Abstract
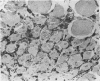
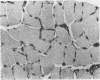
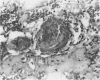
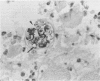
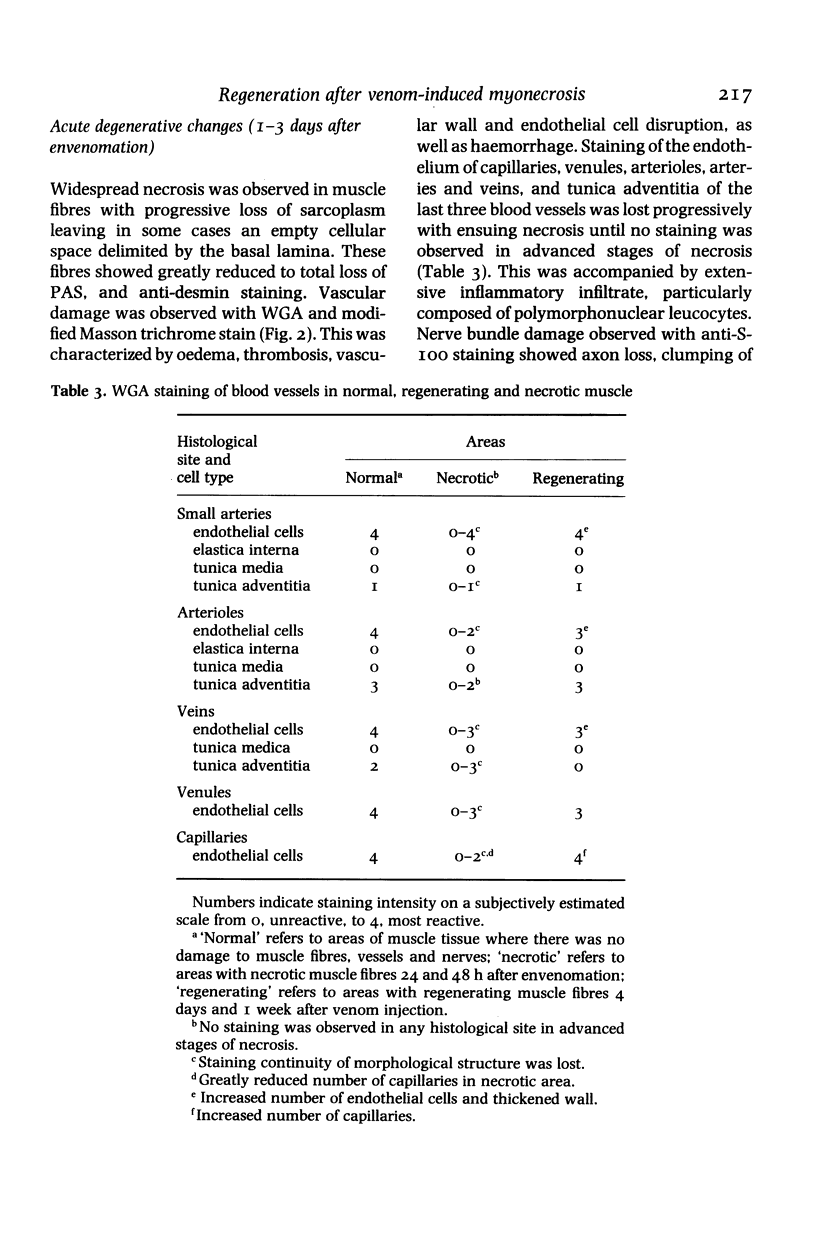
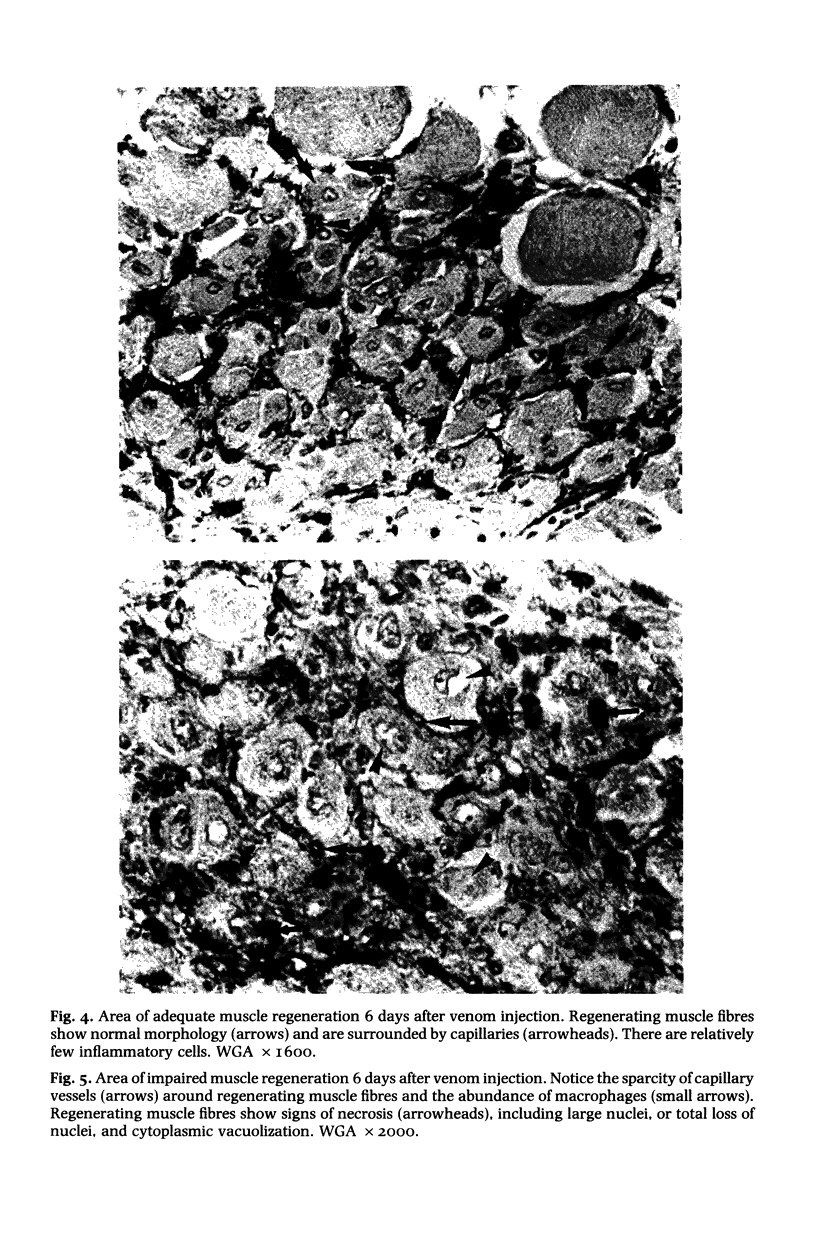
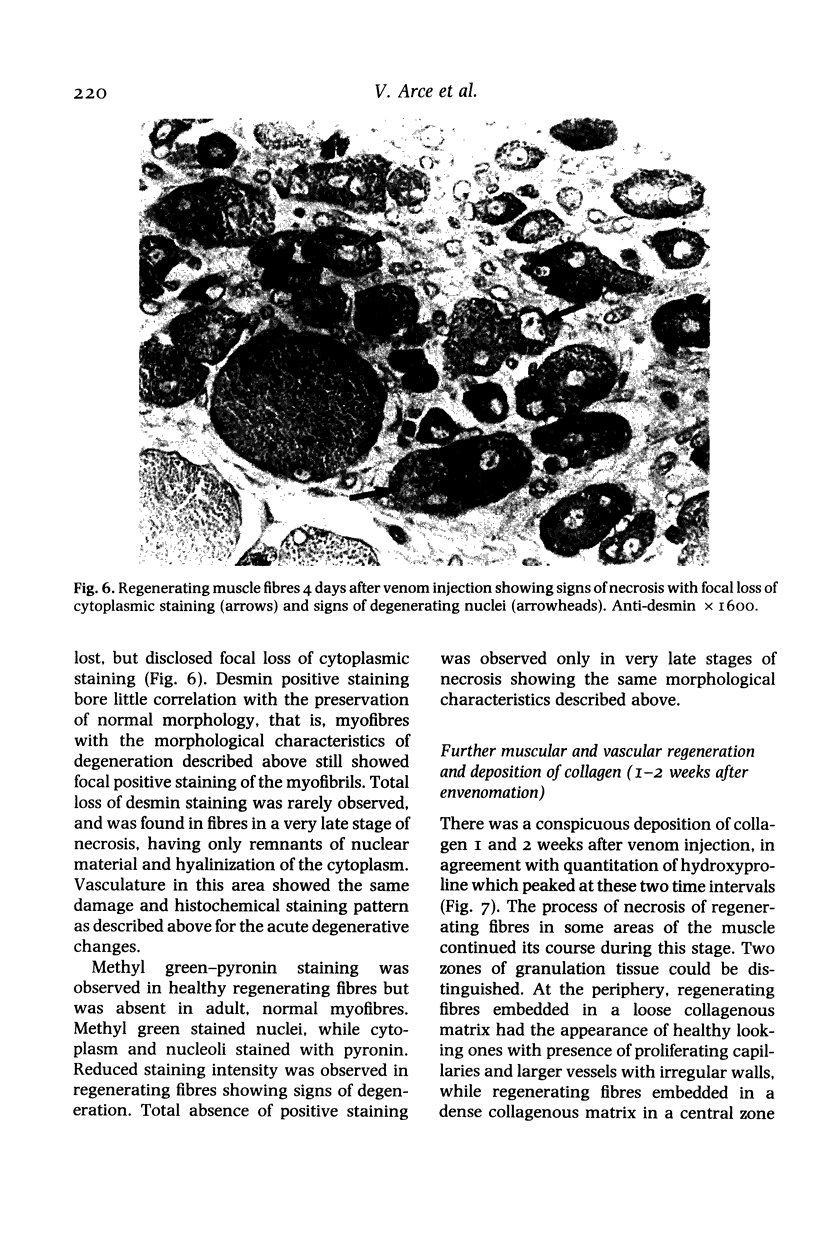
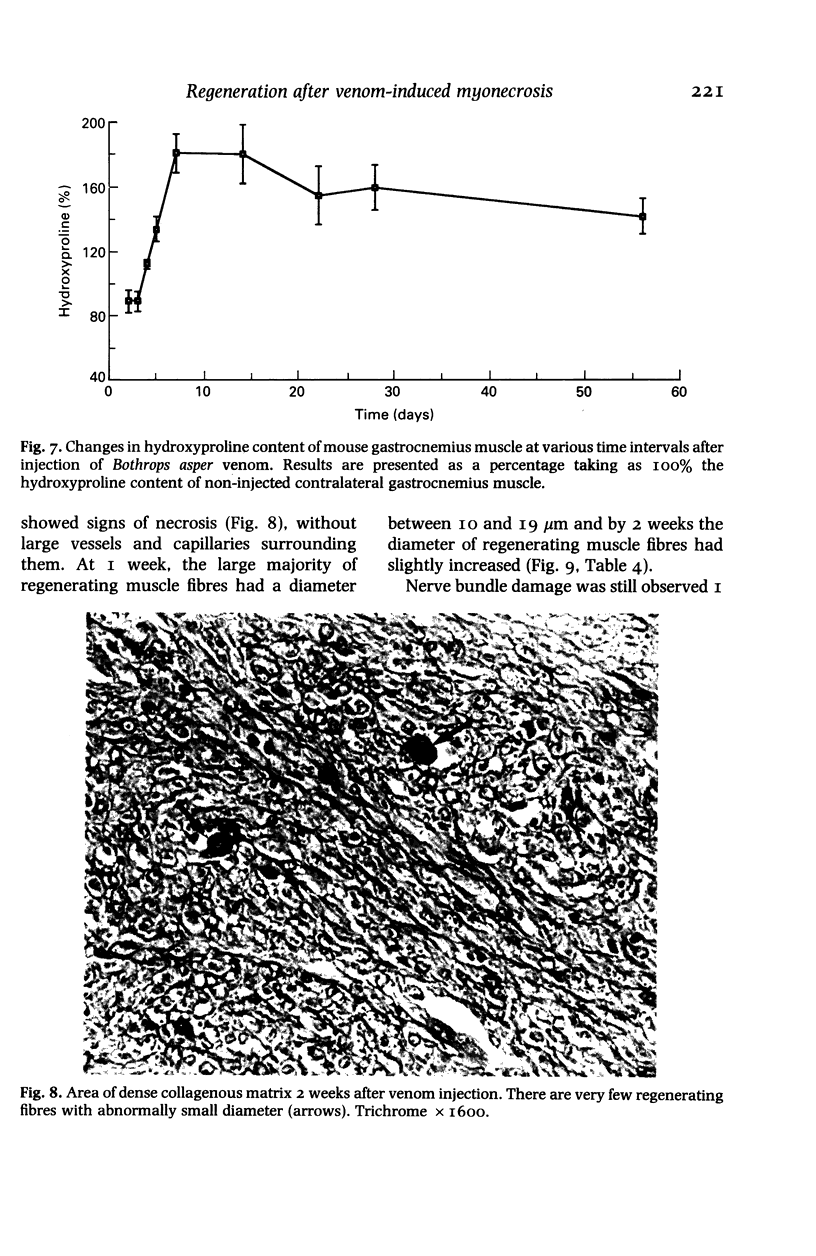
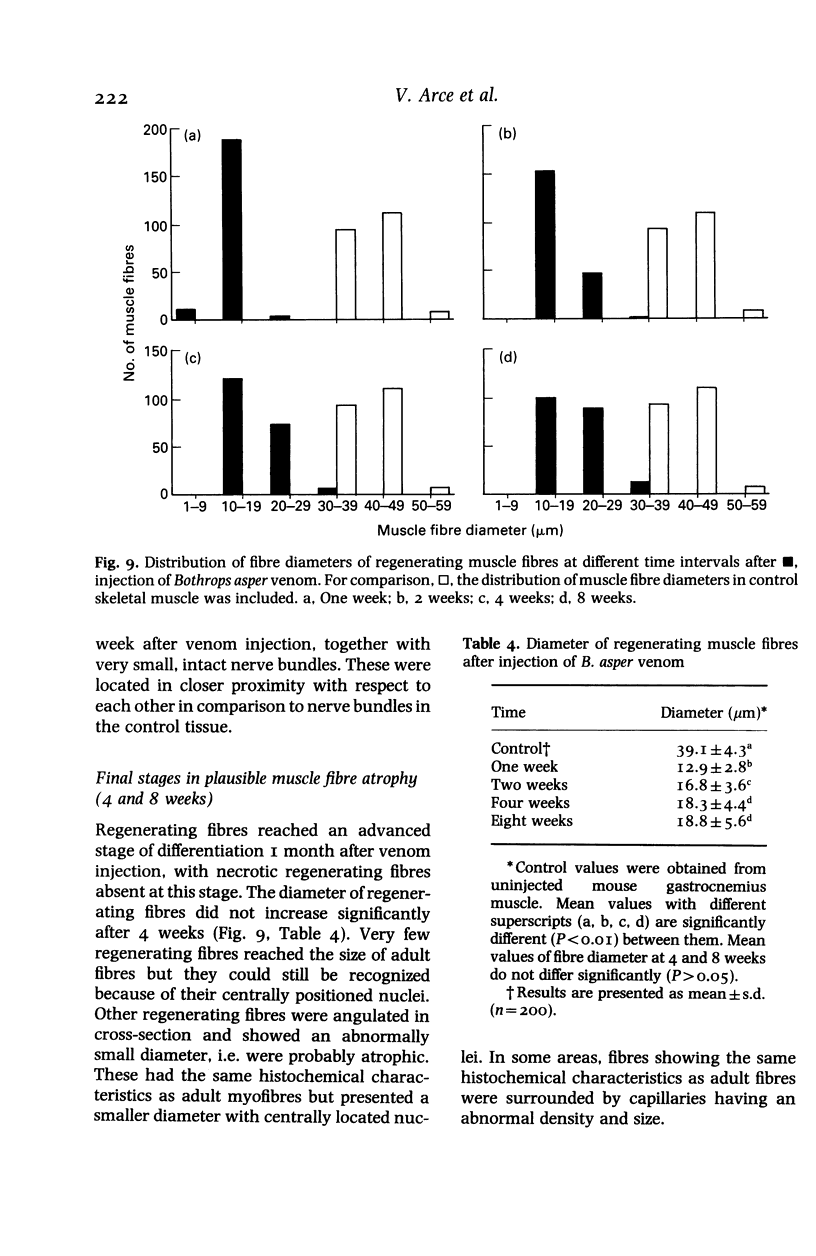
The degenerative and regenerative changes in murine skeletal muscle after injection of Bothrops asper venom were studied by histological, lectin histochemical and immunocytochemical techniques. According to our observations, the process was divided into four main stages: (a) During the first 3 days prominent degenerative events took place in skeletal muscle fibres, capillaries, arteries, veins and intramuscular nerves. An inflammatory infiltrate was abundant after the first day and removal of necrotic material was well advanced by the third day. (b) Muscle regeneration was evident by the fourth day. From 4 to 6 days there were two populations of regenerating muscle fibres, one of apparently normal fibres located in areas where capillary vessels were abundant, and another population of groups of regenerative fibres showing signs of degeneration. This second type of fibre was predominant in areas where the number of capillaries was greatly reduced. (c) One and 2 weeks after envenomation areas of small regenerative fibres of normal morphology and areas of degenerating regenerative fibres were observed. The latter were abundant in regions of dense fibrotic tissue and scarce capillaries. (d) Finally, at 4 and 8 weeks after envenomation there were both areas of fibrosis and areas where regenerating muscle fibres predominated. However, the diameter of these fibres was abnormally small, an indication that they may have been atrophic fibres. It is suggested that muscle regeneration is partially impaired after myonecrosis induced by Bothrops asper venom, probably due to the damage induced by this venom on muscle microvasculature and nerves.
Full text
PDF



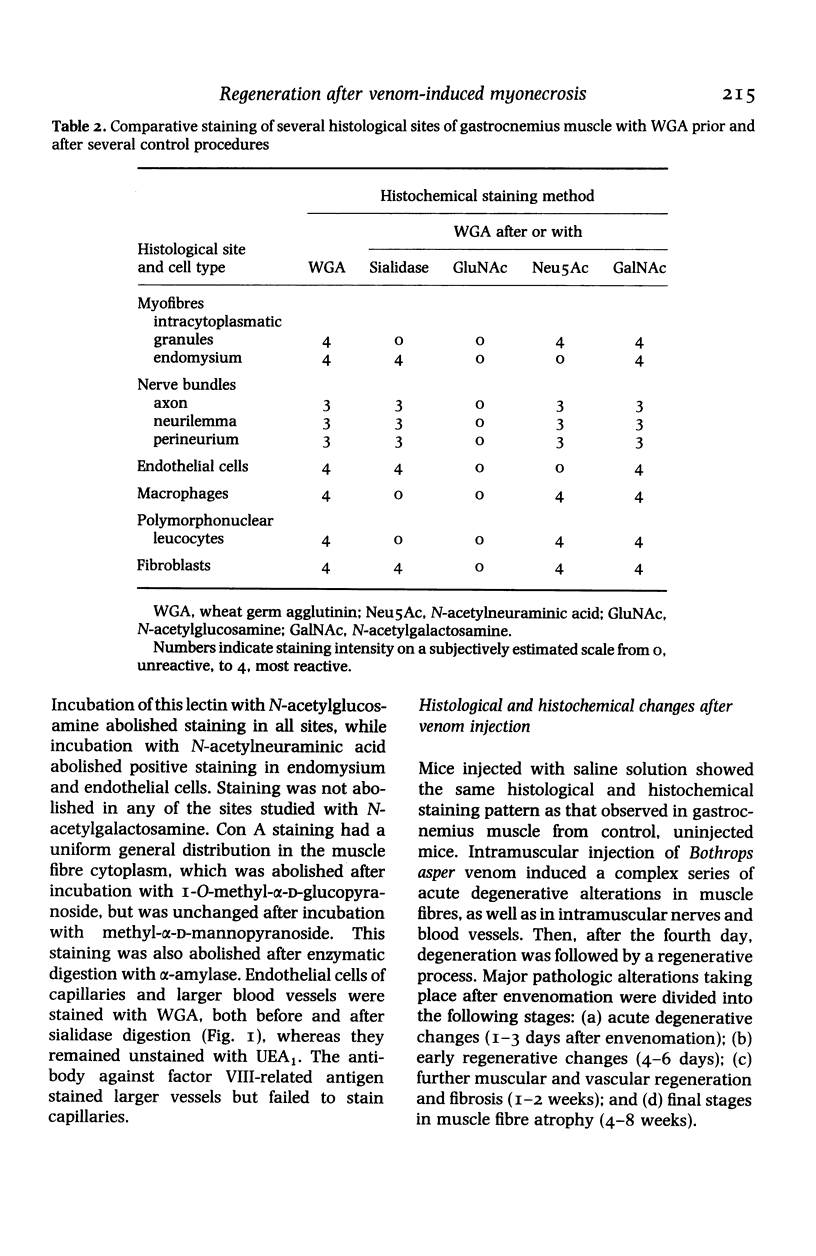
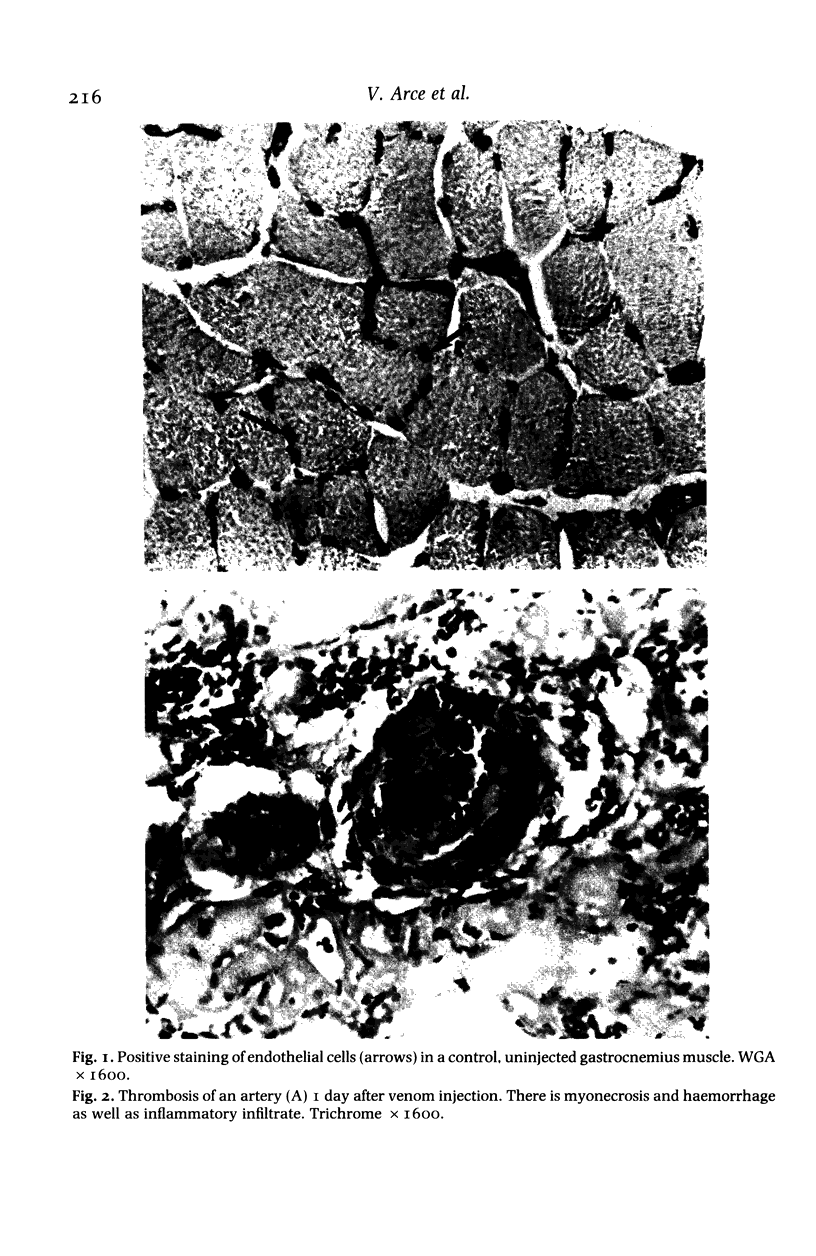





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allbrook D. Skeletal muscle regeneration. Muscle Nerve. 1981 May-Jun;4(3):234–245. doi: 10.1002/mus.880040311. [DOI] [PubMed] [Google Scholar]
- Allen H. J., Johnson E. A., Matta K. L. A comparison of the binding specificities of lectins from Ulex europaeus and Lotus tetragonolobus. Immunol Commun. 1977;6(6):585–602. doi: 10.3109/08820137709093469. [DOI] [PubMed] [Google Scholar]
- Bhavanandan V. P., Katlic A. W. The interaction of wheat germ agglutinin with sialoglycoproteins. The role of sialic acid. J Biol Chem. 1979 May 25;254(10):4000–4008. [PubMed] [Google Scholar]
- Brenes F., Gutiérrez J. M., Lomonte B. Immunohistochemical demonstration of the binding of Bothrops asper myotoxin to skeletal muscle sarcolemma. Toxicon. 1987;25(5):574–577. doi: 10.1016/0041-0101(87)90294-7. [DOI] [PubMed] [Google Scholar]
- Burger M. M., Goldberg A. R. Identification of a tumor-specific determinant on neoplastic cell surfaces. Proc Natl Acad Sci U S A. 1967 Feb;57(2):359–366. doi: 10.1073/pnas.57.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., SMITH E. E. PROTEIN-CARBOHYDRATE INTERACTION. II. INHIBITION STUDIES ON THE INTERACTION OF CONCANAVALIN A WITH POLYSACCHARIDES. Biochemistry. 1965 May;4:876–883. doi: 10.1021/bi00881a013. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J. M., Arce V., Brenes F., Chaves F. Changes in myofibrillar components after skeletal muscle necrosis induced by a myotoxin isolated from the venom of the snake Bothrops asper. Exp Mol Pathol. 1990 Feb;52(1):25–36. doi: 10.1016/0014-4800(90)90055-i. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J. M., Chaves F., Gené J. A., Lomonte B., Camacho Z., Schosinsky K. Myonecrosis induced in mice by a basic myotoxin isolated from the venom of the snake Bothrops nummifer (jumping viper) from Costa Rica. Toxicon. 1989;27(7):735–745. doi: 10.1016/0041-0101(89)90040-8. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J. M., Ownby C. L., Odell G. V. Pathogenesis of myonecrosis induced by crude venom and a myotoxin of Bothrops asper. Exp Mol Pathol. 1984 Jun;40(3):367–379. doi: 10.1016/0014-4800(84)90054-6. [DOI] [PubMed] [Google Scholar]
- Hall-Craggs E. C., Seyan H. S. Histochemical changes in innervated and denervated skeletal muscle fibers following treatment with bupivacaine (marcain). Exp Neurol. 1975 Feb;46(2):345–354. doi: 10.1016/0014-4886(75)90140-5. [DOI] [PubMed] [Google Scholar]
- Harris J. B., Maltin C. A. Myotoxic activity of the crude venom and the principal neurotoxin, taipoxin, of the Australian taipan, Oxyuranus scutellatus. Br J Pharmacol. 1982 May;76(1):61–75. doi: 10.1111/j.1476-5381.1982.tb09191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma M., Kosuge T., Okonogi T., Hattori Z., Sawai Y. A histopathological study on arterial lesions caused by Habu (Trimeresurus flavoviridis) venom. Jpn J Exp Med. 1967 Aug;37(4):323–336. [PubMed] [Google Scholar]
- Hoyer L. W., De los Santos R. P., Hoyer J. R. Antihemophilic factor antigen. Localization in endothelial cells by immunofluorescent microscopy. J Clin Invest. 1973 Nov;52(11):2737–2744. doi: 10.1172/JCI107469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonte B., Gutiérrez J. M. A new muscle damaging toxin, myotoxin II, from the venom of the snake Bothrops asper (terciopelo). Toxicon. 1989;27(7):725–733. doi: 10.1016/0041-0101(89)90039-1. [DOI] [PubMed] [Google Scholar]
- López-De León A., Rojkind M. A simple micromethod for collagen and total protein determination in formalin-fixed paraffin-embedded sections. J Histochem Cytochem. 1985 Aug;33(8):737–743. doi: 10.1177/33.8.2410480. [DOI] [PubMed] [Google Scholar]
- Monsigny M., Roche A. C., Sene C., Maget-Dana R., Delmotte F. Sugar-lectin interactions: how does wheat-germ agglutinin bind sialoglycoconjugates? Eur J Biochem. 1980 Feb;104(1):147–153. doi: 10.1111/j.1432-1033.1980.tb04410.x. [DOI] [PubMed] [Google Scholar]
- Nonaka I., Takagi A., Ishiura S., Nakase H., Sugita H. Pathophysiology of muscle fiber necrosis induced by bupivacaine hydrochloride (Marcaine). Acta Neuropathol. 1983;60(3-4):167–174. doi: 10.1007/BF00691863. [DOI] [PubMed] [Google Scholar]
- PROCKOP D. J., UDENFRIEND S. A specific method for the analysis of hydroxyproline in tissues and urine. Anal Biochem. 1960 Nov;1:228–239. doi: 10.1016/0003-2697(60)90050-6. [DOI] [PubMed] [Google Scholar]
- Queiroz L. S., Petta C. A. Histopathological changes caused by venom of urutu snake (Bothrops alternatus) in mouse skeletal muscle. Rev Inst Med Trop Sao Paulo. 1984 Sep-Oct;26(5):247–253. doi: 10.1590/s0036-46651984000500004. [DOI] [PubMed] [Google Scholar]
- Schulte B. A., Spicer S. S. Light microscopic histochemical detection of sugar residues in secretory glycoproteins of rodent and human tracheal glands with lectin-horseradish peroxidase conjugates and the galactose oxidase-Schiff sequence. J Histochem Cytochem. 1983 Mar;31(3):391–403. doi: 10.1177/31.3.6186733. [DOI] [PubMed] [Google Scholar]