Abstract
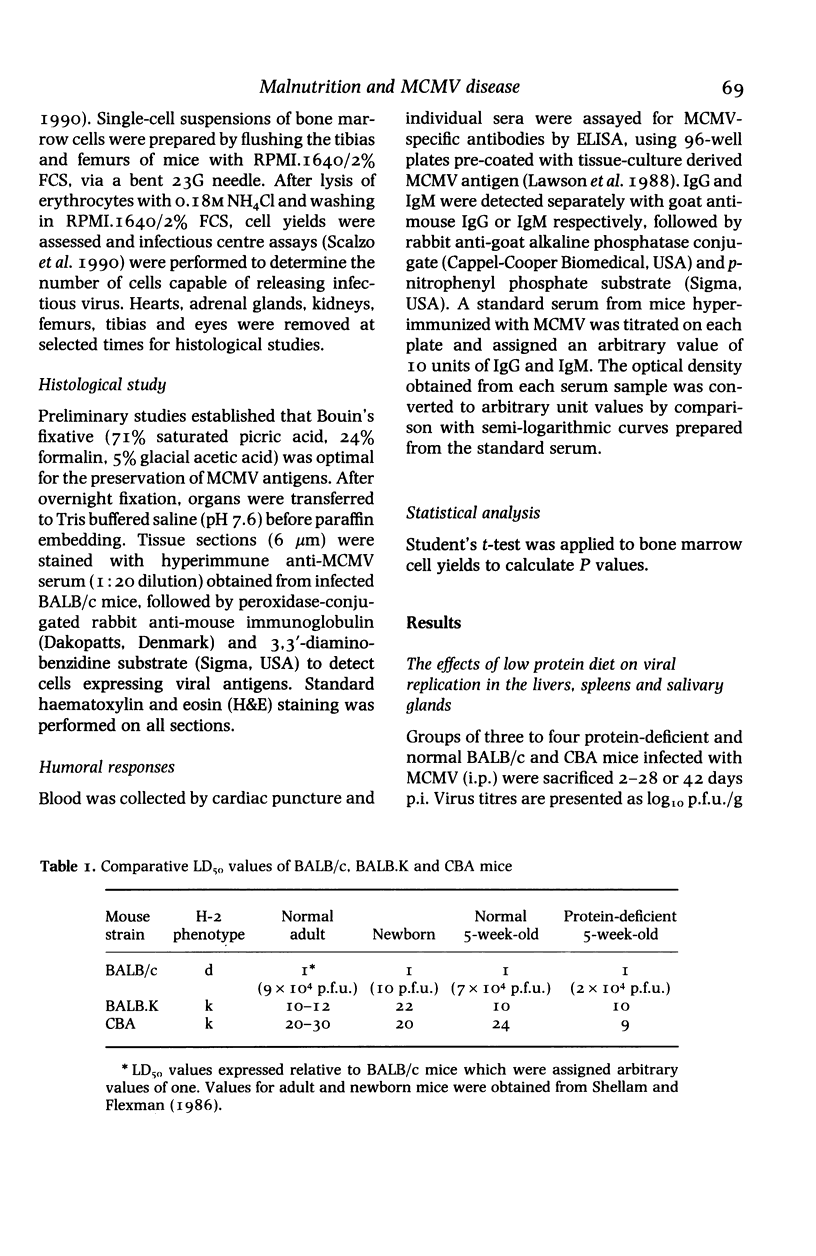
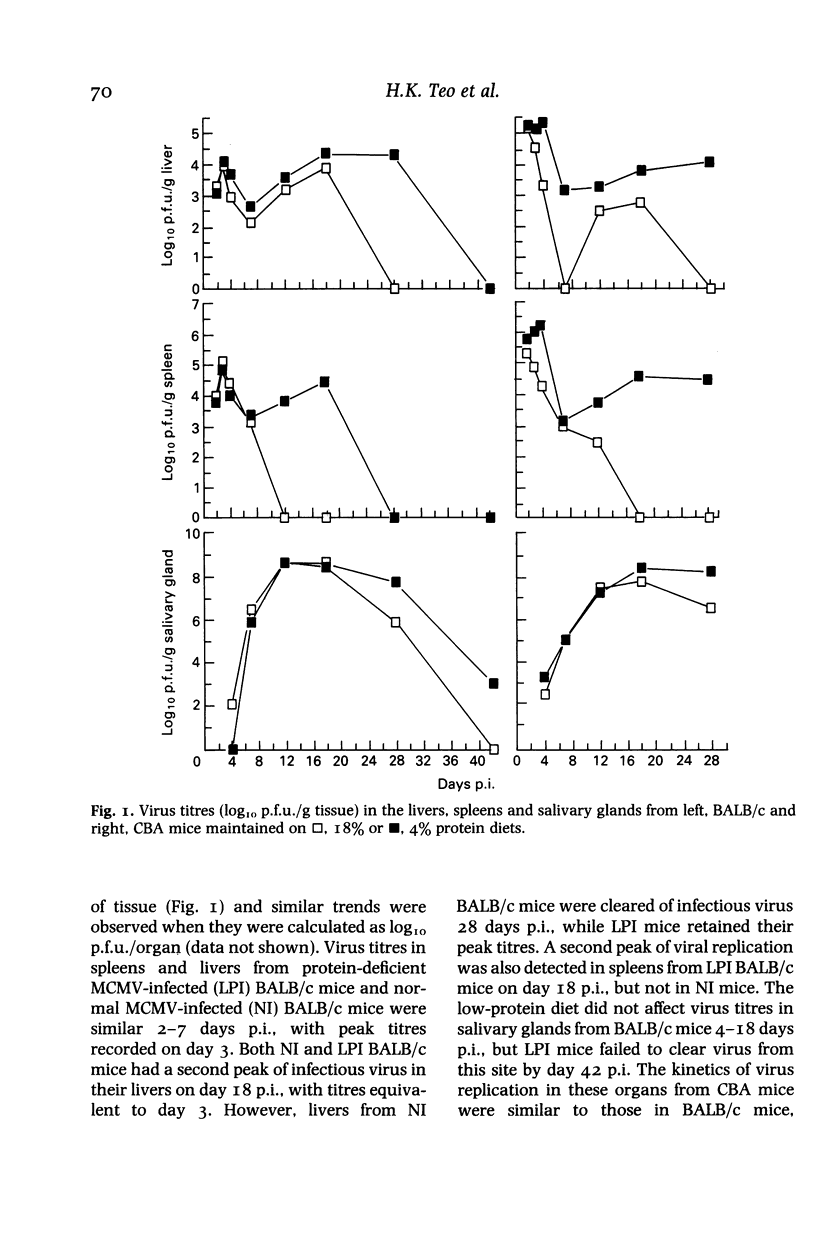
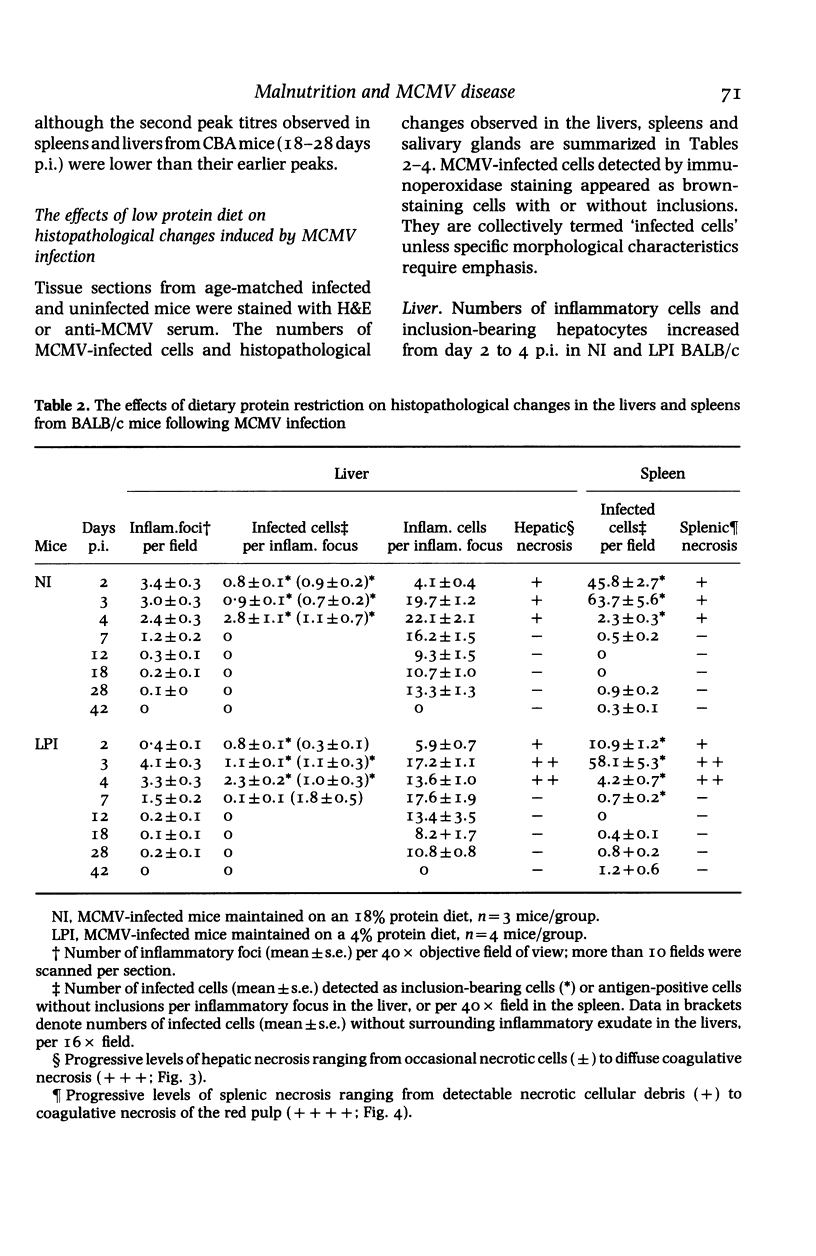
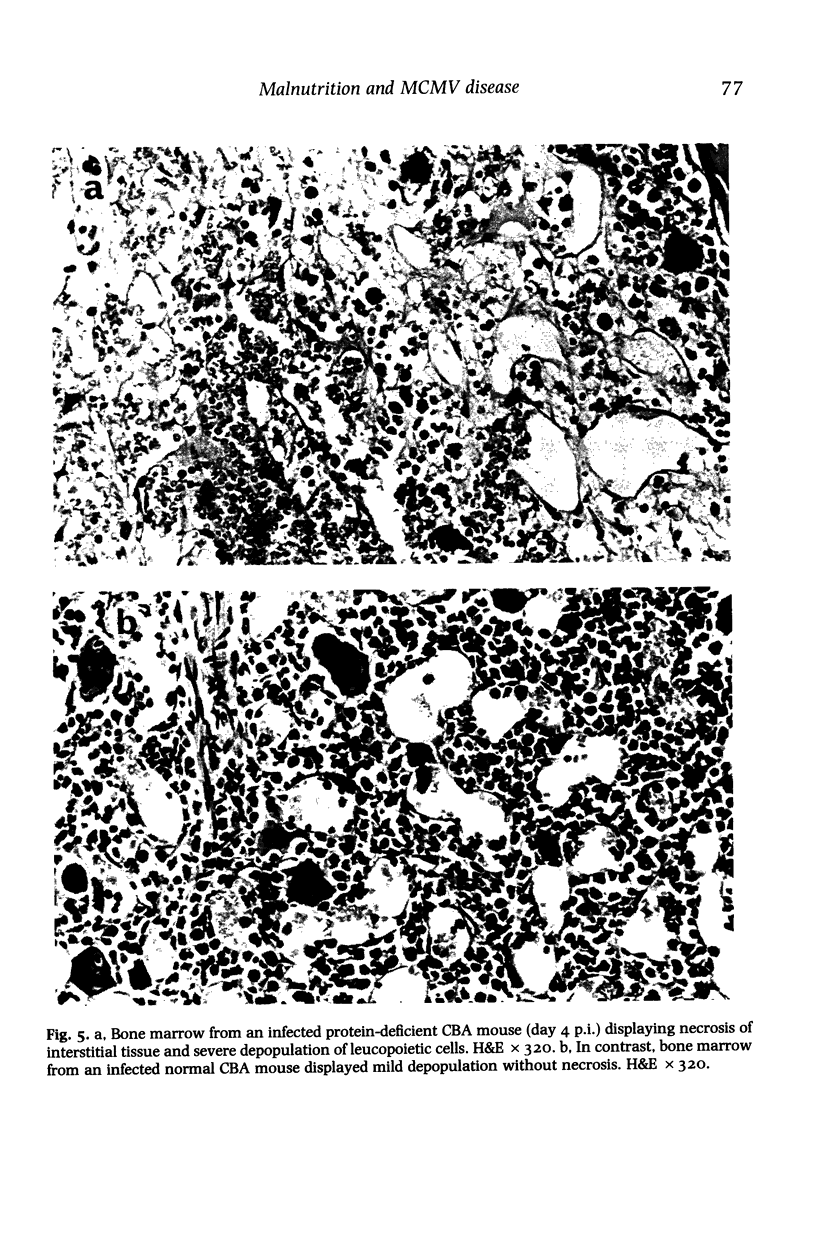
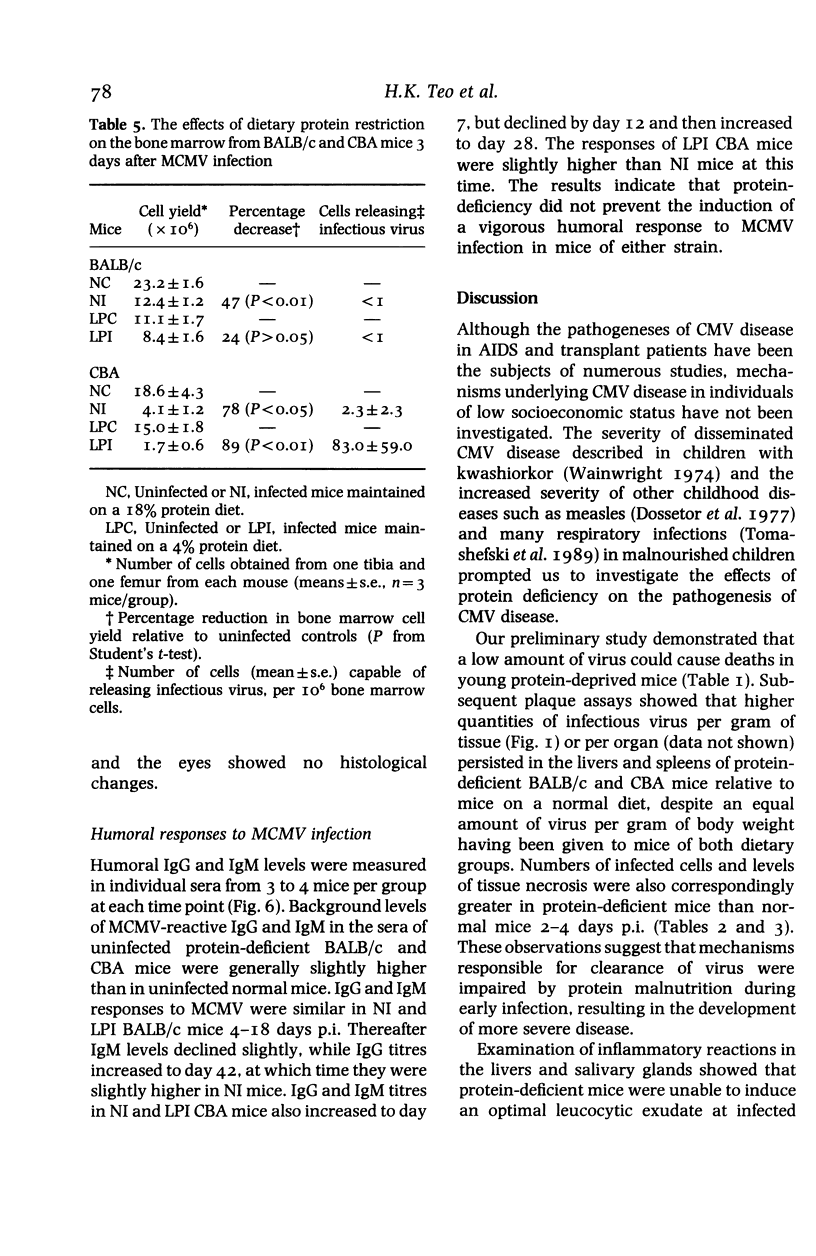
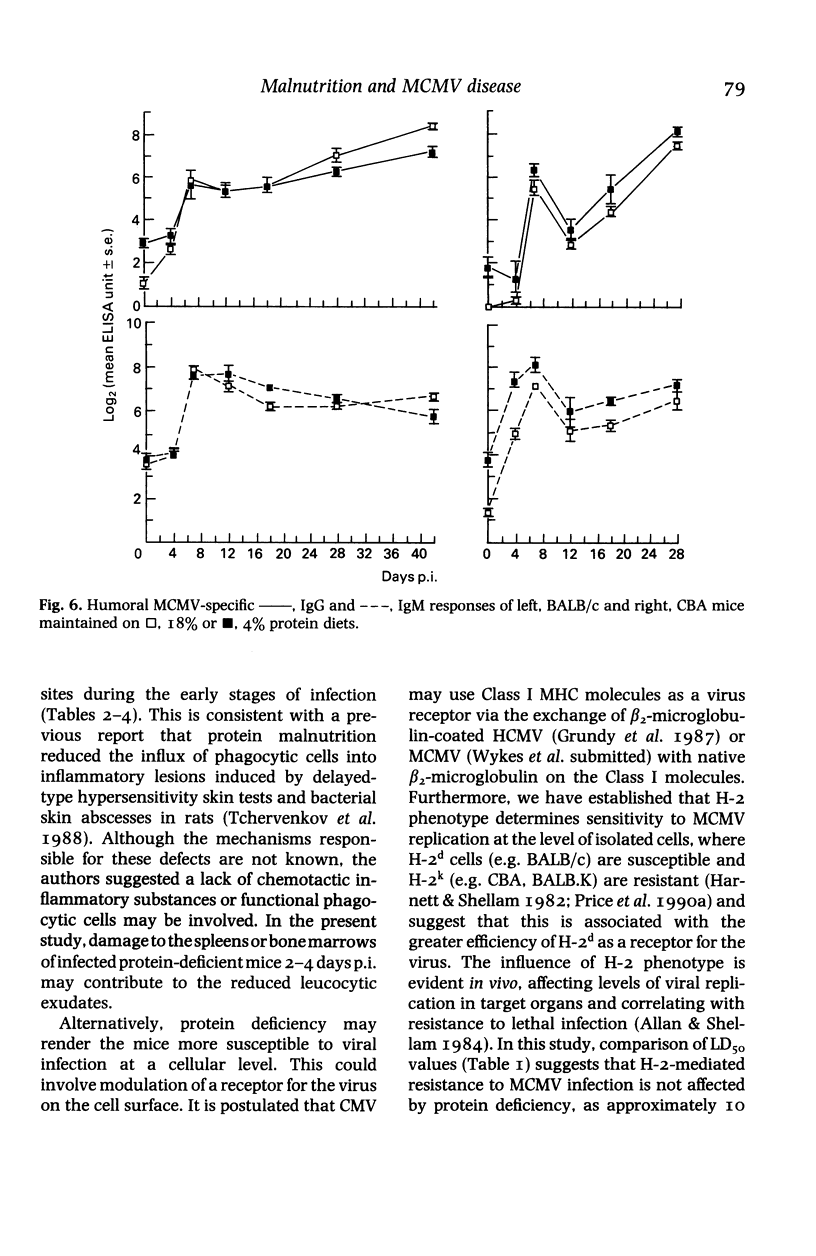
BALB/c and CBA mice maintained on low (4%) or normal (18%) protein diets for 2 weeks after weaning were infected with a sublethal dose of murine cytomegalovirus, adjusted in proportion to body weight. Viral replication, histopathological changes and humoral responses to the virus were compared between the dietary groups 2-42 days post infection (p.i.). Higher numbers of viral antigen-positive cells and/or more prominent tissue necrosis were noted in the livers, spleens, hearts, adrenal glands, kidneys and bone marrows of infected protein-deficient mice. These mice also showed a delayed onset of leucocytic exudation in their livers and salivary glands, relative to infected mice on the normal diet. Second peaks of viral replication were detected by plaque assays in livers and spleens from protein-deficient mice and in livers from normal mice 12-18 days p.i., but few antigen-positive cells and no tissue necrosis were observed. Virus also persisted at higher titres in the salivary glands from protein-deficient mice. Although cellular immunity may be defective in these mice, humoral IgG and IgM responses to the virus were not inhibited. The influence of genetic factors on the pathogenesis of murine cytomegalovirus disease in protein-deficient mice is discussed.
Full text
PDF

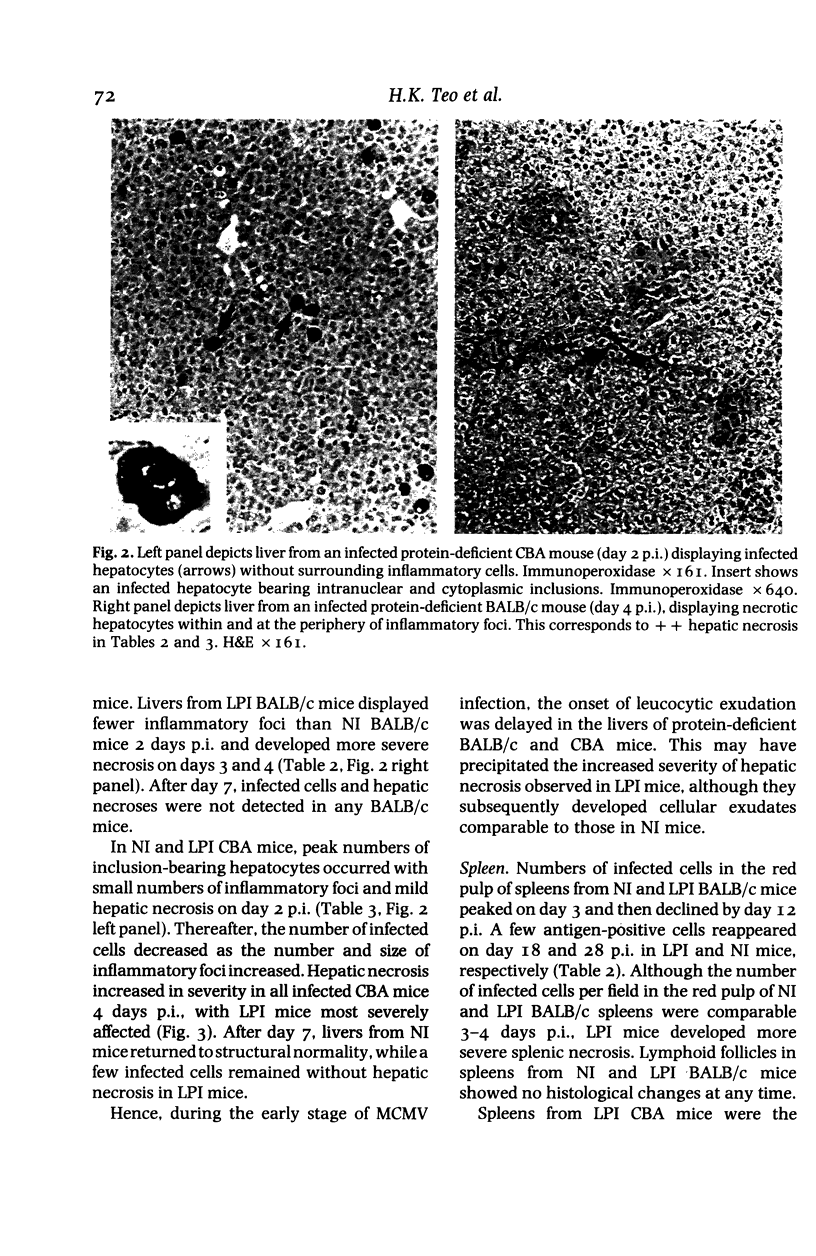
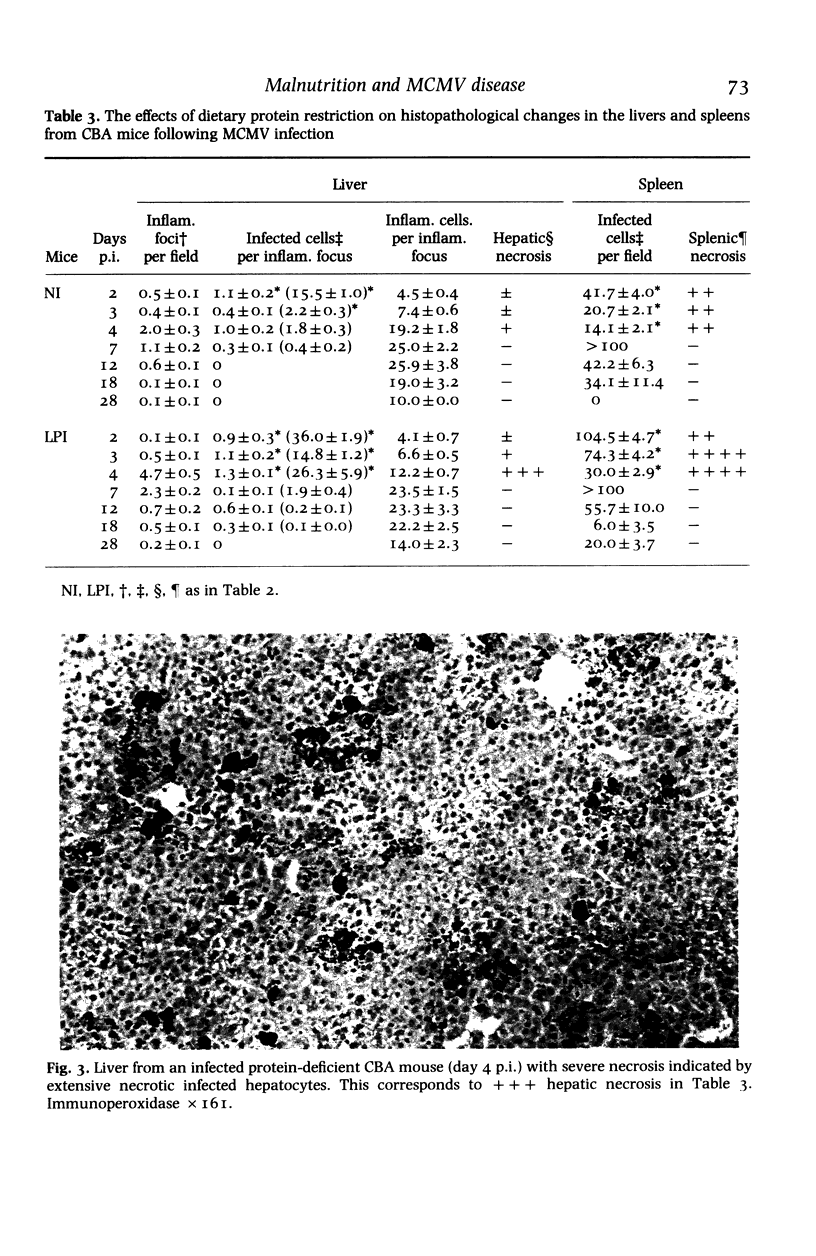
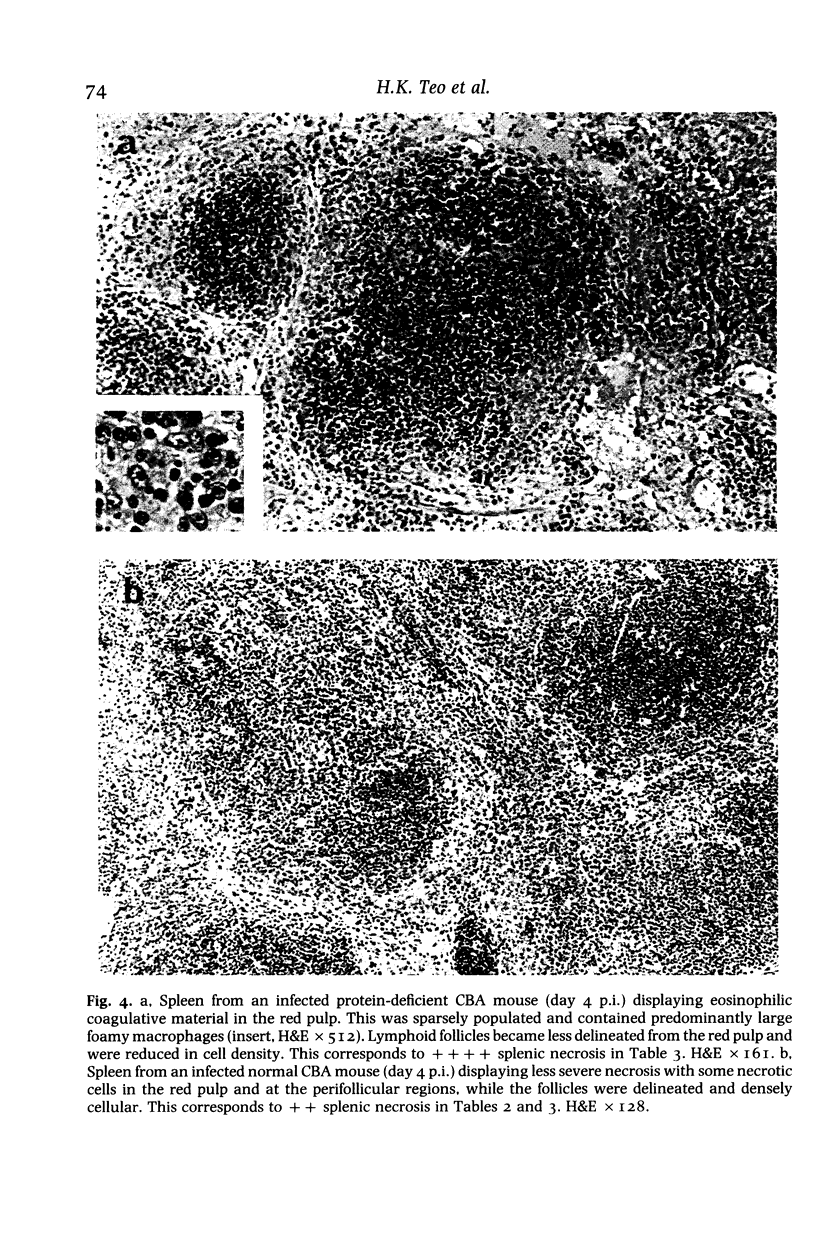
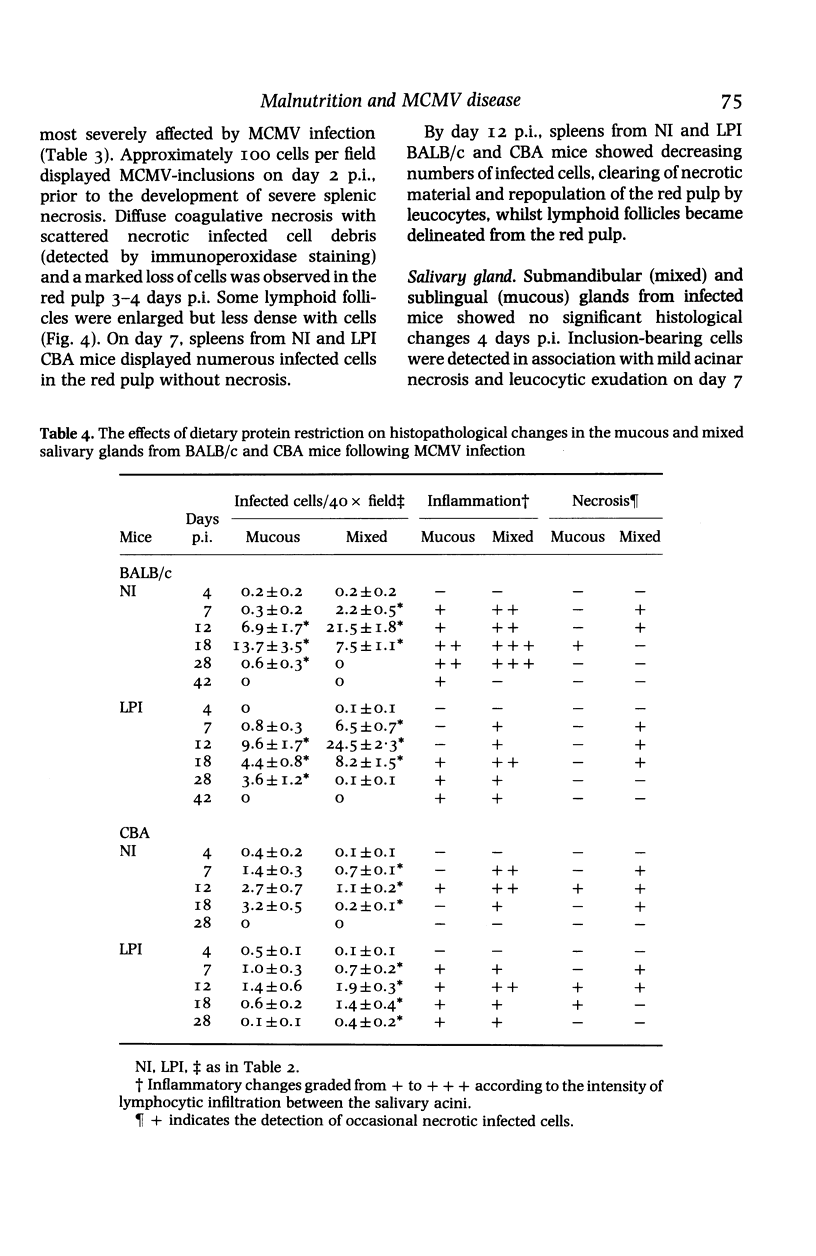




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. G., Hazell L. A., Price P. Influence of dietary protein restriction on immune competence. II. Effect on lymphoid tissue. Clin Exp Immunol. 1976 Nov;26(2):314–326. [PMC free article] [PubMed] [Google Scholar]
- Chandler S. H., Alexander E. R., Holmes K. K. Epidemiology of cytomegaloviral infection in a heterogeneous population of pregnant women. J Infect Dis. 1985 Aug;152(2):249–256. doi: 10.1093/infdis/152.2.249. [DOI] [PubMed] [Google Scholar]
- Chandra R. K. Nutrition and immunity. Trop Geogr Med. 1988 Jul;40(3):S46–S51. [PubMed] [Google Scholar]
- Dossetor J., Whittle H. C., Greenwood B. M. Persistent measles infection in malnourished children. Br Med J. 1977 Jun 25;1(6077):1633–1635. doi: 10.1136/bmj.1.6077.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy J. E., McKeating J. A., Ward P. J., Sanderson A. R., Griffiths P. D. Beta 2 microglobulin enhances the infectivity of cytomegalovirus and when bound to the virus enables class I HLA molecules to be used as a virus receptor. J Gen Virol. 1987 Mar;68(Pt 3):793–803. doi: 10.1099/0022-1317-68-3-793. [DOI] [PubMed] [Google Scholar]
- Lawson C. M., Grundy J. E., Shellam G. R. Antibody responses to murine cytomegalovirus in genetically resistant and susceptible strains of mice. J Gen Virol. 1988 Aug;69(Pt 8):1987–1998. doi: 10.1099/0022-1317-69-8-1987. [DOI] [PubMed] [Google Scholar]
- Mercer J. A., Spector D. H. Pathogenesis of acute murine cytomegalovirus infection in resistant and susceptible strains of mice. J Virol. 1986 Feb;57(2):497–504. doi: 10.1128/jvi.57.2.497-504.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R. B., Nath I., Bhuyan U. N., Talwar G. P. Depression of T-cell function and normality of B-cell response in protein calorie malnutrition. Immunology. 1977 Mar;32(3):345–350. [PMC free article] [PubMed] [Google Scholar]
- Rose A. H., Holt P. G., Turner K. J. The effect of a low protein diet on the immunogenic activity of murine peritoneal macrophages. Int Arch Allergy Appl Immunol. 1982;67(4):356–361. doi: 10.1159/000233047. [DOI] [PubMed] [Google Scholar]
- Salimonu L. S., Ojo-Amaize E., Williams A. I., Johnson A. O., Cooke A. R., Adekunle F. A., Alm G. V., Wigzell H. Depressed natural killer cell activity in children with protein-calorie malnutrition. Clin Immunol Immunopathol. 1982 Jul;24(1):1–7. doi: 10.1016/0090-1229(82)90082-4. [DOI] [PubMed] [Google Scholar]
- Saxena Q. B., Saxena R. K., Adler W. H. Effect of protein calorie malnutrition on the levels of natural and inducible cytotoxic activities in mouse spleen cells. Immunology. 1984 Apr;51(4):727–733. [PMC free article] [PubMed] [Google Scholar]
- Seth V., Beotra A. Malnutrition and immune system. Indian Pediatr. 1986 Apr;23(4):277–302. [PubMed] [Google Scholar]
- Shanley J. D., Pesanti E. L. Murine cytomegalovirus adrenalitis in athymic nude mice. Arch Virol. 1986;88(1-2):27–35. doi: 10.1007/BF01310887. [DOI] [PubMed] [Google Scholar]
- Shann F., Walters S., Pifer L. L., Graham D. M., Jack I., Uren E., Birch D., Stallman N. D. Pneumonia associated with infection with pneumocystis, respiratory syncytial virus, chlamydia, mycoplasma, and cytomegalovirus in children in Papua New Guinea. Br Med J (Clin Res Ed) 1986 Feb 1;292(6516):314–317. doi: 10.1136/bmj.292.6516.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellam G. R., Flexman J. P. Genetically determined resistance to murine cytomegalovirus and herpes simplex virus in newborn mice. J Virol. 1986 Apr;58(1):152–156. doi: 10.1128/jvi.58.1.152-156.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchervenkov J. I., Latter D. A., Psychogios J., Christou N. V. The influence of long-term protein deprivation on in vivo phagocytic cell delivery to inflammatory lesions. Surgery. 1988 Apr;103(4):463–469. [PubMed] [Google Scholar]