Abstract
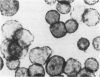
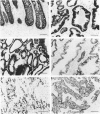
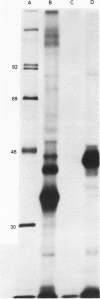
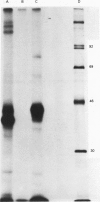
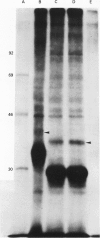
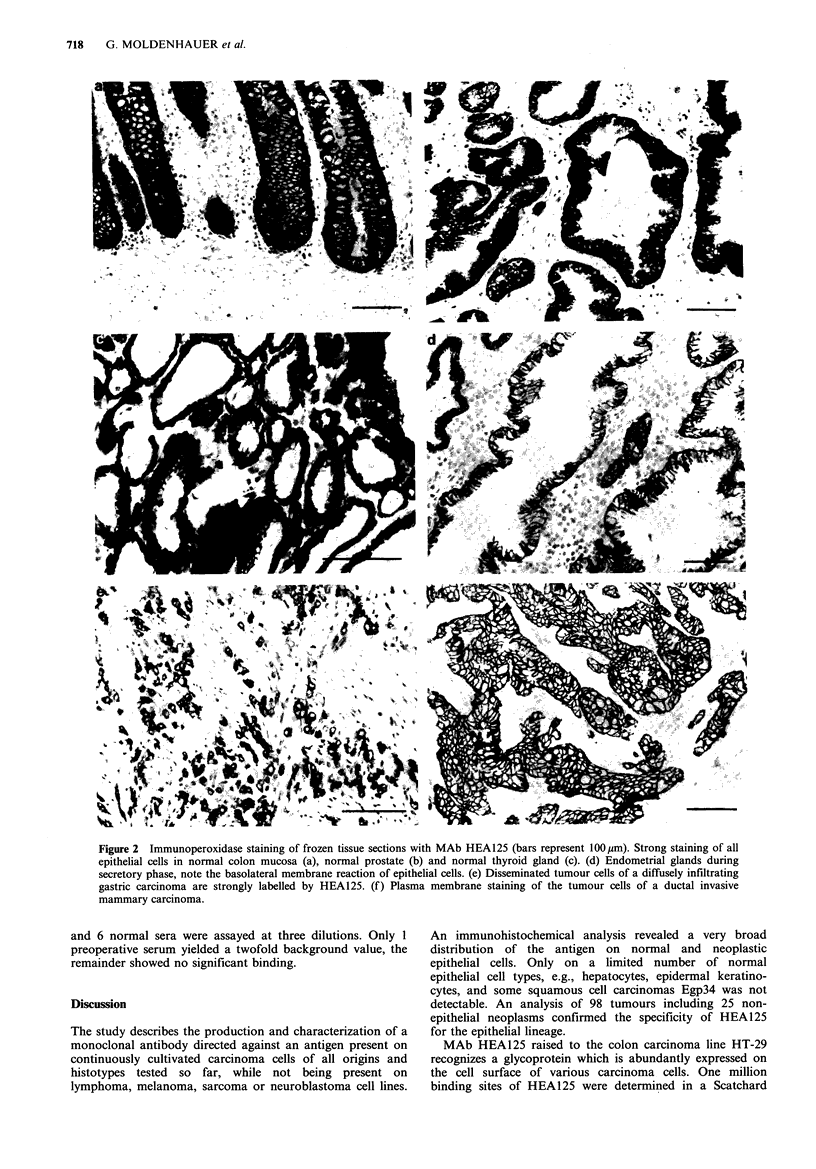
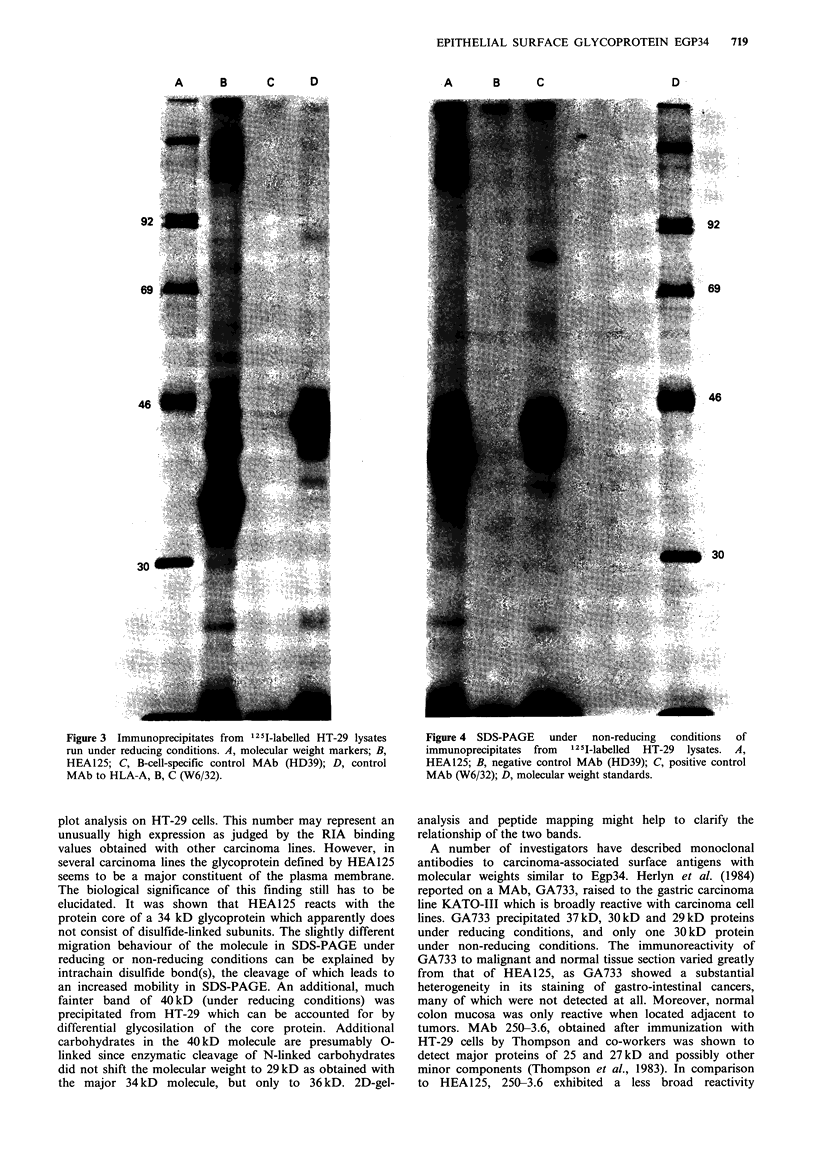
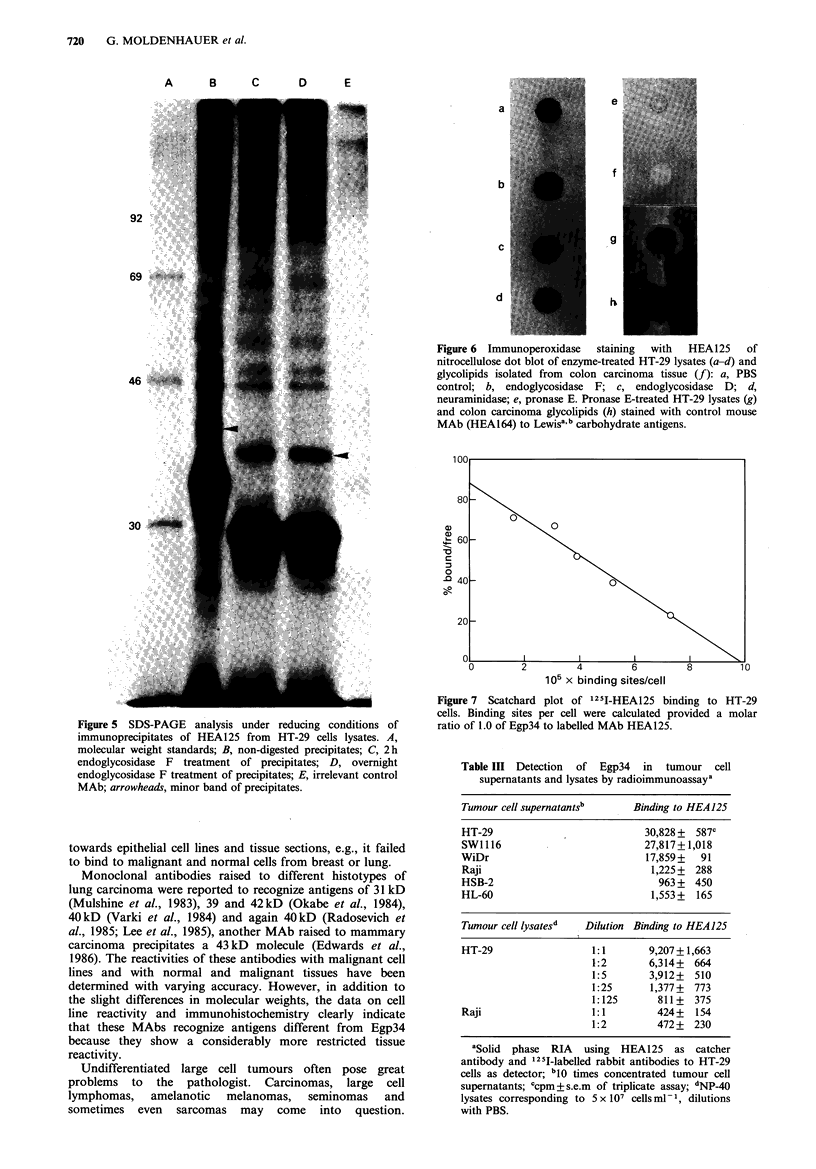
An epithelial cell surface antigen is described which is defined by monoclonal antibody HEA125 (IgG1). The antibody was raised against the colon carcinoma cell line HT-29. Under reducing conditions HEA125 immunoprecipitates a surface glycoprotein of Mr 34,000 which was designated Egp34. The antigen does not contain disulfide-linked subunits. A slightly different migration behavior under non-reducing conditions (Mr 39,000) may be due to intrachain disulfide bonds. After enzymatic cleavage of N-linked carbohydrate residues the apparent molecular weight of the antigen was 29,000. Egp34 is a major cell surface component of HT-29 cells (10(6) molecules per cell). No antigen could be detected in the sera of colorectal cancer patients. A panel of malignant cell lines and normal cells was studied for surface expression of the antigen. 17/17 carcinoma lines of 6 different origins expressed the antigen, whereas 16/16 melanoma, neuroblastoma, sarcoma and lymphoma/leukaemia were unreactive as it was the case for normal fibroblasts and blood cells. Immunoperoxidase staining of frozen tissue sections with HEA125 demonstrated the presence of Egp34 in almost all normal epithelia and tumours derived therefrom. No reactivity with non-epithelial tissues was observed. Undifferentiated carcinomas of various origins homogeneously expressed Egp34. Therefore, HEA125 may become a valuable tool for the immunohistochemical diagnosis of carcinoma.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bast R. C., Jr, Feeney M., Lazarus H., Nadler L. M., Colvin R. B., Knapp R. C. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981 Nov;68(5):1331–1337. doi: 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal S. D., Speak J. A. Membrane antigen in small cell carcinoma of the lung defined by monoclonal antibody SM1. Cancer Res. 1984 Jan;44(1):265–270. [PubMed] [Google Scholar]
- Bruck C., Portetelle D., Glineur C., Bollen A. One-step purification of mouse monoclonal antibodies from ascitic fluid by DEAE Affi-Gel blue chromatography. J Immunol Methods. 1982 Sep 30;53(3):313–319. doi: 10.1016/0022-1759(82)90178-8. [DOI] [PubMed] [Google Scholar]
- Colcher D., Hand P. H., Nuti M., Schlom J. A spectrum of monoclonal antibodies reactive with human mammary tumor cells. Proc Natl Acad Sci U S A. 1981 May;78(5):3199–3203. doi: 10.1073/pnas.78.5.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörken B., Pezzutto A., Moldenhauer G., Schwartz R., Kiesel S., Hunstein W. An immunoenzymatic staining assay (ISA) for the rapid screening of monoclonal antibodies detecting membrane and cytoplasmic antigens. J Immunol Methods. 1986 Apr 3;88(1):129–136. doi: 10.1016/0022-1759(86)90061-x. [DOI] [PubMed] [Google Scholar]
- Edwards D. P., Grzyb K. T., Dressler L. G., Mansel R. E., Zava D. T., Sledge G. W., Jr, McGuire W. L. Monoclonal antibody identification and characterization of a Mr 43,000 membrane glycoprotein associated with human breast cancer. Cancer Res. 1986 Mar;46(3):1306–1317. [PubMed] [Google Scholar]
- Edwards P. A. Heterogeneous expression of cell-surface antigens in normal epithelia and their tumours, revealed by monoclonal antibodies. Br J Cancer. 1985 Feb;51(2):149–160. doi: 10.1038/bjc.1985.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A. E., Rouse R. V., Wang M. C., Chu T. M., Herzenberg L. A. Monoclonal antibodies to a human prostate antigen. Cancer Res. 1982 Sep;42(9):3714–3718. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Structural studies of murine lymphocyte surface IgD. J Immunol. 1980 May;124(5):2082–2088. [PubMed] [Google Scholar]
- Hayman M. J., Crumpton M. J. Isolation of glycoproteins from pig lymphocyte plasma membrane using Lens culinaris phytohemagglutinin. Biochem Biophys Res Commun. 1972 May 26;47(4):923–930. doi: 10.1016/0006-291x(72)90581-5. [DOI] [PubMed] [Google Scholar]
- Herlyn D., Herlyn M., Ross A. H., Ernst C., Atkinson B., Koprowski H. Efficient selection of human tumor growth-inhibiting monoclonal antibodies. J Immunol Methods. 1984 Oct 12;73(1):157–167. doi: 10.1016/0022-1759(84)90041-3. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Koprowski H., Steplewski Z., Mitchell K., Herlyn M., Herlyn D., Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979 Nov;5(6):957–971. doi: 10.1007/BF01542654. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee I., Radosevich J. A., Ma Y. X., Combs S. G., Rosen S. T., Gould V. E. Immunohistochemical analysis of human pulmonary carcinomas using monoclonal antibody 44-3A6. Cancer Res. 1985 Nov;45(11 Pt 2):5813–5817. [PubMed] [Google Scholar]
- Masuko T., Yagita H., Hashimoto Y. Monoclonal antibodies against cell surface antigens present on human urinary bladder cancer cells. J Natl Cancer Inst. 1984 Mar;72(3):523–530. [PubMed] [Google Scholar]
- Metzgar R. S., Gaillard M. T., Levine S. J., Tuck F. L., Bossen E. H., Borowitz M. J. Antigens of human pancreatic adenocarcinoma cells defined by murine monoclonal antibodies. Cancer Res. 1982 Feb;42(2):601–608. [PubMed] [Google Scholar]
- Momburg F., Degener T., Bacchus E., Moldenhauer G., Hämmerling G. J., Möller P. Loss of HLA-A,B,C and de novo expression of HLA-D in colorectal cancer. Int J Cancer. 1986 Feb 15;37(2):179–184. doi: 10.1002/ijc.2910370203. [DOI] [PubMed] [Google Scholar]
- Momburg F., Moldenhauer G., Hämmerling G. J., Möller P. Immunohistochemical study of the expression of a Mr 34,000 human epithelium-specific surface glycoprotein in normal and malignant tissues. Cancer Res. 1987 Jun 1;47(11):2883–2891. [PubMed] [Google Scholar]
- Mulshine J. L., Cuttitta F., Bibro M., Fedorko J., Fargion S., Little C., Carney D. N., Gazdar A. F., Minna J. D. Monoclonal antibodies that distinguish non-small cell from small cell lung cancer. J Immunol. 1983 Jul;131(1):497–502. [PubMed] [Google Scholar]
- Okabe T., Kaizu T., Fujisawa M., Watanabe J., Kojima K., Yamashita T., Takaku F. Monoclonal antibodies to surface antigens of small cell carcinoma of the lung. Cancer Res. 1984 Nov;44(11):5273–5278. [PubMed] [Google Scholar]
- Radosevich J. A., Ma Y. X., Lee I., Salwen H. R., Gould V. E., Rosen S. T. Monoclonal antibody 44-3A6 as a probe for a novel antigen found on human lung carcinomas with glandular differentiation. Cancer Res. 1985 Nov;45(11 Pt 2):5808–5812. [PubMed] [Google Scholar]
- Reeve J. G., Wulfrank D. A., Stewart J., Twentyman P. R., Baillie-Johnson H., Bleehen N. M. Monoclonal-antibody-defined human lung tumour cell-surface antigens. Int J Cancer. 1985 Jun 15;35(6):769–775. doi: 10.1002/ijc.2910350612. [DOI] [PubMed] [Google Scholar]
- Schmiegel W. H., Kalthoff H., Arndt R., Gieseking J., Greten H., Klöppel G., Kreiker C., Ladak A., Lampe V., Ulrich S. Monoclonal antibody-defined human pancreatic cancer-associated antigens. Cancer Res. 1985 Mar;45(3):1402–1407. [PubMed] [Google Scholar]
- Schwartz R., Kniep B., Müthing J., Mühlradt P. F. Glycoconjugates of murine tumor lines with different metastatic capacities. II. Diversity of glycolipid composition. Int J Cancer. 1985 Nov 15;36(5):601–607. doi: 10.1002/ijc.2910360514. [DOI] [PubMed] [Google Scholar]
- Stacker S. A., Thompson C., Riglar C., McKenzie I. F. A new breast carcinoma antigen defined by a monoclonal antibody. J Natl Cancer Inst. 1985 Nov;75(5):801–811. doi: 10.1093/jnci/75.5.801. [DOI] [PubMed] [Google Scholar]
- Thompson C. H., Jones S. L., Pihl E., McKenzie I. F. Monoclonal antibodies to human colon and colorectal carcinoma. Br J Cancer. 1983 May;47(5):595–605. doi: 10.1038/bjc.1983.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi H., Terasaki P. I., Chia D., Kukes G., Mugishima H., Kasai K., Suyama N., Fukushima K., Hirota M. A monoclonal antibody, CSTO-1, against a stomach adenocarcinoma-associated antigen. Cancer Res. 1984 Sep;44(9):3952–3956. [PubMed] [Google Scholar]
- Ueda R., Ogata S., Morrissey D. M., Finstad C. L., Szkudlarek J., Whitmore W. F., Oettgen H. F., Lloyd K. O., Old L. J. Cell surface antigens of human renal cancer defined by mouse monoclonal antibodies: identification of tissue-specific kidney glycoproteins. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5122–5126. doi: 10.1073/pnas.78.8.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki N. M., Reisfeld R. A., Walker L. E. Antigens associated with a human lung adenocarcinoma defined by monoclonal antibodies. Cancer Res. 1984 Feb;44(2):681–687. [PubMed] [Google Scholar]