Abstract
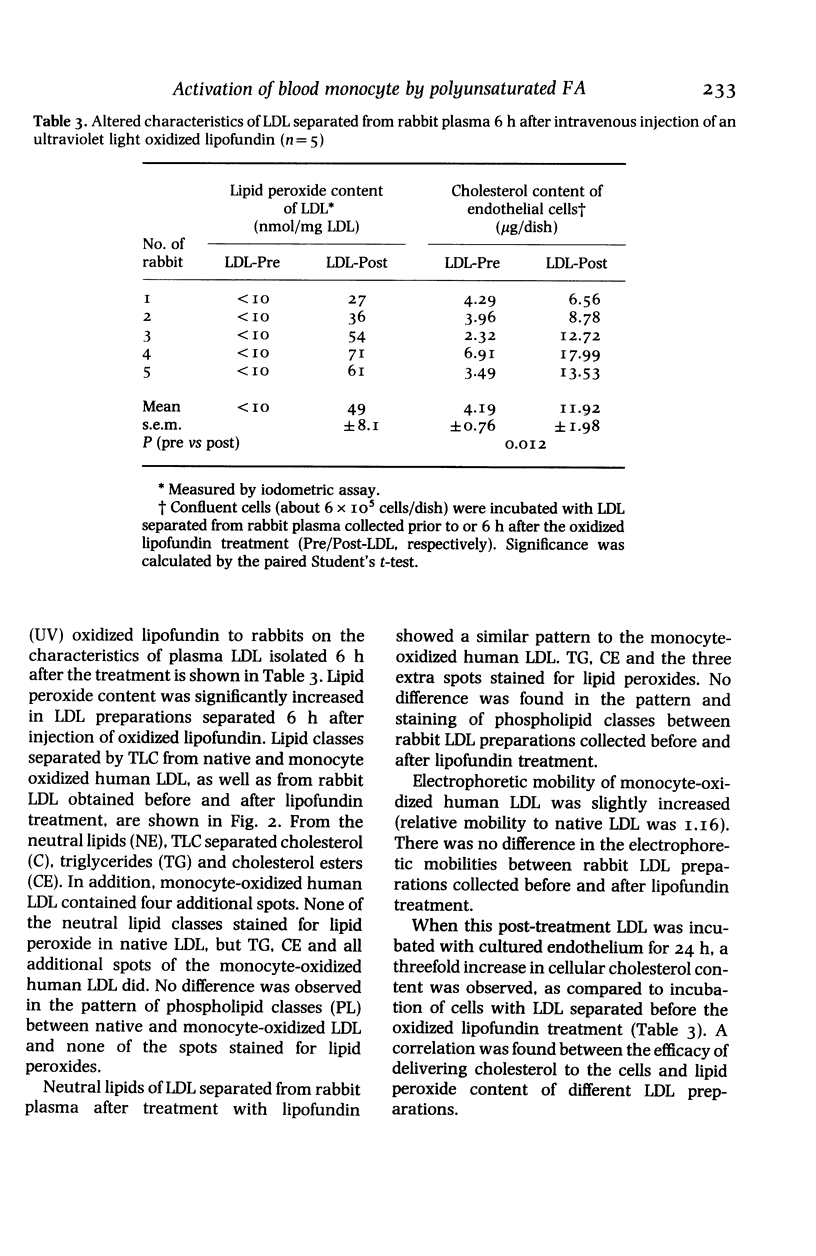
The ability of native and oxidized lipids and lipoproteins to stimulate production of reactive oxygen species (ROS; superoxide and hydrogen peroxide) by human blood monocytes has been studied in vitro. Neither native human low density lipoprotein (LDL), 'altered' LDL (oxidized either by lipoxygenase, activated human monocytes or air) nor oxidized cholesterol had any significant effect on ROS production of monocytes. However, different oxidation products of a lipid emulsion (Lipofundin; largely consisting of linoleic acid oxidized either by lipoxygenase, Fe3+ or ultraviolet irradiation) greatly enhanced ROS production of monocytes. A hypothesis that activation of circulating leucocytes by oxidized fatty acids may generate oxidized plasma LDL, was tested in rabbits. Characteristics of LDL, separated from rabbit plasma 6 h after intravenous injection of an oxidized lipid emulsion, was compared to that of LDL isolated before the lipid treatment. Post-treatment LDL-fraction of plasma had increased lipid peroxide content and compared to the pretreatment LDL, caused a threefold increase in the incorporation of cholesterol into cultured (rat aortic) endothelial cells. The observed intense and lasting stimulation of monocytes by oxidized polyunsaturated fatty acids in vitro, and the generation of 'altered' LDL by these oxidized lipids in vivo suggests a mechanism by which atherogenic oxidized LDL could form in the circulation.
Full text
PDF





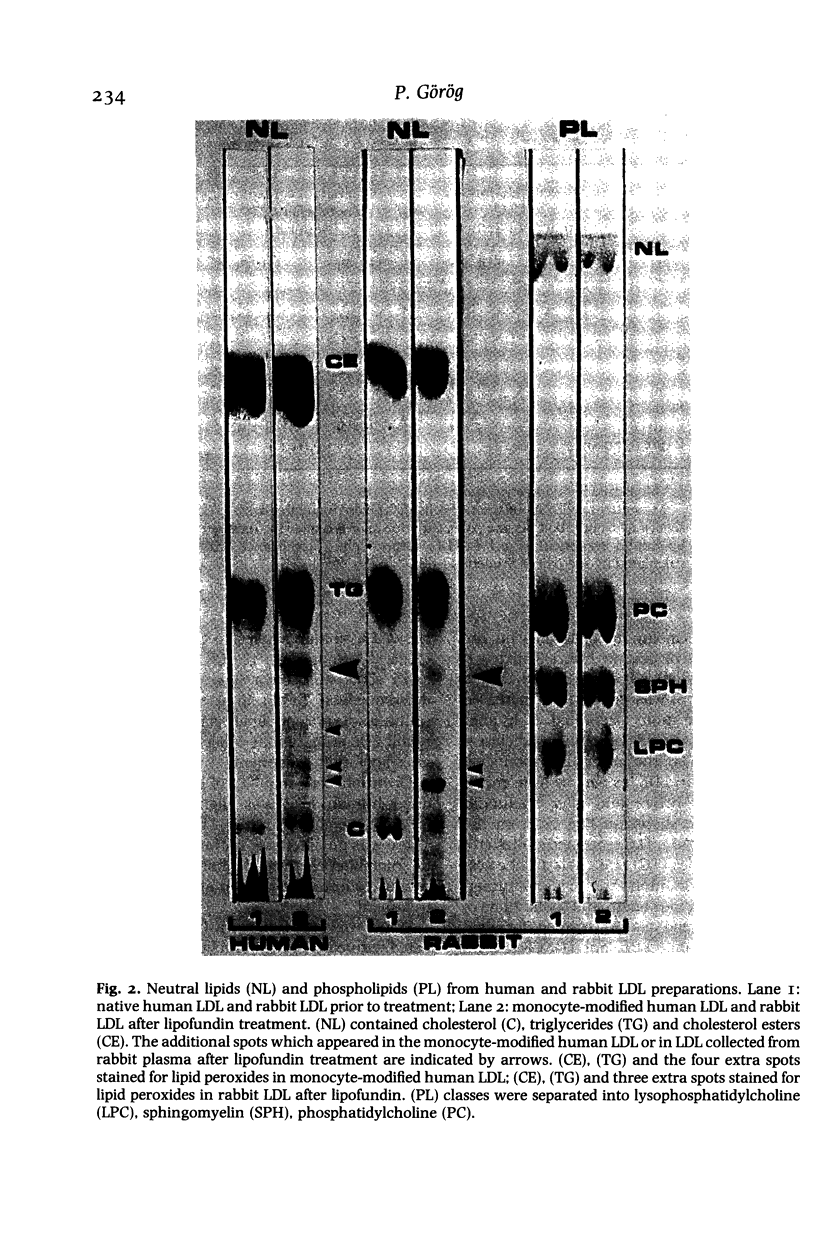



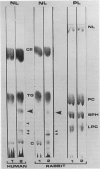
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreesen R., Picht J., Löhr G. W. Primary cultures of human blood-born macrophages grown on hydrophobic teflon membranes. J Immunol Methods. 1983 Feb 11;56(3):295–304. doi: 10.1016/s0022-1759(83)80019-2. [DOI] [PubMed] [Google Scholar]
- Bass D. A., Parce J. W., Dechatelet L. R., Szejda P., Seeds M. C., Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983 Apr;130(4):1910–1917. [PubMed] [Google Scholar]
- Block L. H., Knorr M., Vogt E., Locher R., Vetter W., Groscurth P., Qiao B. Y., Pometta D., James R., Regenass M. Low density lipoprotein causes general cellular activation with increased phosphatidylinositol turnover and lipoprotein catabolism. Proc Natl Acad Sci U S A. 1988 Feb;85(3):885–889. doi: 10.1073/pnas.85.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbisier P., Houbion A., Remacle J. A new technique for highly sensitive detection of superoxide dismutase activity by chemiluminescence. Anal Biochem. 1987 Jul;164(1):240–247. doi: 10.1016/0003-2697(87)90392-7. [DOI] [PubMed] [Google Scholar]
- Davies S. W., Ranjadayalan K., Wickens D. G., Dormandy T. L., Timmis A. D. Lipid peroxidation associated with successful thrombolysis. Lancet. 1990 Mar 31;335(8692):741–743. doi: 10.1016/0140-6736(90)90866-4. [DOI] [PubMed] [Google Scholar]
- Frei B., Stocker R., Ames B. N. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9748–9752. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaton E., Orgad U., Weisman Y., Wolman M. Phagocytic system stimulation in experimental atherosclerosis of chicks. Exp Mol Pathol. 1988 Dec;49(3):291–296. doi: 10.1016/0014-4800(88)90001-9. [DOI] [PubMed] [Google Scholar]
- Görög P., Kakkar V. V. Increased uptake of monocyte-treated low density lipoproteins by aortic endothelium in vivo. Atherosclerosis. 1987 May;65(1-2):99–107. doi: 10.1016/0021-9150(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Hartung H. P., Kladetzky R. G., Melnik B., Hennerici M. Stimulation of the scavenger receptor on monocytes-macrophages evokes release of arachidonic acid metabolites and reduced oxygen species. Lab Invest. 1986 Aug;55(2):209–216. [PubMed] [Google Scholar]
- Huttner J. J., Gwebu E. T., Panganamala R. V., Milo G. E., Cornwell D. C., Sharma H. M., Geer J. C. Fatty acids and their prostaglandin derivatives: inhibitors of proliferation in aortic smooth muscle cells. Science. 1977 Jul 15;197(4300):289–291. doi: 10.1126/science.877555. [DOI] [PubMed] [Google Scholar]
- Janero D. R., Burghardt B. Thiobarbituric acid-reactive malondialdehyde formation during superoxide-dependent, iron-catalyzed lipid peroxidation: influence of peroxidation conditions. Lipids. 1989 Feb;24(2):125–131. doi: 10.1007/BF02535249. [DOI] [PubMed] [Google Scholar]
- Kelley J. L., Rozek M. M., Suenram C. A., Schwartz C. J. Activation of human peripheral blood monocytes by lipoproteins. Am J Pathol. 1988 Feb;130(2):223–231. [PMC free article] [PubMed] [Google Scholar]
- McGuire P. G., Orkin R. W. Isolation of rat aortic endothelial cells by primary explant techniques and their phenotypic modulation by defined substrata. Lab Invest. 1987 Jul;57(1):94–105. [PubMed] [Google Scholar]
- Morin R. J., Peng S. K. The role of cholesterol oxidation products in the pathogenesis of atherosclerosis. Ann Clin Lab Sci. 1989 Jul-Aug;19(4):225–237. [PubMed] [Google Scholar]
- Nagelkerke J. F., Havekes L., van Hinsbergh V. W., van Berkel T. J. In vivo catabolism of biologically modified LDL. Arteriosclerosis. 1984 May-Jun;4(3):256–264. doi: 10.1161/01.atv.4.3.256. [DOI] [PubMed] [Google Scholar]
- Nishigaki I., Hagihara M., Tsunekawa H., Maseki M., Yagi K. Lipid peroxide levels of serum lipoprotein fractions of diabetic patients. Biochem Med. 1981 Jun;25(3):373–378. doi: 10.1016/0006-2944(81)90096-x. [DOI] [PubMed] [Google Scholar]
- Noble R. P. Electrophoretic separation of plasma lipoproteins in agarose gel. J Lipid Res. 1968 Nov;9(6):693–700. [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Reaction of linoleic acid hydroperoxide with thiobarbituric acid. J Lipid Res. 1978 Nov;19(8):1053–1057. [PubMed] [Google Scholar]
- Rössner S. The intravenous fat tolerance test with intralipid in various types of hyperlipidaemias and comparison between metabolism of intralipid and VLDL. Adv Exp Med Biol. 1973;38:69–85. [PubMed] [Google Scholar]
- Voyta J. C., Via D. P., Butterfield C. E., Zetter B. R. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984 Dec;99(6):2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi K. Lipid peroxides and human diseases. Chem Phys Lipids. 1987 Nov-Dec;45(2-4):337–351. doi: 10.1016/0009-3084(87)90071-5. [DOI] [PubMed] [Google Scholar]
- Zhang H. F., Davis W. B., Chen X. S., Whisler R. L., Cornwell D. G. Studies on oxidized low density lipoproteins. Controlled oxidation and a prostaglandin artifact. J Lipid Res. 1989 Feb;30(2):141–148. [PubMed] [Google Scholar]