Abstract
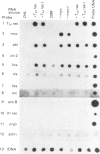
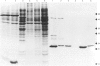
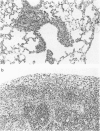
The effects of expression of human c-myc and both mutated (T24) and normal forms of human Ha-ras-1 were studied in an aneuploid rat fibroblast line (208F). Mutated T24 Ha-ras was also studied in a near-diploid cell derived from early passage Chinese hamster lung fibroblasts (CHL). In contrast to the parental fibroblasts, cells expressing any of the human oncogenes engendered rapidly growing tumours in immune-suppressed animals. Blood- and lymph-borne metastases were observed from both ras- and myc-expressing cells. In general ras-expressing cells were more aggressive than those expressing myc. In the 208F background, expression of c-myc was associated with an incidence of mitosis similar to that in tumours expressing T24 Ha-ras, but incidence of single cell death by apoptosis was higher. Quantitatively, expression of human oncogene mRNA was constant during growth in vivo, and similar to that sometimes observed in human neoplasms. Of 9 endogenous proto-oncogenes, 7 showed no change in expression from the parental fibroblasts, but c-abl and c-fos were strongly expressed in all cells expressing human ras or myc. Thus these tumorigenic cells, although transfected with single human oncogenes, all expressed oncogenes with both nuclear- and membrane-associated products.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anzano M. A., Roberts A. B., De Larco J. E., Wakefield L. M., Assoian R. K., Roche N. S., Smith J. M., Lazarus J. E., Sporn M. B. Increased secretion of type beta transforming growth factor accompanies viral transformation of cells. Mol Cell Biol. 1985 Jan;5(1):242–247. doi: 10.1128/mcb.5.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M. O., Kraynak A. R., Storer R. D., Gibbs J. B. Experimental metastasis in nude mice of NIH 3T3 cells containing various ras genes. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5277–5281. doi: 10.1073/pnas.83.14.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur G. M., Seeger R. C., Schwab M., Varmus H. E., Bishop J. M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984 Jun 8;224(4653):1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cichutek K., Duesberg P. H. Harvey ras genes transform without mutant codons, apparently activated by truncation of a 5' exon (exon -1). Proc Natl Acad Sci U S A. 1986 Apr;83(8):2340–2344. doi: 10.1073/pnas.83.8.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard J. G., van Beek W. P., Janssen J. W., Schijven J. F. Transfection by human oncogenes: concomitant induction of tumorigenicity and tumor-associated membrane alterations. Int J Cancer. 1985 Feb 15;35(2):207–213. doi: 10.1002/ijc.2910350211. [DOI] [PubMed] [Google Scholar]
- Colletta G., Cirafici A. M., Vecchio G. Induction of the c-fos oncogene by thyrotropic hormone in rat thyroid cells in culture. Science. 1986 Jul 25;233(4762):458–460. doi: 10.1126/science.3726540. [DOI] [PubMed] [Google Scholar]
- DeBortoli M. E., Abou-Issa H., Haley B. E., Cho-Chung Y. S. Amplified expression of p21 ras protein in hormone-dependent mammary carcinomas of humans and rodents. Biochem Biophys Res Commun. 1985 Mar 15;127(2):699–706. doi: 10.1016/s0006-291x(85)80218-7. [DOI] [PubMed] [Google Scholar]
- Denekamp J., Hobson B. Endothelial-cell proliferation in experimental tumours. Br J Cancer. 1982 Nov;46(5):711–720. doi: 10.1038/bjc.1982.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming W. H., Hamel A., MacDonald R., Ramsey E., Pettigrew N. M., Johnston B., Dodd J. G., Matusik R. J. Expression of the c-myc protooncogene in human prostatic carcinoma and benign prostatic hyperplasia. Cancer Res. 1986 Mar;46(3):1535–1538. [PubMed] [Google Scholar]
- Folkman J. How is blood vessel growth regulated in normal and neoplastic tissue? G.H.A. Clowes memorial Award lecture. Cancer Res. 1986 Feb;46(2):467–473. [PubMed] [Google Scholar]
- Gallick G. E., Kurzrock R., Kloetzer W. S., Arlinghaus R. B., Gutterman J. U. Expression of p21ras in fresh primary and metastatic human colorectal tumors. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1795–1799. doi: 10.1073/pnas.82.6.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyette M., Petropoulos C. J., Shank P. R., Fausto N. Expression of a cellular oncogene during liver regeneration. Science. 1983 Feb 4;219(4584):510–512. doi: 10.1126/science.6297003. [DOI] [PubMed] [Google Scholar]
- Hanna N., Schneider M. Enhancement of tumor metastasis and suppression of natural killer cell activity by beta-estradiol treatment. J Immunol. 1983 Feb;130(2):974–980. [PubMed] [Google Scholar]
- Hay J. H., Morten J. E., Clarke B., Swinton J. The suitability of immunosuppressed mice kept in a standard animal unit as recipients of human tumour xenografts. Lab Anim. 1985 Apr;19(2):119–122. doi: 10.1258/002367785780942561. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Johnson P. W., Baubock C., Roder J. C. Transfection of a rat cell line with the v-Ki-ras oncogene is associated with enhanced susceptibility to natural killer cell lysis. J Exp Med. 1985 Nov 1;162(5):1732–1737. doi: 10.1084/jem.162.5.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasid A., Lippman M. E., Papageorge A. G., Lowy D. R., Gelmann E. P. Transfection of v-rasH DNA into MCF-7 human breast cancer cells bypasses dependence on estrogen for tumorigenicity. Science. 1985 May 10;228(4700):725–728. doi: 10.1126/science.4039465. [DOI] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Klein E. Conditioned tumorigenicity of activated oncogenes. Cancer Res. 1986 Jul;46(7):3211–3224. [PubMed] [Google Scholar]
- Klostergaard J., Foster W. A., Hamilton D. A., Turpin J., Lopez-Berestein G. Effector mechanisms of human monocyte-mediated tumor cytotoxicity in vitro: biochemical, functional, and serological characterization of cytotoxins produced by peripheral blood monocytes isolated by counterflow elutriation. Cancer Res. 1986 Jun;46(6):2871–2875. [PubMed] [Google Scholar]
- Kris R. M., Avivi A., Bar-Eli M., Alon Y., Carmi P., Schlessinger J., Raz A. Expression of ki-ras oncogene in tumor cell variants exhibiting different metastatic capabilities. Int J Cancer. 1985 Feb 15;35(2):227–230. doi: 10.1002/ijc.2910350214. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Schubert D., Verma I. M. Induction of the proto-oncogene fos by nerve growth factor. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7330–7334. doi: 10.1073/pnas.82.21.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzrock R., Gallick G. E., Gutterman J. U. Differential expression of p21ras gene products among histological subtypes of fresh primary human lung tumors. Cancer Res. 1986 Mar;46(3):1530–1534. [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lang J. C., Spandidos D. A. The structure and function of eukaryotic enhancer elements and their role in oncogenesis. Anticancer Res. 1986 May-Jun;6(3 Pt B):437–449. [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986 Jan;46(1):1–7. [PubMed] [Google Scholar]
- Mareel M. M., Van Roy F. M. Are oncogenes involved in invasion and metastasis? Anticancer Res. 1986 May-Jun;6(3 Pt B):419–435. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschel R. J., Nakahara K., Chu E., Pozzatti R., Liotta L. A. Karyotypic analysis of diploid or near diploid metastatic Harvey ras transformed rat embryo fibroblasts. Cancer Res. 1986 Aug;46(8):4104–4108. [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Ozanne B., Wheeler T., Kaplan P. L. Cells transformed by RNA and DNA tumor viruses produce transforming factors. Fed Proc. 1982 Nov;41(13):3004–3007. [PubMed] [Google Scholar]
- Pozzatti R., Muschel R., Williams J., Padmanabhan R., Howard B., Liotta L., Khoury G. Primary rat embryo cells transformed by one or two oncogenes show different metastatic potentials. Science. 1986 Apr 11;232(4747):223–227. doi: 10.1126/science.3456644. [DOI] [PubMed] [Google Scholar]
- Pragnell I. B., Spandidos D. A., Wilkie N. M. Consequences of altered oncogene expression in rodent cells. Proc R Soc Lond B Biol Sci. 1985 Oct 22;226(1242):107–119. doi: 10.1098/rspb.1985.0085. [DOI] [PubMed] [Google Scholar]
- Quade K. Transformation of mammalian cells by avian myelocytomatosis virus and avian erythroblastosis virus. Virology. 1979 Oct 30;98(2):461–465. doi: 10.1016/0042-6822(79)90569-5. [DOI] [PubMed] [Google Scholar]
- Racker E., Resnick R. J., Feldman R. Glycolysis and methylaminoisobutyrate uptake in rat-1 cells transfected with ras or myc oncogenes. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3535–3538. doi: 10.1073/pnas.82.11.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A., Williams A. R., Piris J., Spandidos D. A., Wyllie A. H. Evaluation of a monoclonal antibody to ras peptide, RAP-5, claimed to bind preferentially to cells of infiltrating carcinomas. Br J Cancer. 1986 Dec;54(6):877–883. doi: 10.1038/bjc.1986.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen N., Reynolds C. P., Thiele C. J., Biedler J. L., Israel M. A. Increased N-myc expression following progressive growth of human neuroblastoma. Cancer Res. 1986 Aug;46(8):4139–4142. [PubMed] [Google Scholar]
- Rothberg P. G., Erisman M. D., Diehl R. E., Rovigatti U. G., Astrin S. M. Structure and expression of the oncogene c-myc in fresh tumor material from patients with hematopoietic malignancies. Mol Cell Biol. 1984 Jun;4(6):1096–1103. doi: 10.1128/mcb.4.6.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotin D., Robinson B., Tannock I. F. Influence of hypoxia and an acidic environment on the metabolism and viability of cultured cells: potential implications for cell death in tumors. Cancer Res. 1986 Jun;46(6):2821–2826. [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Spandidos D. A., Agnantis N. J. Human malignant tumours of the breast, as compared to their respective normal tissue, have elevated expression of the Harvey ras oncogene. Anticancer Res. 1984 Jul-Oct;4(4-5):269–272. [PubMed] [Google Scholar]
- Spandidos D. A., Freshney M., Wilkie N. M. Heterogeneity of cell lines derived after transformation of early passage rodent cells by the Ha-ras1 human oncogene. Anticancer Res. 1985 Jul-Aug;5(4):387–391. [PubMed] [Google Scholar]
- Spandidos D. A., Harrison P. R., Paul J. Transfer and expression of herpes simplex virus thymidine kinase and human globin genes in mammalian cells studied by spot hybridization assays. Biosci Rep. 1981 Dec;1(12):911–920. doi: 10.1007/BF01114960. [DOI] [PubMed] [Google Scholar]
- Spandidos D. A., Kerr I. B. Elevated expression of the human ras oncogene family in premalignant and malignant tumours of the colorectum. Br J Cancer. 1984 Jun;49(6):681–688. doi: 10.1038/bjc.1984.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandidos D. A., Lamothe A., Field J. K. Multiple transcriptional activation of cellular oncogenes in human head and neck solid tumours. Anticancer Res. 1985 Mar-Apr;5(2):221–224. [PubMed] [Google Scholar]
- Spandidos D. A. Mechanism of carcinogenesis: the role of oncogenes, transcriptional enhancers and growth factors. Anticancer Res. 1985 Sep-Oct;5(5):485–498. [PubMed] [Google Scholar]
- Spandidos D. A., Wilkie N. M. Malignant transformation of early passage rodent cells by a single mutated human oncogene. Nature. 1984 Aug 9;310(5977):469–475. doi: 10.1038/310469a0. [DOI] [PubMed] [Google Scholar]
- Stacey D. W., Watson T., Kung H. F., Curran T. Microinjection of transforming ras protein induces c-fos expression. Mol Cell Biol. 1987 Jan;7(1):523–527. doi: 10.1128/mcb.7.1.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel G. G., Courtenay V. D., Rostom A. Y. Improved immune-suppression techniques for the exongrafting of human tumours. Br J Cancer. 1978 Feb;37(2):224–230. doi: 10.1038/bjc.1978.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson U. P., Turpeenniemi-Hujanen T., Williams J. E., Westin E. H., Heilman C. A., Talmadge J. E., Liotta L. A. NIH/3T3 cells transfected with human tumor DNA containing activated ras oncogenes express the metastatic phenotype in nude mice. Mol Cell Biol. 1985 Jan;5(1):259–262. doi: 10.1128/mcb.5.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble W. S., Johnson P. W., Hozumi N., Roder J. C. Inducible cellular transformation by a metallothionein-ras hybrid oncogene leads to natural killer cell susceptibility. Nature. 1986 Jun 19;321(6072):782–784. doi: 10.1038/321782a0. [DOI] [PubMed] [Google Scholar]
- Wakelam M. J., Davies S. A., Houslay M. D., McKay I., Marshall C. J., Hall A. Normal p21N-ras couples bombesin and other growth factor receptors to inositol phosphate production. Nature. 1986 Sep 11;323(6084):173–176. doi: 10.1038/323173a0. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. The action of oncogenes in the cytoplasm and nucleus. Science. 1985 Nov 15;230(4727):770–776. doi: 10.1126/science.2997917. [DOI] [PubMed] [Google Scholar]
- Williams A. R., Piris J., Spandidos D. A., Wyllie A. H. Immunohistochemical detection of the ras oncogene p21 product in an experimental tumour and in human colorectal neoplasms. Br J Cancer. 1985 Nov;52(5):687–693. doi: 10.1038/bjc.1985.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter E., Perucho M. Oncogene amplification during tumorigenesis of established rat fibroblasts reversibly transformed by activated human ras oncogenes. Mol Cell Biol. 1986 Jul;6(7):2562–2570. doi: 10.1128/mcb.6.7.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. The biology of cell death in tumours. Anticancer Res. 1985 Jan-Feb;5(1):131–136. [PubMed] [Google Scholar]
- Zhan X., Goldfarb M. Growth factor requirements of oncogene-transformed NIH 3T3 and BALB/c 3T3 cells cultured in defined media. Mol Cell Biol. 1986 Oct;6(10):3541–3544. doi: 10.1128/mcb.6.10.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]