Abstract
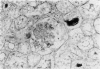
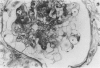
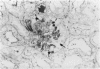
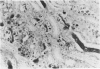
In-vivo BrdU incorporation and visualization by immunohistochemistry, previously reported in normal mouse kidney, were applied to a mouse model of anti-GBM GN, induced by immunization with rabbit anti-mouse GBM antiserum, to assess the contribution of capsular cell proliferation in the development of crescents. A significant increase (P = 0.003) in the BrdU-labelling index (LI) for capsular cells was observed, as compared to normal mice (5.76 +/- 1.1 vs 0.70% +/- 0.12%). Elevated LI were also observed for tuft and tubular cells but these increases were not statistically significant. It was concluded that, in this model, capsular cell proliferation is a major contributory factor to the formation of cellular crescents. In addition, other pathological features, indicative of glomerular damage, were assessed semi-quantitatively alongside numbers of labelled capsular cells per glomerulus. It was found that podocyte vacuolation is strongly associated with, and may precede, proliferation, suggesting some common causative factor. Fibrin, when present, was confined within the tuft capillary loops and was only weakly associated with either podocyte vacuolation or capsular cell proliferation. It was concluded that this protein does not play a major role in the initiation of pathological damage. Finally, glomerular lesions were found to be randomly distributed. Thus, the idea of intraglomerular signalling, resulting in 'clustering' of damaged glomeruli, is not supported.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cawood A. H., Savage J. R. A comparison of the use of bromodeoxyuridine and [3H]thymidine in studies of the cell cycle. Cell Tissue Kinet. 1983 Jan;16(1):51–57. [PubMed] [Google Scholar]
- Danova M., Riccardi A., Brugnatelli S., Fiocca R., Girino M., Villani L., Giordano P., Dionigi P., Giordano M., Buttini R. In vivo bromodeoxyuridine incorporation in human gastric cancer: a study on formalin-fixed and paraffin-embedded sections. Histochem J. 1988 Mar;20(3):125–130. doi: 10.1007/BF01746675. [DOI] [PubMed] [Google Scholar]
- Dean P. N., Dolbeare F., Gratzner H., Rice G. C., Gray J. W. Cell-cycle analysis using a monoclonal antibody to BrdUrd. Cell Tissue Kinet. 1984 Jul;17(4):427–436. doi: 10.1111/j.1365-2184.1984.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Furness P. N., Turner S. N., Appleby P., Turner D. R. A morphological study of experimental proteinuria using a novel form of surface fixation. J Pathol. 1989 Jan;157(1):37–45. doi: 10.1002/path.1711570106. [DOI] [PubMed] [Google Scholar]
- Min K. W., Györkey F., Györkey P., Yium J. J., Eknoyan G. The morphogenesis of glomerular crescents in rapidly progressive glomerulonephritis. Kidney Int. 1974 Jan;5(1):47–56. doi: 10.1038/ki.1974.6. [DOI] [PubMed] [Google Scholar]
- Morley A. R., Wheeler J. Cell proliferation within Bowman's capsule in mice. J Pathol. 1985 Apr;145(4):315–327. doi: 10.1002/path.1711450405. [DOI] [PubMed] [Google Scholar]
- Robertson H., Wheeler J., Morley A. R. In vivo bromodeoxyuridine incorporation in normal mouse kidney: immunohistochemical detection and measurement of labelling indices. Histochem J. 1990 Apr;22(4):209–214. doi: 10.1007/BF02386007. [DOI] [PubMed] [Google Scholar]
- Thornton J. G., Wells M., Hume W. J. Flash labelling of S-phase cells in short-term organ culture of normal and pathological human endometrium using bromodeoxyuridine and tritiated thymidine. J Pathol. 1988 Apr;154(4):321–328. doi: 10.1002/path.1711540407. [DOI] [PubMed] [Google Scholar]
- Vassalli P., McCluskey R. T. The pathogenetic role of the coagulation process in glomerular diseases of immunologic origin. Adv Nephrol Necker Hosp. 1971;1:47–63. [PubMed] [Google Scholar]
- Veronese S., Gambacorta M., Falini B. In situ demonstration of tissue proliferative activity using anti-bromo-deoxyuridine monoclonal antibody. J Clin Pathol. 1989 Aug;42(8):820–826. doi: 10.1136/jcp.42.8.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler J., Morley A. R., Appleton D. R. Anti-glomerular basement membrane (GBM) glomerulonephritis in the mouse: development of disease and cell proliferation. J Exp Pathol (Oxford) 1990 Jun;71(3):411–422. [PMC free article] [PubMed] [Google Scholar]
- Wheeler J., Simpson J., Morley A. R. Routine and rapid enzyme linked immunosorbent assays for circulating anti-glomerular basement membrane antibodies. J Clin Pathol. 1988 Feb;41(2):163–170. doi: 10.1136/jcp.41.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]