Abstract
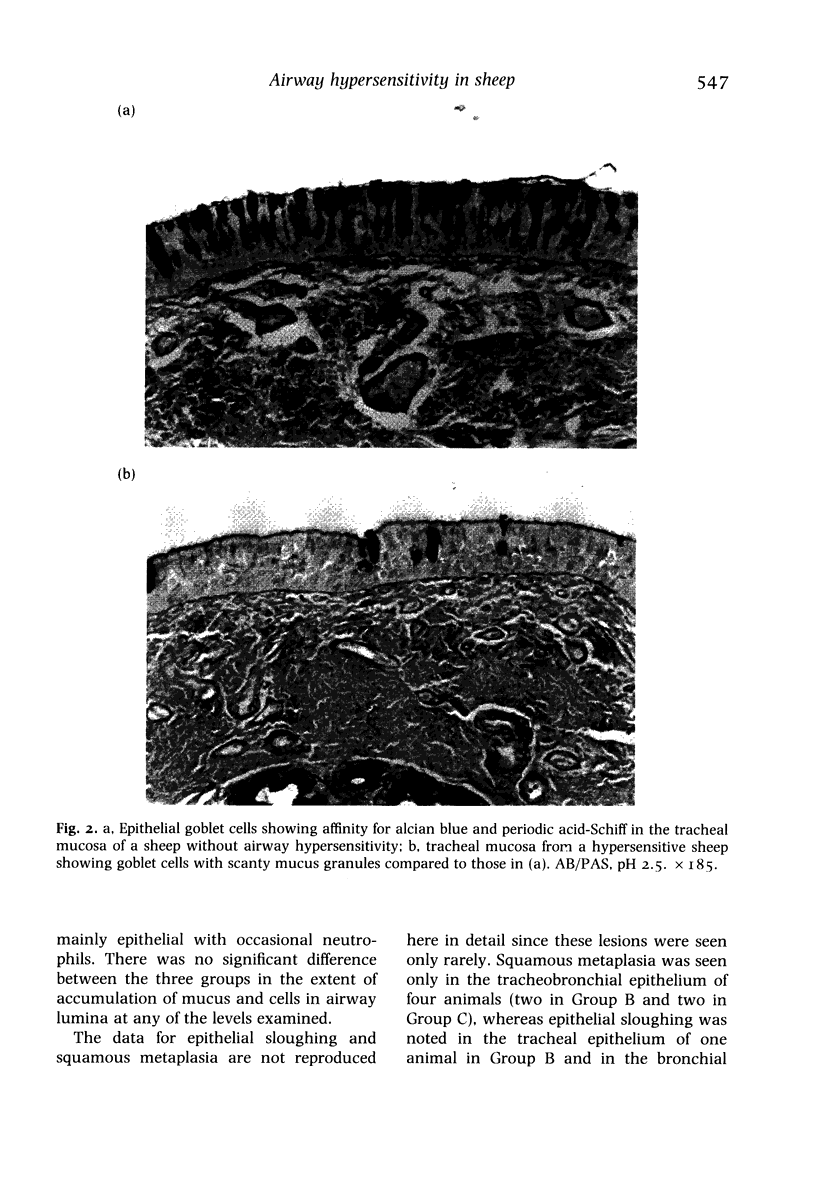
The airways of 12 sheep with naturally-occurring allergic airway hypersensitivity, six of which had changes in both airway resistance and dynamic lung compliance (Group A) and six of which had changes in only dynamic lung compliance (Group B), were compared quantitatively with six non-reacting sheep (Group C) in order to examine the relation between airway hypersensitivity and various morphological features thought to be related to airway hypersensitivity. Compared to the non-reacting sheep (Group C), the hypersensitive sheep (Groups A and B) had a thinner epithelium in medium bronchi and bronchioles, fewer goblet cells in bronchioles, and greater gland area at most airway levels. The differences of the gland dimensions and the types of mucosubstance between hypersensitive and non-reacting animals were more variable. No significant differences between the three groups were noted with regard to luminal occlusion or epithelial sloughing and squamous metaplasia. Although there was a positive association between epithelial thickness and goblet cell density in the small airways, the development of allergic airway hypersensitivity in sheep may occur in the absence of major morphological changes in the airway epithelium.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen W., Pack R. J., Alley M. R., Carr D. H., Manktelow B. W. Airway hypersensitivity induced by Ascaris suum extract in New Zealand Romney sheep. N Z Vet J. 1990 Jun;38(2):57–61. doi: 10.1080/00480169.1990.35617. [DOI] [PubMed] [Google Scholar]
- Christensen T. G., Janeczek A. H. Response of the tracheobronchial epithelium to hemoprotein tracers. Lung. 1985;163(2):95–108. doi: 10.1007/BF02713811. [DOI] [PubMed] [Google Scholar]
- Cutz E., Levison H., Cooper D. M. Ultrastructure of airways in children with asthma. Histopathology. 1978 Nov;2(6):407–421. doi: 10.1111/j.1365-2559.1978.tb01735.x. [DOI] [PubMed] [Google Scholar]
- Dunnill M. S., Massarella G. R., Anderson J. A. A comparison of the quantitative anatomy of the bronchi in normal subjects, in status asthmaticus, in chronic bronchitis, and in emphysema. Thorax. 1969 Mar;24(2):176–179. doi: 10.1136/thx.24.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwood R. K., Kennedy S., Belzberg A., Hogg J. C., Paré P. D. Respiratory mucosal permeability in asthma. Am Rev Respir Dis. 1983 Sep;128(3):523–527. doi: 10.1164/arrd.1983.128.3.523. [DOI] [PubMed] [Google Scholar]
- Jeffery P. K., Reid L. M. The effect of tobacco smoke, with or without phenylmethyloxadiazole (PMO), on rat bronchial epithelium: a light and electron microscopic study. J Pathol. 1981 Apr;133(4):341–359. doi: 10.1002/path.1711330406. [DOI] [PubMed] [Google Scholar]
- Kallós P., Kallós L. Experimental asthma in guinea pigs revisited. Int Arch Allergy Appl Immunol. 1984;73(1):77–85. doi: 10.1159/000233441. [DOI] [PubMed] [Google Scholar]
- Laitinen L. A., Heino M., Laitinen A., Kava T., Haahtela T. Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am Rev Respir Dis. 1985 Apr;131(4):599–606. doi: 10.1164/arrd.1985.131.4.599. [DOI] [PubMed] [Google Scholar]
- Lebargy F., Lenormand E., Pariente R., Fournier M. Morphological changes in rat tracheal mucosa immediately after antigen challenge. Bull Eur Physiopathol Respir. 1987 Sep-Oct;23(5):417–421. [PubMed] [Google Scholar]
- Lozewicz S., Wells C., Gomez E., Ferguson H., Richman P., Devalia J., Davies R. J. Morphological integrity of the bronchial epithelium in mild asthma. Thorax. 1990 Jan;45(1):12–15. doi: 10.1136/thx.45.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Byrne P. M., Dolovich M., Dirks R., Roberts R. S., Newhouse M. T. Lung epithelial permeability: relation to nonspecific airway responsiveness. J Appl Physiol Respir Environ Exerc Physiol. 1984 Jul;57(1):77–84. doi: 10.1152/jappl.1984.57.1.77. [DOI] [PubMed] [Google Scholar]
- Ranga V., Powers M. A., Padilla M., Strope G. L., Fowler L., Kleinerman J. Effect of allergic bronchoconstriction on airways epithelial permeability to large polar solutes in the guinea pig. Am Rev Respir Dis. 1983 Dec;128(6):1065–1070. doi: 10.1164/arrd.1983.128.6.1065. [DOI] [PubMed] [Google Scholar]
- Salvato G. Some histological changes in chronic bronchitis and asthma. Thorax. 1968 Mar;23(2):168–172. doi: 10.1136/thx.23.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobonya R. E. Quantitative structural alterations in long-standing allergic asthma. Am Rev Respir Dis. 1984 Aug;130(2):289–292. doi: 10.1164/arrd.1984.130.2.289. [DOI] [PubMed] [Google Scholar]
- Spicer S. S., Chakrin L. W., Wardell J. R., Jr, Kendrick W. Histochemistry of mucosubstances in the canine and human respiratory tract. Lab Invest. 1971 Dec;25(6):483–490. [PubMed] [Google Scholar]
- Yanta M. A., Snapper J. R., Ingram R. H., Jr, Drazen J. M., Coles S., Reid L. Airway responsiveness to inhaled mediators: relationship to epithelial thickness and secretory cell number. Am Rev Respir Dis. 1981 Sep;124(3):337–340. doi: 10.1164/arrd.1981.124.3.337. [DOI] [PubMed] [Google Scholar]



