Abstract
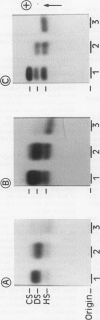
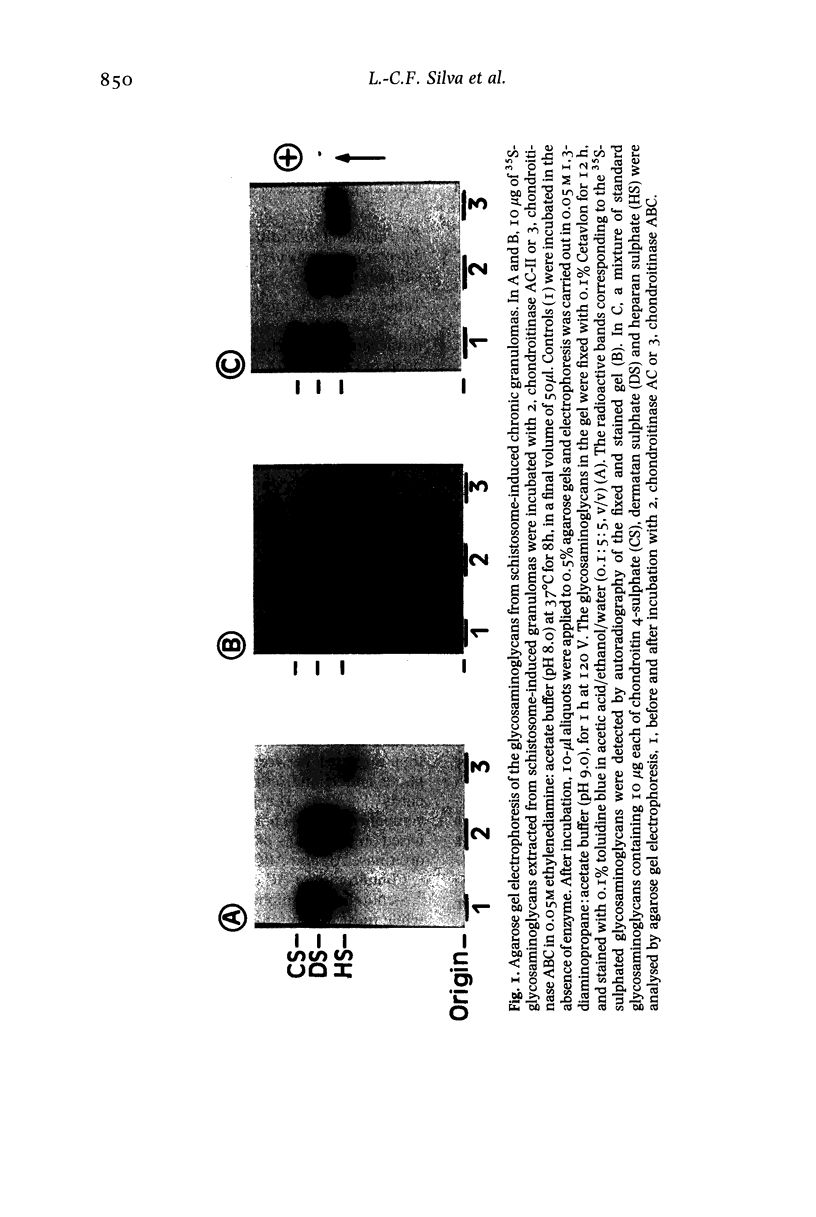
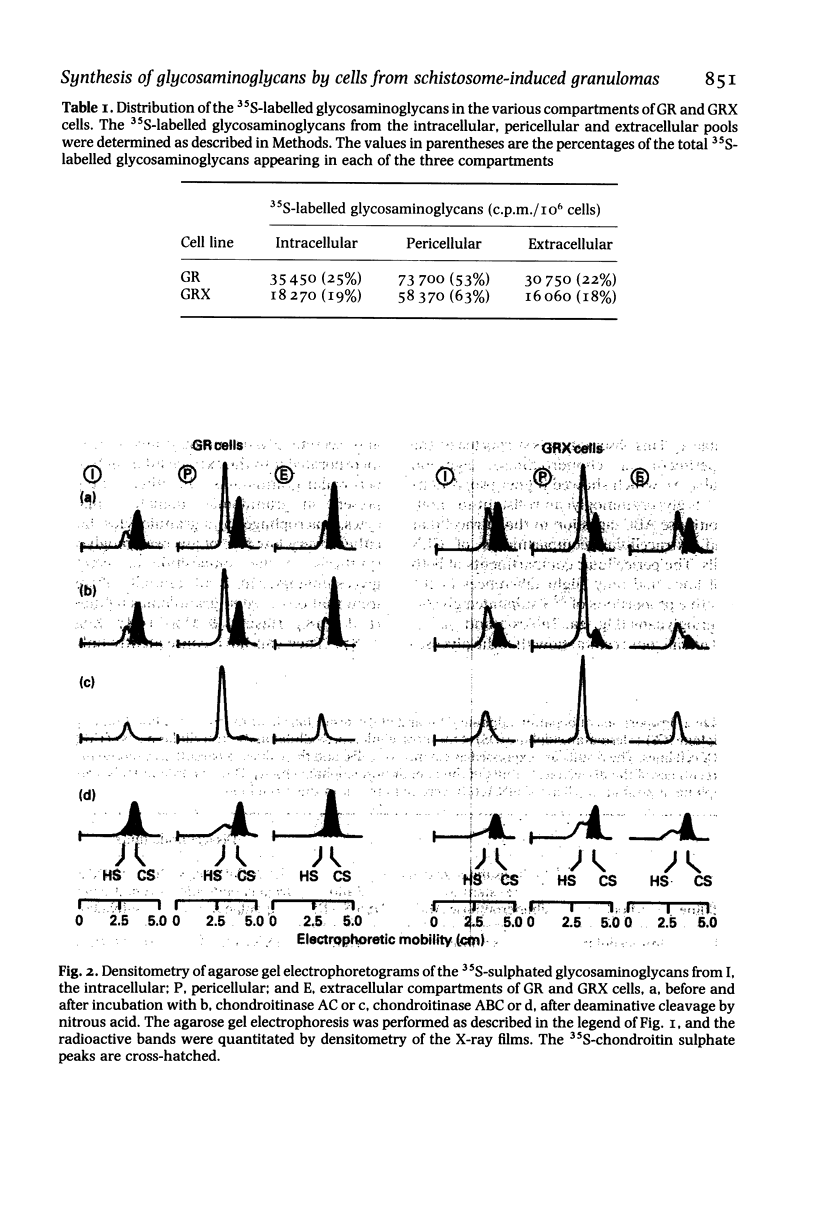
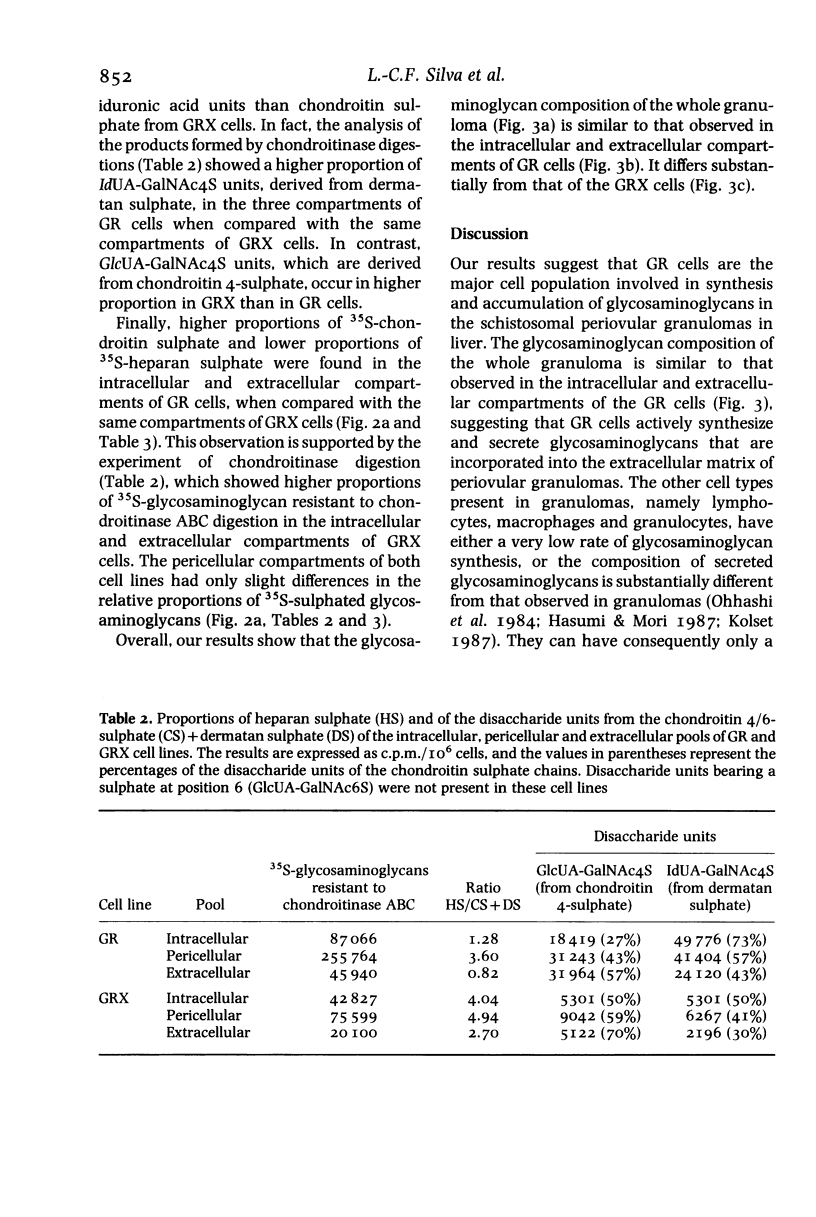
Sulphated glycosaminoglycans were isolated from schistosome-induced hepatic granuloma and from the pericellular, intracellular and extracellular compartments of two murine cell lines derived from granulomas: the primary cell line GR, and the permanent cell line GRX, established spontaneously from GR. The glycosaminoglycans composition in the whole granuloma was similar to that observed in the intracellular and extracellular compartments of GR cells. This result suggests that GR cells may be the major cell population involved in the synthesis and accumulation of glycosaminoglycans in the granulomas, and play an important role in the process of hepatic fibrosis. The conversion of the primary cell line GR into the established GRX cells did not modify the ratios that prevail among different glycosaminoglycans of the cell surface. However, it decreased the synthesis and secretion of glycosaminoglycans, reduced the proportion of iduronic acid units in the chondroitin sulphate, and increased the proportion of heparan sulphate in intracellular and extracellular pools. These characteristics of the GRX cells are similar to those observed in long-term cultures of smooth-muscle cells. In agreement with the general phenomenon of progressive de-differentiation during in-vitro culture of primary cell lines, these data indicate that the connective tissue cells of liver may belong to the myofibroblastic cell lineage.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al Adnani M. S. Concomitant immunohistochemical localization of fibronectin and collagen in schistosome granulomata. J Pathol. 1985 Oct;147(2):77–85. doi: 10.1002/path.1711470202. [DOI] [PubMed] [Google Scholar]
- BRENER Z., PELLEGRINO J. Method for isolating schistosome granulomas from mouse liver. J Parasitol. 1956 Dec;42(6):564–564. [PubMed] [Google Scholar]
- Beldekas J. C., Gerstenfeld L., Sonenshein G. E., Franzblau C. Cell density and estradiol modulation of procollagen type III in cultured calf smooth muscle cells. J Biol Chem. 1982 Oct 25;257(20):12252–12256. [PubMed] [Google Scholar]
- Benya P. D., Padilla S. R., Nimni M. E. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell. 1978 Dec;15(4):1313–1321. doi: 10.1016/0092-8674(78)90056-9. [DOI] [PubMed] [Google Scholar]
- Dietrich C. P., Dietrich S. M. Electrophoretic behaviour of acidic mucopolysaccharides in diamine buffers. Anal Biochem. 1976 Feb;70(2):645–647. doi: 10.1016/0003-2697(76)90496-6. [DOI] [PubMed] [Google Scholar]
- Gressner A. M., Haarmann R. Hyaluronic acid synthesis and secretion by rat liver fat storing cells (perisinusoidal lipocytes) in culture. Biochem Biophys Res Commun. 1988 Feb 29;151(1):222–229. doi: 10.1016/0006-291x(88)90582-7. [DOI] [PubMed] [Google Scholar]
- Grimaud J. A., Boros D. L., Takiya C., Mathew R. C., Emonard H. Collagen isotypes, laminin, and fibronectin in granulomas of the liver and intestines of Schistosoma mansoni-infected mice. Am J Trop Med Hyg. 1987 Sep;37(2):335–344. doi: 10.4269/ajtmh.1987.37.335. [DOI] [PubMed] [Google Scholar]
- Hasumi F., Mori Y. Changes in the biosynthesis of glycosaminoglycans in polymorphonuclear leukocytes in relation to their induction into the peritoneal cavity. J Leukoc Biol. 1987 Aug;42(2):150–155. doi: 10.1002/jlb.42.2.150. [DOI] [PubMed] [Google Scholar]
- Hök M., Kjellén L., Johansson S. Cell-surface glycosaminoglycans. Annu Rev Biochem. 1984;53:847–869. doi: 10.1146/annurev.bi.53.070184.004215. [DOI] [PubMed] [Google Scholar]
- Kraemer P. M. Heparan sulfates of cultured cells. I. Membrane-associated and cell-sap species in Chinese hamster cells. Biochemistry. 1971 Apr 13;10(8):1437–1445. doi: 10.1021/bi00784a026. [DOI] [PubMed] [Google Scholar]
- Mak K. M., Leo M. A., Lieber C. S. Alcoholic liver injury in baboons: transformation of lipocytes to transitional cells. Gastroenterology. 1984 Jul;87(1):188–200. [PubMed] [Google Scholar]
- Maurão P. A., Machado-Santelli G. M., Toledo O. M. Sulfated glycosaminoglycans of cultured fibroblasts from guinea-pig embryo kidney. Biochim Biophys Acta. 1980 May 7;629(2):259–265. [PubMed] [Google Scholar]
- Mehlhorn H., Frenkel J. K., Andrews P., Thomas H. Light and electron microscopic studies on Schistosoma mansoni Granulomas of mouse livers following treatment with praziquantel. Tropenmed Parasitol. 1982 Dec;33(4):229–239. [PubMed] [Google Scholar]
- Mourão P. A., Pillai S., Donnelly P. V. Sulfated glycosaminoglycans synthesized by fibroblast, smooth muscle and endothelium-like cells grown in culture. Cell Differ. 1983 Feb;12(2):99–108. doi: 10.1016/0045-6039(83)90062-3. [DOI] [PubMed] [Google Scholar]
- Rihn B., Beck G., Monteil H., Lecerf F., Girardot R. Effect of the cytotoxin of Clostridium difficile on cultured hepatoma cells. Biol Cell. 1985;53(1):23–32. doi: 10.1111/j.1768-322x.1985.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Roberts R., Gallagher J., Spooncer E., Allen T. D., Bloomfield F., Dexter T. M. Heparan sulphate bound growth factors: a mechanism for stromal cell mediated haemopoiesis. Nature. 1988 Mar 24;332(6162):376–378. doi: 10.1038/332376a0. [DOI] [PubMed] [Google Scholar]
- Schürch W., Seemayer T. A., Lagacé R. Stromal myofibroblasts in primary invasive and metastatic carcinomas. A combined immunological, light and electron microscopic study. Virchows Arch A Pathol Anat Histol. 1981;391(2):125–139. doi: 10.1007/BF00437591. [DOI] [PubMed] [Google Scholar]
- Scott J. E. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988 Jun 1;252(2):313–323. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976 Sep 7;15(18):3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Toledo O. M., Mourão P. A. Sulfated glycosaminoglycans of human aorta: chondroitin 6-sulfate increase with age. Biochem Biophys Res Commun. 1979 Jul 12;89(1):50–55. doi: 10.1016/0006-291x(79)90941-0. [DOI] [PubMed] [Google Scholar]
- Tseng S. C., Savion N., Stern R., Gospodarowicz D. Fibroblast growth factor modulates synthesis of collagen in cultured vascular endothelial cells. Eur J Biochem. 1982 Feb;122(2):355–360. doi: 10.1111/j.1432-1033.1982.tb05888.x. [DOI] [PubMed] [Google Scholar]
- Warren K. S., Klein L. Chronic murine hepatosplenic schistosomiasis mansoni: relative irreversibility after treatment. Trans R Soc Trop Med Hyg. 1969;63(3):333–337. doi: 10.1016/0035-9203(69)90006-6. [DOI] [PubMed] [Google Scholar]
- Warren K. S. The pathology, pathobiology and pathogenesis of schistosomiasis. Nature. 1978 Jun 22;273(5664):609–612. doi: 10.1038/273609a0. [DOI] [PubMed] [Google Scholar]
- da Silva L. C., Mourão P. A., Borojevic R. Patterns of sulfated glycosaminoglycan synthesis and accumulation in hepatic granulomas induced by schistosomal infection. Exp Mol Pathol. 1989 Jun;50(3):411–420. doi: 10.1016/0014-4800(89)90049-x. [DOI] [PubMed] [Google Scholar]