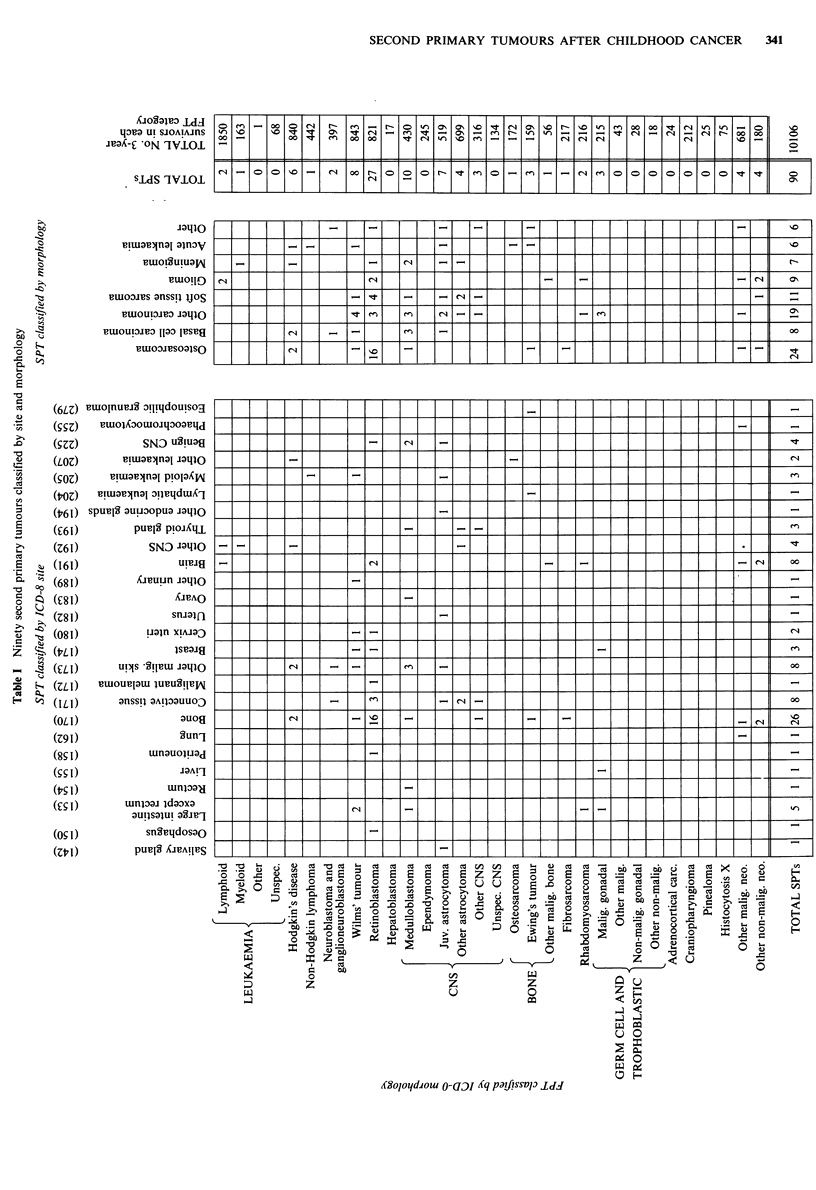
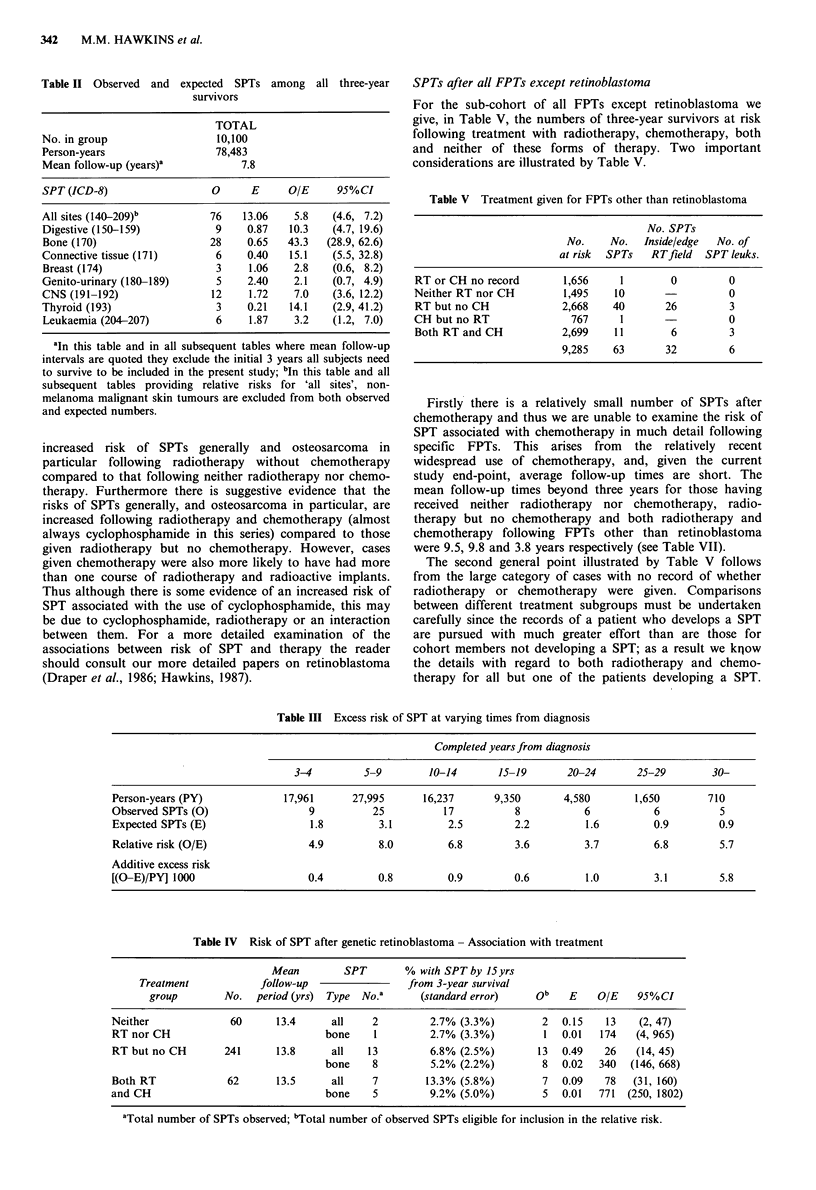
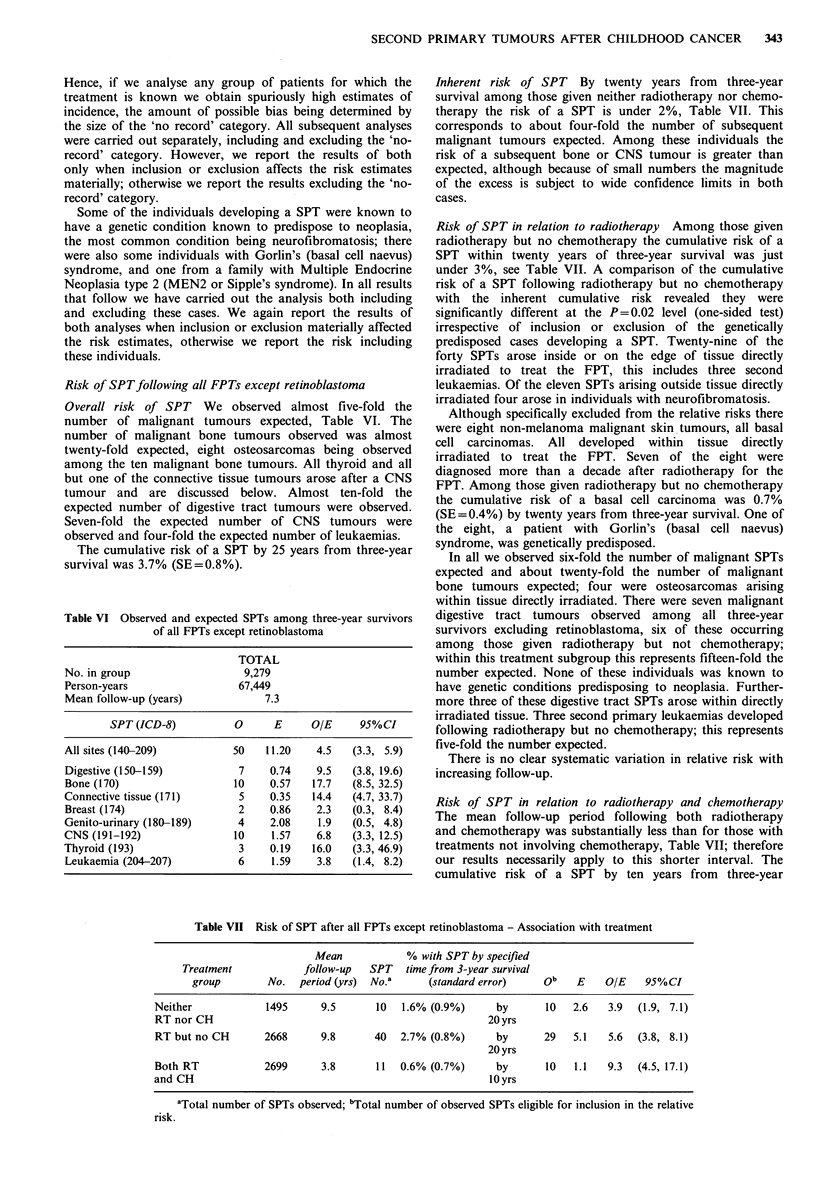
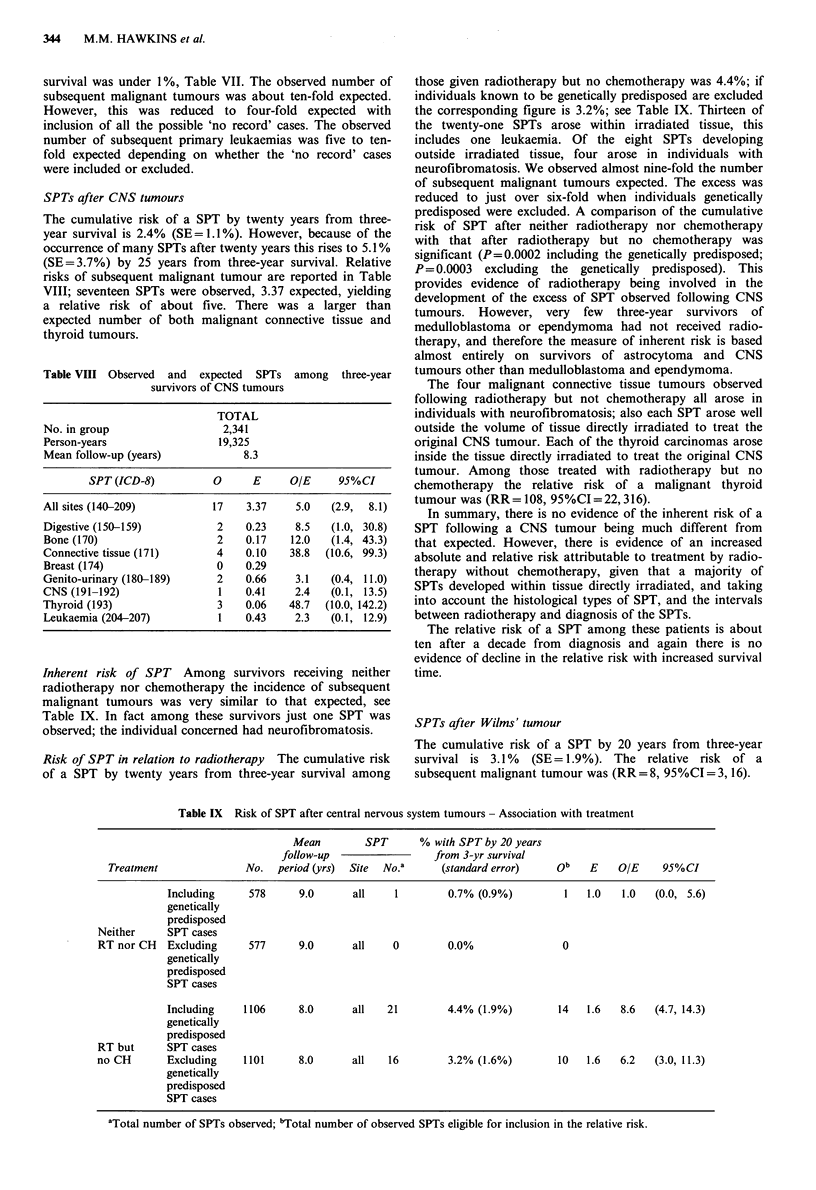
Among a cohort of 10,106 three-year survivors of childhood cancer, 90 second primary tumours (SPTs) were observed. Within 25 years of 3-year survival about 4% developed a SPT, about 6-fold expected, the relative risk not varying much with increasing follow-up. Following genetic retinoblastoma we observed 30-fold the expected number of SPTs, and over 400-fold the expected number of osteosarcomas. The risk of SPT in the absence of radiotherapy and chemotherapy (inherent risk) following genetic retinoblastoma was 13-fold expected and over 200-fold the expected number of osteosarcomas were observed. There was evidence that both radiotherapy and cyclophosphamide were associated with an increased risk of SPT. After all first primary tumours (FPTs) excluding retinoblastoma we observed almost 5-fold the expected number of SPTs. The inherent risk was 4-fold expected, the relative risks associated with radiotherapy but no chemotherapy, and both radiotherapy and chemotherapy were 6- and 9-fold expected respectively. There were about 20-fold the number of malignant bone tumours expected, most were osteosarcoma; also 7-fold the number of central nervous system tumours expected. There were 8 basal cell carcinomas and it seems likely that radiotherapy was involved in the development of some of these. Radiotherapy appears to have been involved in the development of many of the SPTs observed following all FPTs excluding retinoblastoma, particularly after CNS tumours, Wilms' tumour and Hodgkin's disease. Currently there is insufficient follow-up to examine the risk following chemotherapy. After acute leukaemia there was 20-fold the expected number of central nervous system tumours, though this is based on only 3 cases; whether therapy is directly involved in their development is uncertain. The risks we report are rarely greater than those reported in previous large-scale studies; in most instances they are substantially less. It is very unlikely that many SPTs were missed with our follow-up system so alternative explanations require further investigation; in particular it is possible the lower risks in our data compared to series treated in the United States may be explained, in part, by less combination therapy and lower doses of radiotherapy.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson D. H., Ellsworth R. M., Kitchin F. D., Tung G. Second nonocular tumors in retinoblastoma survivors. Are they radiation-induced? Ophthalmology. 1984 Nov;91(11):1351–1355. doi: 10.1016/s0161-6420(84)34127-6. [DOI] [PubMed] [Google Scholar]
- Birch J. M., Hartley A. L., Marsden H. B., Harris M., Swindell R. Excess risk of breast cancer in the mothers of children with soft tissue sarcomas. Br J Cancer. 1984 Mar;49(3):325–331. doi: 10.1038/bjc.1984.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boice J. D. Cancer following medical irradiation. Cancer. 1981 Mar 1;47(5 Suppl):1081–1090. doi: 10.1002/1097-0142(19810301)47:5+<1081::aid-cncr2820471305>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Draper G. J., Sanders B. M., Kingston J. E. Second primary neoplasms in patients with retinoblastoma. Br J Cancer. 1986 May;53(5):661–671. doi: 10.1038/bjc.1986.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farwell J., Flannery J. T. Cancer in relatives of children with central-nervous-system neoplasms. N Engl J Med. 1984 Sep 20;311(12):749–753. doi: 10.1056/NEJM198409203111201. [DOI] [PubMed] [Google Scholar]
- Farwell J., Flannery J. T. Second primaries in children with central nervous system tumors. J Neurooncol. 1984;2(4):371–375. doi: 10.1007/BF00178120. [DOI] [PubMed] [Google Scholar]
- Kingston J. E., Hawkins M. M., Draper G. J., Marsden H. B., Kinnier Wilson L. M. Patterns of multiple primary tumours in patients treated for cancer during childhood. Br J Cancer. 1987 Sep;56(3):331–338. doi: 10.1038/bjc.1987.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. P., Cassady J. R., Jaffe N. Risk of second tumors in survivors of childhood cancer. Cancer. 1975 Apr;35(4):1230–1235. doi: 10.1002/1097-0142(197504)35:4<1230::aid-cncr2820350430>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Li F. P., Fraumeni J. F., Jr Prospective study of a family cancer syndrome. JAMA. 1982 May 21;247(19):2692–2694. [PubMed] [Google Scholar]
- Li F. P., Fraumeni J. F., Jr Soft-tissue sarcomas, breast cancer, and other neoplasms. A familial syndrome? Ann Intern Med. 1969 Oct;71(4):747–752. doi: 10.7326/0003-4819-71-4-747. [DOI] [PubMed] [Google Scholar]
- Li F. P. Second malignant tumors after cancer in childhood. Cancer. 1977 Oct;40(4 Suppl):1899–1902. doi: 10.1002/1097-0142(197710)40:4+<1899::aid-cncr2820400821>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Li F. P., Yan J. C., Sallan S., Cassady J. R., Jr, Danahy J., Fine W., Gelber R. D., Green D. M. Second neoplasms after Wilms' tumor in childhood. J Natl Cancer Inst. 1983 Dec;71(6):1205–1209. [PubMed] [Google Scholar]
- Meadows A. T., Baum E., Fossati-Bellani F., Green D., Jenkin R. D., Marsden B., Nesbit M., Newton W., Oberlin O., Sallan S. G. Second malignant neoplasms in children: an update from the Late Effects Study Group. J Clin Oncol. 1985 Apr;3(4):532–538. doi: 10.1200/JCO.1985.3.4.532. [DOI] [PubMed] [Google Scholar]
- Meadows A. T., D'Angio G. J., Miké V., Banfi A., Harris C., Jenkin R. D., Schwartz A. Patterns of second malignant neoplasms in children. Cancer. 1977 Oct;40(4 Suppl):1903–1911. doi: 10.1002/1097-0142(197710)40:4+<1903::aid-cncr2820400822>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Miké V., Meadows A. T., D'Angio G. J. Incidence of second malignant neoplasms in children: results of an international study. Lancet. 1982 Dec 11;2(8311):1326–1331. doi: 10.1016/s0140-6736(82)91524-0. [DOI] [PubMed] [Google Scholar]
- Potish R. A., Dehner L. P., Haselow R. E., Kim T. H., Levitt S. H., Nesbit M. The incidence of second neoplasms following megavoltage radiation for pediatric tumors. Cancer. 1985 Oct 1;56(7):1534–1537. doi: 10.1002/1097-0142(19851001)56:7<1534::aid-cncr2820560711>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Schmähl D., Habs M., Lorenz M., Wagner I. Occurrence of second tumors in man after anticancer drug treatment. Cancer Treat Rev. 1982 Sep;9(3):167–194. doi: 10.1016/s0305-7372(82)80006-6. [DOI] [PubMed] [Google Scholar]
- Stiller C. A. Descriptive epidemiology of childhood leukaemia and lymphoma in Great Britain. Leuk Res. 1985;9(6):671–674. doi: 10.1016/0145-2126(85)90273-5. [DOI] [PubMed] [Google Scholar]
- TURCOT J., DESPRES J. P., ST PIERRE F. Malignant tumors of the central nervous system associated with familial polyposis of the colon: report of two cases. Dis Colon Rectum. 1959 Sep-Oct;2:465–468. doi: 10.1007/BF02616938. [DOI] [PubMed] [Google Scholar]
- Todd D. W., Christoferson L. A., Leech R. W., Rudolf L. A family affected with intestinal polyposis and gliomas. Ann Neurol. 1981 Oct;10(4):390–392. doi: 10.1002/ana.410100413. [DOI] [PubMed] [Google Scholar]
- Tucker M. A., Meadows A. T., Boice J. D., Jr, Stovall M., Oberlin O., Stone B. J., Birch J., Voûte P. A., Hoover R. N., Fraumeni J. F., Jr Leukemia after therapy with alkylating agents for childhood cancer. J Natl Cancer Inst. 1987 Mar;78(3):459–464. [PubMed] [Google Scholar]



