Abstract
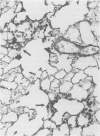
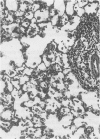
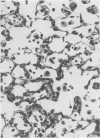
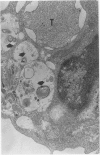
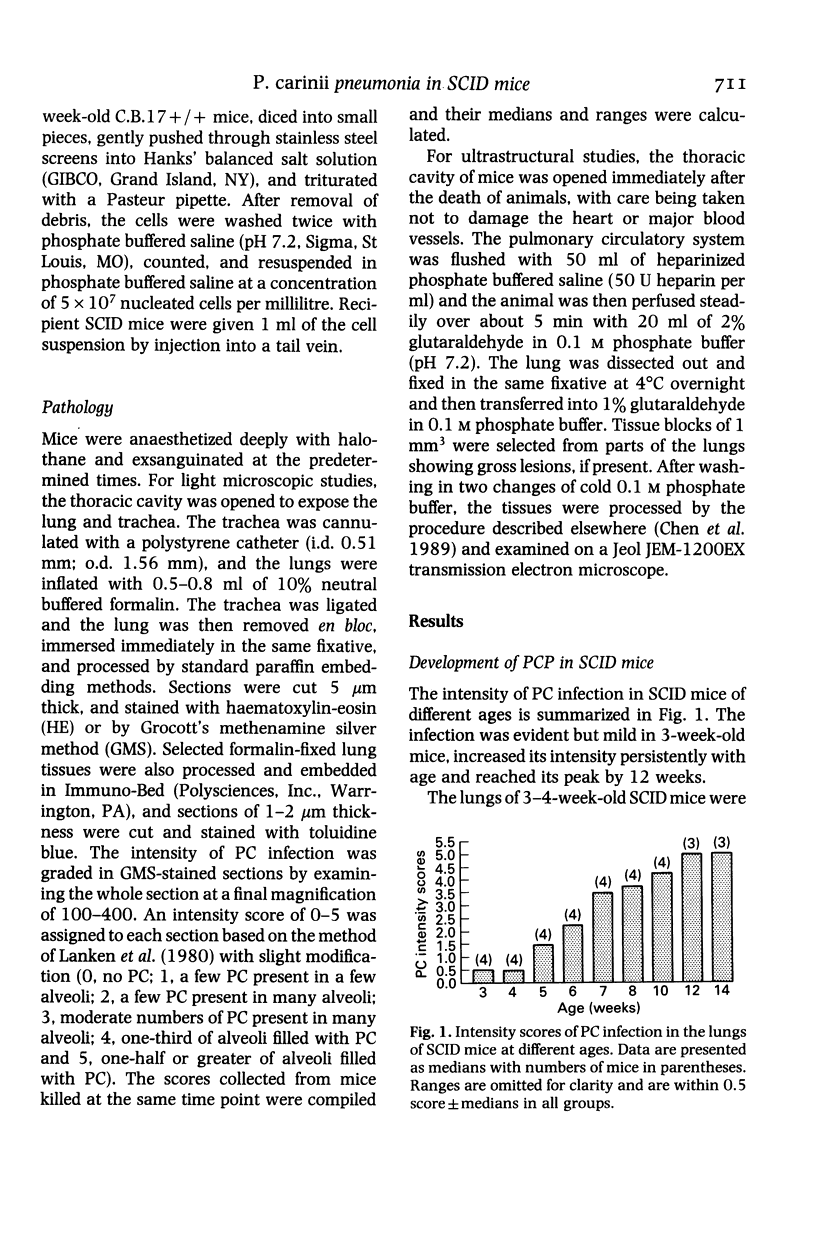
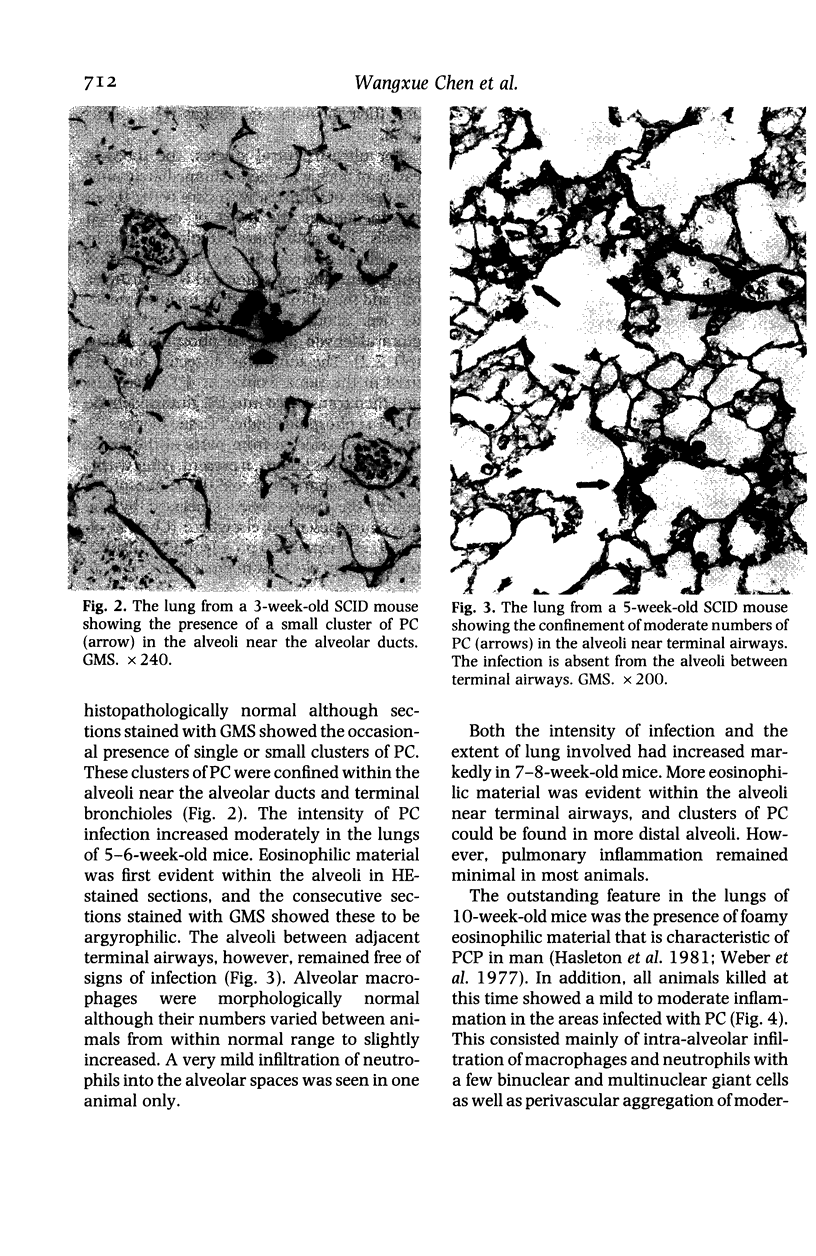
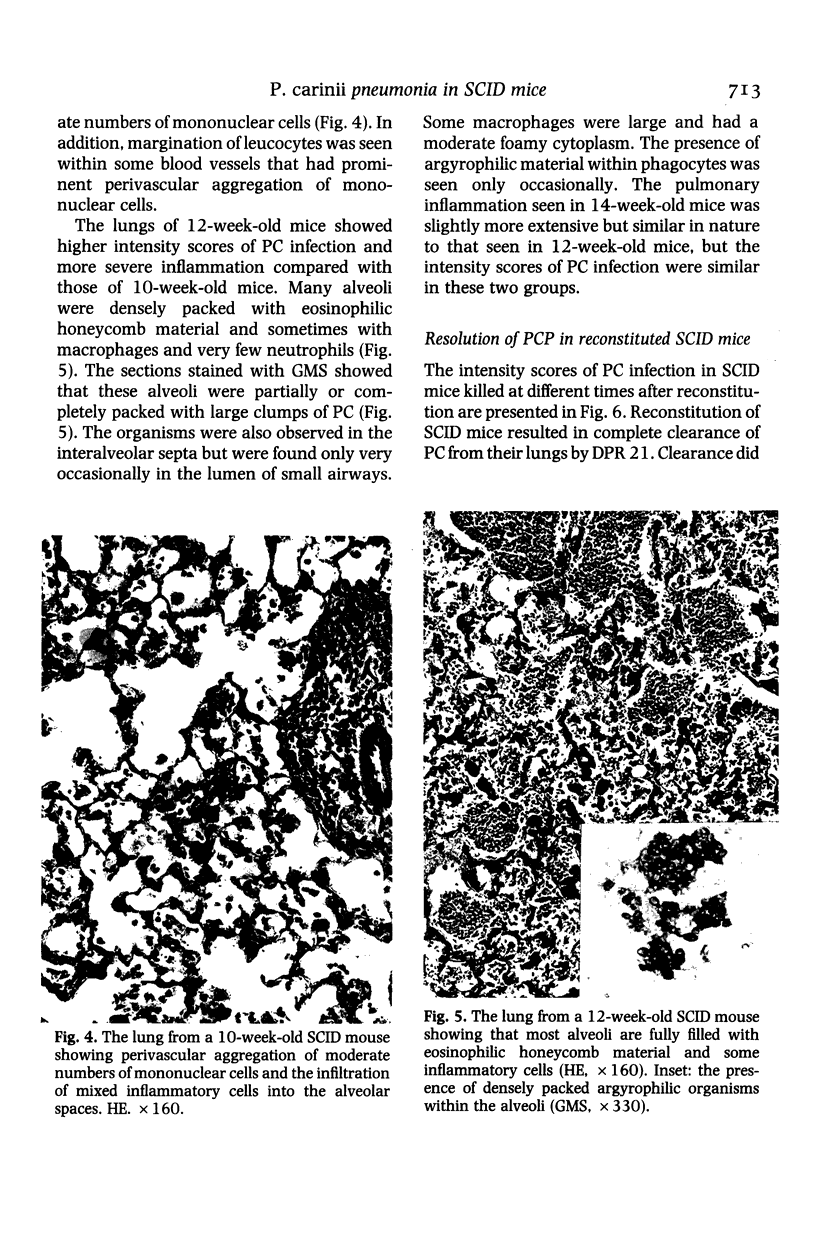
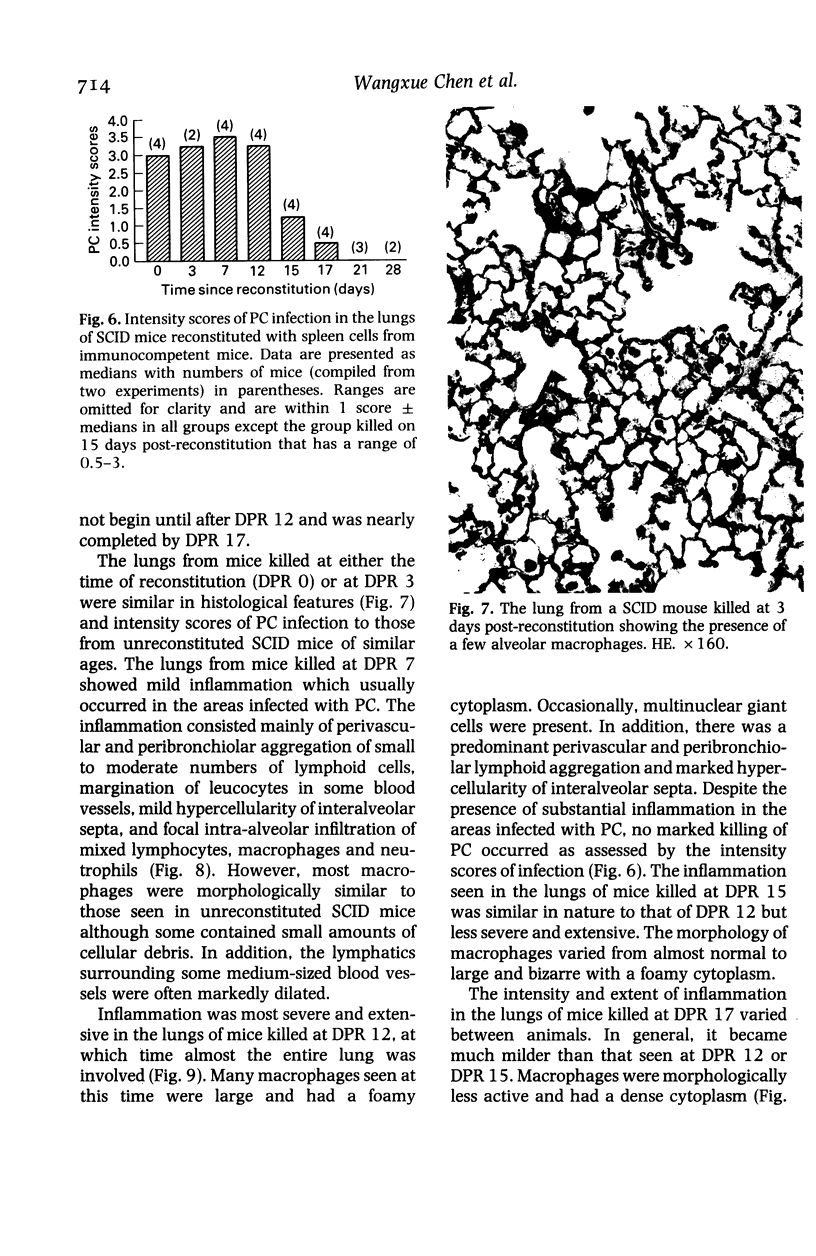
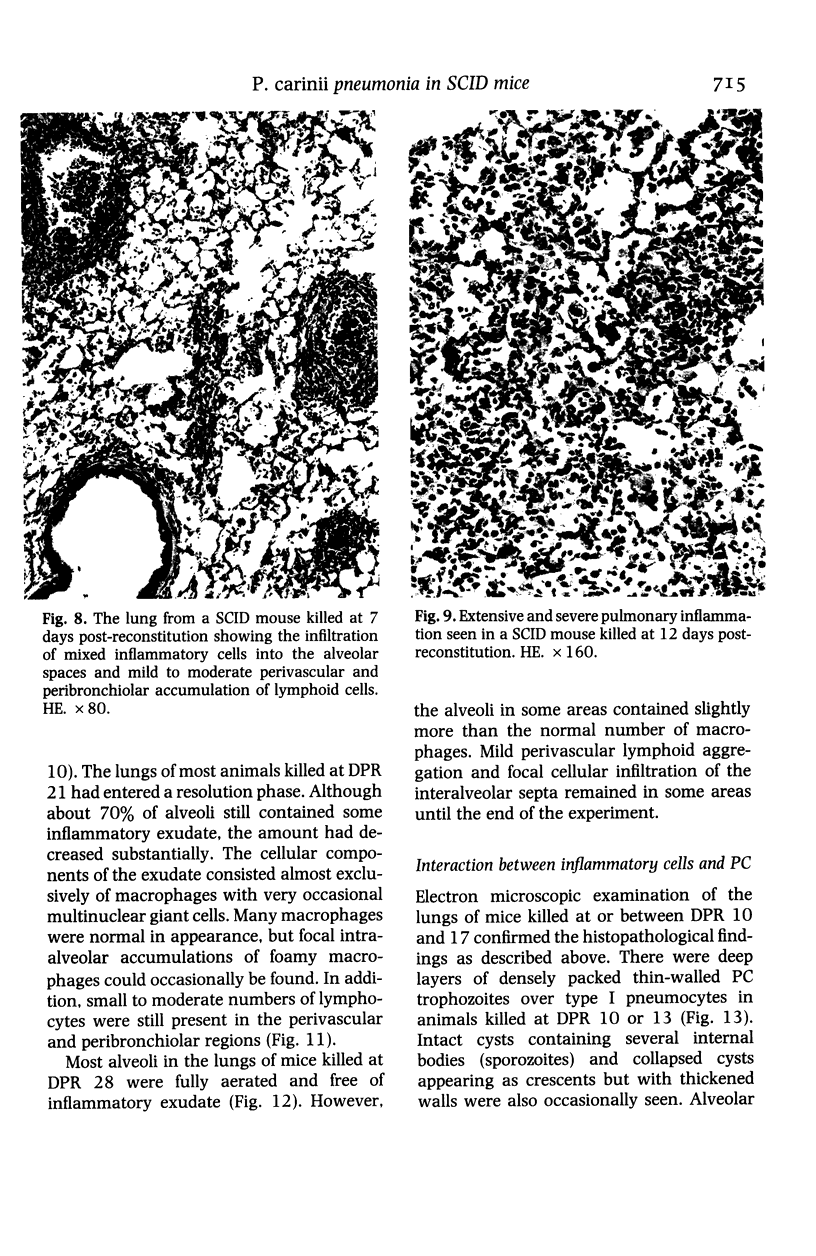
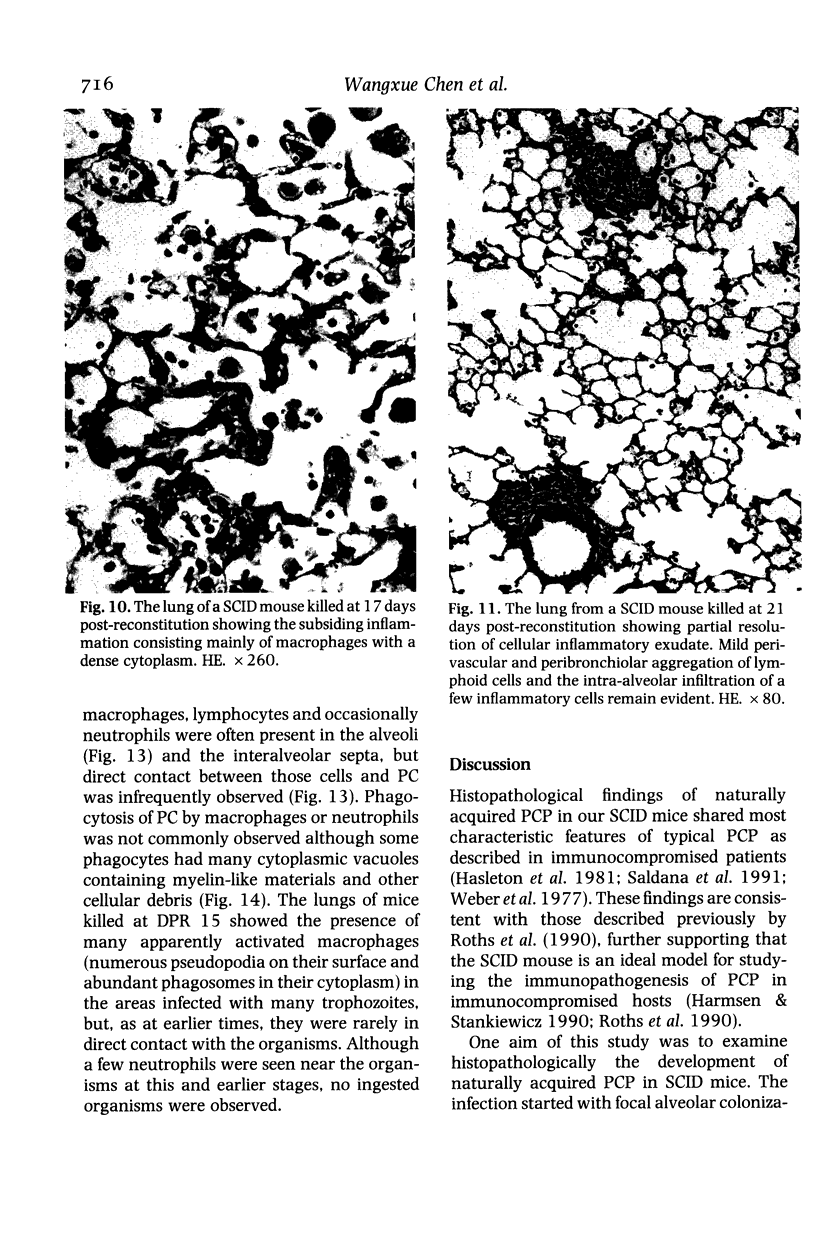
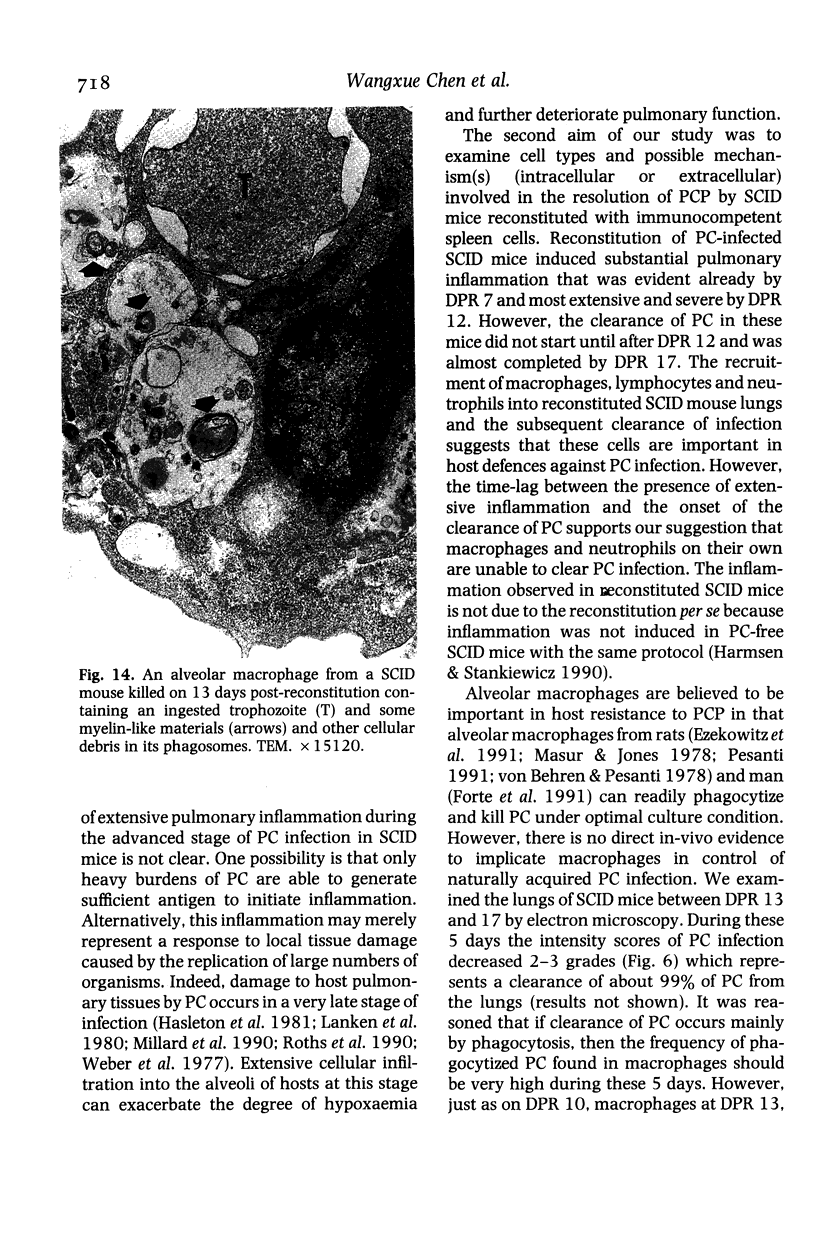
The development and resolution of naturally-acquired Pneumocystis carinii pneumonia was studied in a severe combined immunodeficient (SCID) mouse model by light and electron microscopies. Initial infection was evident in 3-week-old SCID mice and started as focal alveolar colonization in the areas near terminal airways. Pronounced pulmonary inflammation occurred in animals of 10 weeks or older and the infection intensity reached a plateau in animals 12 weeks of age. At this stage of disease, the histopathological features of P. carinii infection in SCID mice were similar to those of immunodeficient man. Reconstitution of SCID mice with immunocompetent spleen cells at day 0 induced substantial pulmonary inflammation that was evident already by day 7 and most severe and extensive by day 12. The clearance of P. carinii did not begin until after day 12 and was almost completed by day 17. Alveolar macrophages in mice killed between days 12 and 15, at the time when P. carinii are being rapidly cleared, appeared active but phagocytosis of P. carinii was not commonly observed by either light or electron microscopy. These results suggest that (1) the presence of non-lymphoid inflammatory cells in SCID mice is not sufficient to control P. carinii infection; (2) the clearance of P. carinii from the lungs of reconstituted SCID mice requires local recruitment of large numbers of inflammatory cells with an active appearance; and (3) intracellular killing of P. carinii by phagocytosis does not appear to be a major mechanism in host defences against P. carinii infection in this model.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Chen W. X., Alley M. R., Manktelow B. W. Experimental induction of pneumonia in mice with Bordetella parapertussis isolated from sheep. J Comp Pathol. 1989 Jan;100(1):77–89. doi: 10.1016/0021-9975(89)90092-3. [DOI] [PubMed] [Google Scholar]
- Ezekowitz R. A., Williams D. J., Koziel H., Armstrong M. Y., Warner A., Richards F. F., Rose R. M. Uptake of Pneumocystis carinii mediated by the macrophage mannose receptor. Nature. 1991 May 9;351(6322):155–158. doi: 10.1038/351155a0. [DOI] [PubMed] [Google Scholar]
- Gigliotti F., Hughes W. T. Passive immunoprophylaxis with specific monoclonal antibody confers partial protection against Pneumocystis carinii pneumonitis in animal models. J Clin Invest. 1988 Jun;81(6):1666–1668. doi: 10.1172/JCI113503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen A. G., Stankiewicz M. Requirement for CD4+ cells in resistance to Pneumocystis carinii pneumonia in mice. J Exp Med. 1990 Sep 1;172(3):937–945. doi: 10.1084/jem.172.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasleton P. S., Curry A., Rankin E. M. Pneumocystis carinii pneumonia: a light microscopical and ultrastructural study. J Clin Pathol. 1981 Oct;34(10):1138–1146. doi: 10.1136/jcp.34.10.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath D. The human carotid body in health and disease. J Pathol. 1991 May;164(1):1–8. doi: 10.1002/path.1711640102. [DOI] [PubMed] [Google Scholar]
- Hidalgo H. A., Helmke R. J., German V. F., Mangos J. A. Pneumocystis carinii induces an oxidative burst in alveolar macrophages. Infect Immun. 1992 Jan;60(1):1–7. doi: 10.1128/iai.60.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr Oxygen metabolism and the microbicidal activity of macrophages. Fed Proc. 1978 Nov;37(13):2759–2764. [PubMed] [Google Scholar]
- Lanken P. N., Minda M., Pietra G. G., Fishman A. P. Alveolar response to experimental Pneumocystis carinii pneumonia in the rat. Am J Pathol. 1980 Jun;99(3):561–588. [PMC free article] [PubMed] [Google Scholar]
- Long E. G., Smith J. S., Meier J. L. Attachment of Pneumocystis carinii to rat pneumocytes. Lab Invest. 1986 Jun;54(6):609–615. [PubMed] [Google Scholar]
- Masur H., Jones T. C. The interaction in vitro of Pneumocystis carinii with macrophages and L-cells. J Exp Med. 1978 Jan 1;147(1):157–170. doi: 10.1084/jem.147.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard P. R., Wakefield A. E., Hopkin J. M. A sequential ultrastructural study of rat lungs infected with Pneumocystis carinii to investigate the appearances of the organism, its relationships and its effects on pneumocytes. Int J Exp Pathol. 1990 Dec;71(6):895–904. [PMC free article] [PubMed] [Google Scholar]
- Pesanti E. L. Interaction of cytokines and alveolar cells with Pneumocystis carinii in vitro. J Infect Dis. 1991 Mar;163(3):611–616. doi: 10.1093/infdis/163.3.611. [DOI] [PubMed] [Google Scholar]
- Roths J. B., Marshall J. D., Allen R. D., Carlson G. A., Sidman C. L. Spontaneous Pneumocystis carinii pneumonia in immunodeficient mutant scid mice. Natural history and pathobiology. Am J Pathol. 1990 May;136(5):1173–1186. [PMC free article] [PubMed] [Google Scholar]
- Saldana M. J., Mones J. M., Martinez G. R. The pathology of treated Pneumocystis carinii pneumonia. Semin Diagn Pathol. 1989 Aug;6(3):300–312. [PubMed] [Google Scholar]
- Shellito J., Suzara V. V., Blumenfeld W., Beck J. M., Steger H. J., Ermak T. H. A new model of Pneumocystis carinii infection in mice selectively depleted of helper T lymphocytes. J Clin Invest. 1990 May;85(5):1686–1693. doi: 10.1172/JCI114621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes D. C., Gigliotti F., Rehg J. E., Snellgrove R. L., Hughes W. T. Experimental Pneumocystis carinii pneumonia in the ferret. Br J Exp Pathol. 1987 Apr;68(2):267–276. [PMC free article] [PubMed] [Google Scholar]
- Von Behren L. A., Pesanti E. L. Uptake and degradation of Pneumocystis carinii by macrophages in vitro. Am Rev Respir Dis. 1978 Dec;118(6):1051–1059. doi: 10.1164/arrd.1978.118.6.1051. [DOI] [PubMed] [Google Scholar]
- Walzer P. D., Kim C. K., Linke M. J., Pogue C. L., Huerkamp M. J., Chrisp C. E., Lerro A. V., Wixson S. K., Hall E., Shultz L. D. Outbreaks of Pneumocystis carinii pneumonia in colonies of immunodeficient mice. Infect Immun. 1989 Jan;57(1):62–70. doi: 10.1128/iai.57.1.62-70.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer P. D., Powell R. D., Jr, Yoneda K., Rutledge M. E., Milder J. E. Growth characteristics and pathogenesis of experimental Pneumocystis carinii pneumonia. Infect Immun. 1980 Mar;27(3):928–937. doi: 10.1128/iai.27.3.928-937.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber W. R., Askin F. B., Dehner L. P. Lung biopsy in Pneumocystis carinii pneumonia: a histopathologic study of typical and atypical features. Am J Clin Pathol. 1977 Jan;67(1):11–19. doi: 10.1093/ajcp/67.1.11. [DOI] [PubMed] [Google Scholar]
- Yoneda K., Walzer P. D. Interaction of Pneumocystis carinii with host lungs: an ultrastructural study. Infect Immun. 1980 Aug;29(2):692–703. doi: 10.1128/iai.29.2.692-703.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda K., Walzer P. D. Mechanism of pulmonary alveolar injury in experimental Pneumocystis carinii pneumonia in the rat. Br J Exp Pathol. 1981 Aug;62(4):339–346. [PMC free article] [PubMed] [Google Scholar]