Abstract
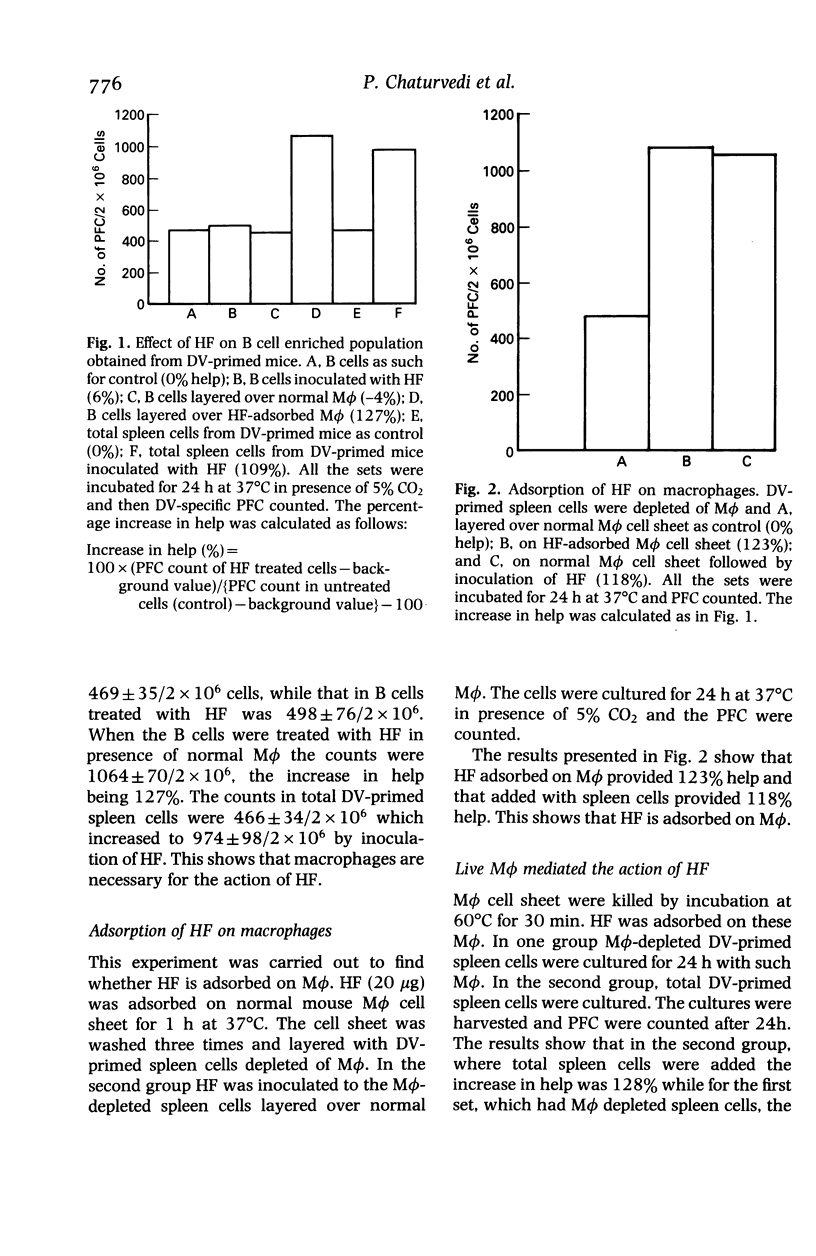
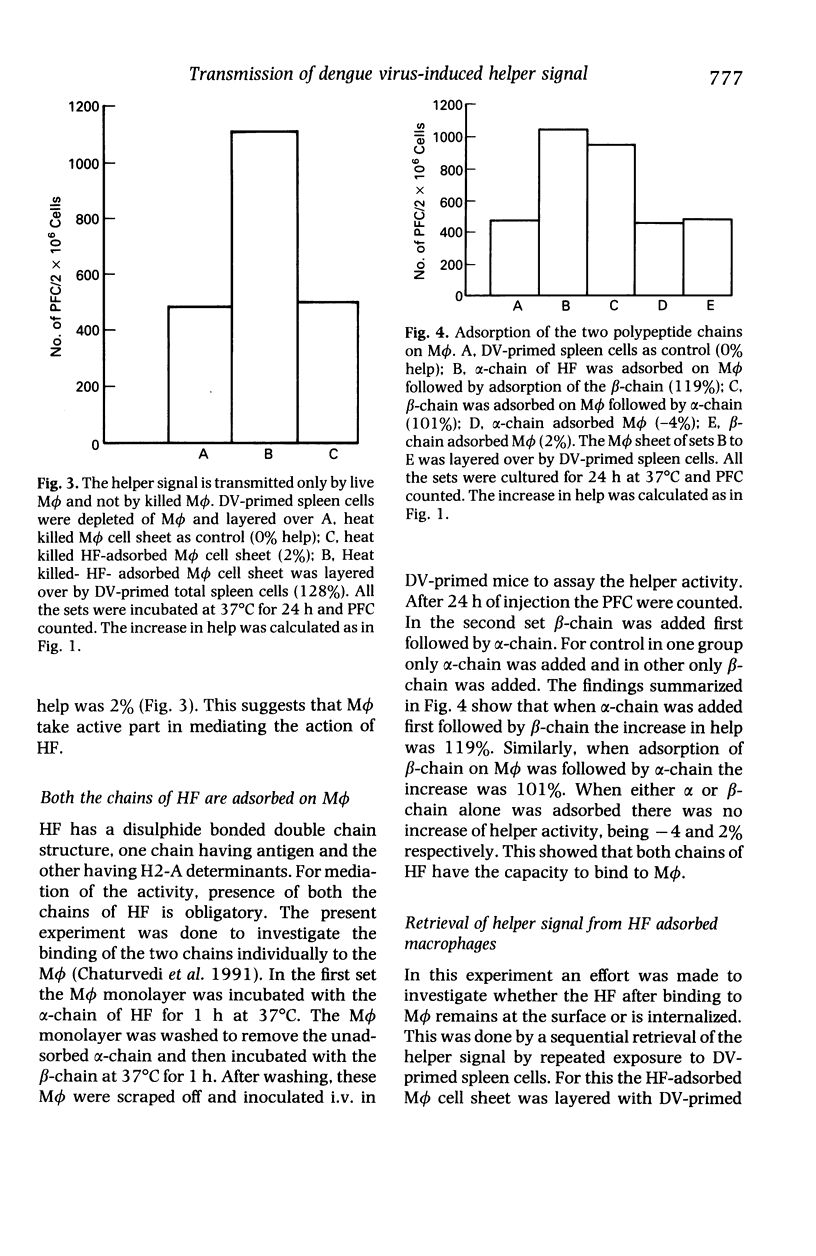
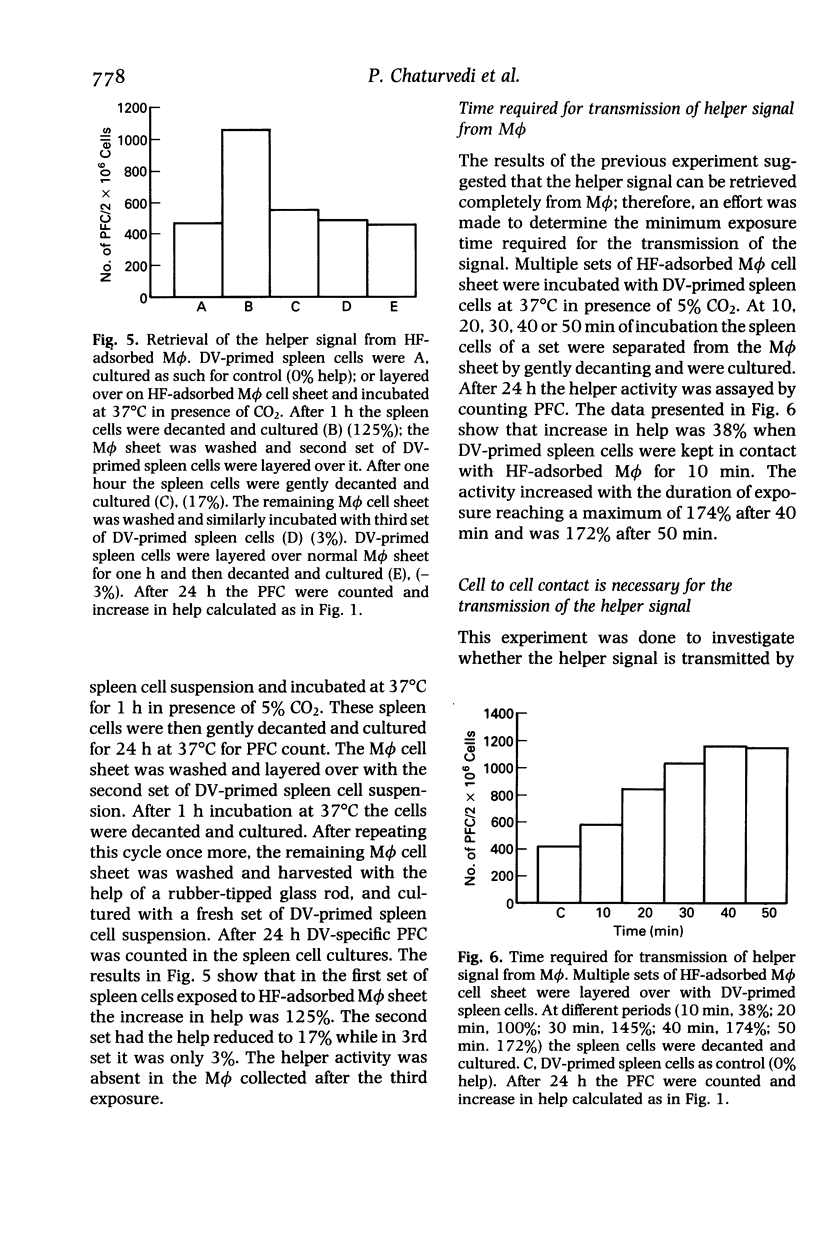
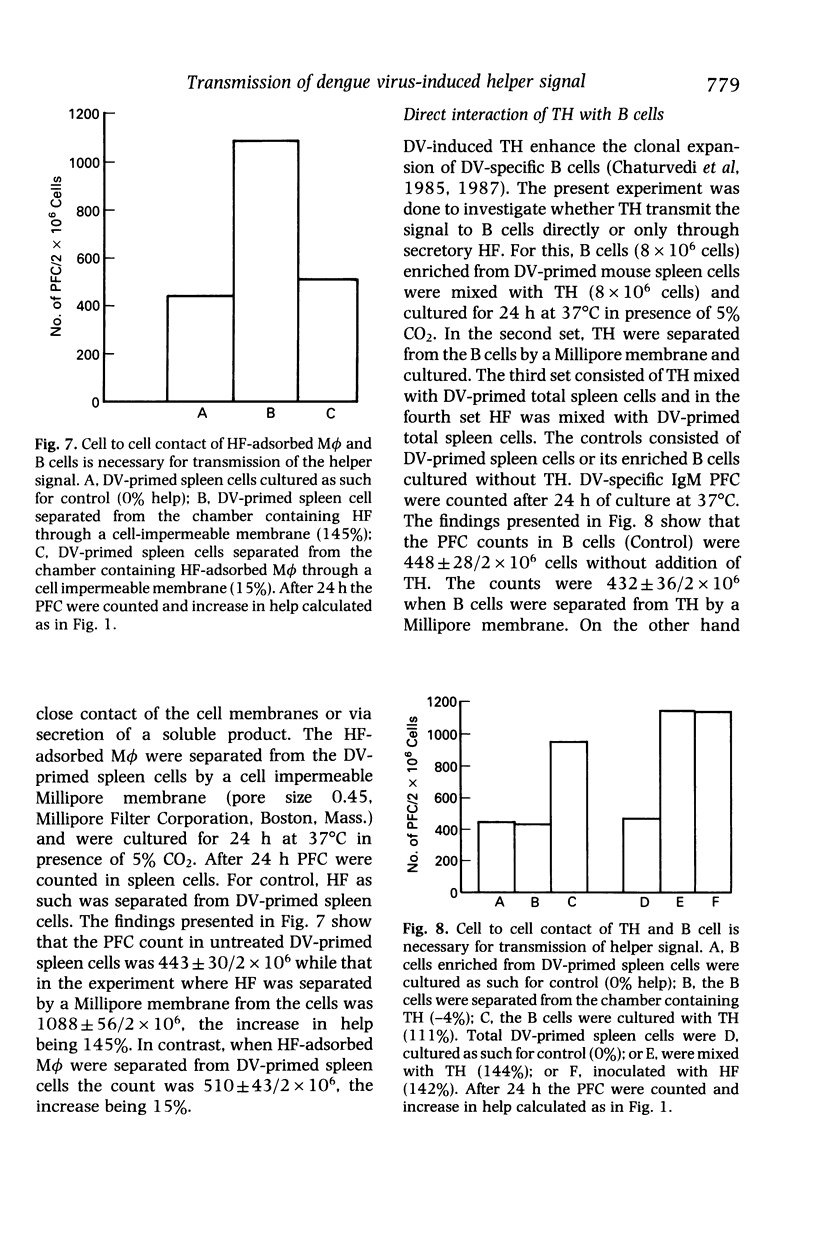
The helper T cells (TH) generated in dengue type 2 virus (DV) infection of mice produce a soluble helper cytokine (HF) which enhances the clonal expansion of DV-specific IgM antibody plaque forming cells (PFC). The present study was undertaken to investigate the mechanism of transmission of the helper signal from TH and HF to B cells. It was observed that TH could transmit the helper signal to B cells by direct cell to cell contact, but HF could not do so without the presence of live macrophages (M phi). HF was adsorbed by both heat killed and live M phi but the former could not transmit it to B cells. Both the polypeptide chains of HF bind to M phi. HF remains on the surface of M phi and can be retrieved completely by contact with B cells for 40 min. The helper signal from TH or HF-adsorbed M phi could not be transmitted to B cells when they were separated from each other by a cell impermeable membrane. The enhancement of PFC count is greater when the signal is transmitted by HF-adsorbed M phi as compared to that by TH alone. Thus, even with lower frequency of TH a significant number of B cells may be triggered with the help of HF and M phi. The findings thus show that the DV-specific helper signal could be transmitted only by a close physical contact of the plasma membranes of the signal presenting cells (TH or HF-adsorbed M phi) and B cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaturvedi P., Mukherjee R., Chaturvedi U. C., Mathur A. Characterization of the dengue virus-induced helper cytokine. Int J Exp Pathol. 1992 Jun;73(3):263–272. [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi P., Mukherjee R., Chaturvedi U. C., Mathur A. Dengue virus-induced helper cytokine has two polypeptide chains which bear different determinants. Int J Exp Pathol. 1991 Dec;72(6):665–672. [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi U. C., Nagar R., Mathur A. Effect of dengue virus infection on Fc-receptor functions of mouse macrophages. J Gen Virol. 1983 Nov;64(Pt 11):2399–2407. doi: 10.1099/0022-1317-64-11-2399. [DOI] [PubMed] [Google Scholar]
- Chaturvedi U. C., Pahwa M., Mathur A. Dengue virus-induced helper T cells. Indian J Med Res. 1987 Jul;86:1–8. [PubMed] [Google Scholar]
- Chaturvedi U. C., Shukla M. I., Mathur A. Role of macrophages in the transmission of dengue virus-induced suppressor signal to a subpopulation of T lymphocytes. Ann Immunol (Paris) 1982 Jan-Feb;133C(1):83–96. doi: 10.1016/0769-2625(82)90008-3. [DOI] [PubMed] [Google Scholar]
- Chaturvedi U. C., Shukla M. I., Pahwa M., Mathur A. Inhibition of B & helper T lymphocytes by dengue virus-induced suppressor factor. Indian J Med Res. 1985 Nov;82:471–474. [PubMed] [Google Scholar]
- Guy R., Ullrich S. J., Foo-Philips M., Hathcock K. S., Appella E., Hodes R. J. Antigen-specific helper function of cell-free T cell products bearing TCR V beta 8 determinants. Science. 1989 Jun 23;244(4911):1477–1480. doi: 10.1126/science.2472009. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Noelle R. J., McCann J., Marshall L., Bartlett W. C. Cognate interactions between helper T cells and B cells. III. Contact-dependent, lymphokine-independent induction of B cell cycle entry by activated helper T cells. J Immunol. 1989 Sep 15;143(6):1807–1814. [PubMed] [Google Scholar]
- Noelle R. J., Snow E. C. Cognate interactions between helper T cells and B cells. Immunol Today. 1990 Oct;11(10):361–368. doi: 10.1016/0167-5699(90)90142-v. [DOI] [PubMed] [Google Scholar]
- Rizvi N., Chaturvedi U. C., Mathur A. Obligatory role of macrophages in dengue virus antigen presentation to B lymphocytes. Immunology. 1989 May;67(1):38–43. [PMC free article] [PubMed] [Google Scholar]
- Rizvi N., Chaturvedi U. C., Nagar R., Mathur A. Macrophage functions during dengue virus infection: antigenic stimulation of B cells. Immunology. 1987 Nov;62(3):493–498. [PMC free article] [PubMed] [Google Scholar]
- Sanders V. M., Snyder J. M., Uhr J. W., Vitetta E. S. Characterization of the physical interaction between antigen-specific B and T cells. J Immunol. 1986 Oct 15;137(8):2395–2404. [PubMed] [Google Scholar]
- Shukla M. I., Chaturvedi U. C. In vivo role of macrophages in transmission of dengue virus-induced suppressor signal to T lymphocytes. Br J Exp Pathol. 1982 Oct;63(5):522–530. [PMC free article] [PubMed] [Google Scholar]
- Shukla M. I., Chaturvedi U. C. Transmission of dengue virus-induced suppressor signal from macrophage to lymphocyte occurs by cell contact. Br J Exp Pathol. 1983 Feb;64(1):87–92. [PMC free article] [PubMed] [Google Scholar]
- Trizio D., Cudkowicz G. Separation of T and B lymphocytes by nylon wool columns: evaluation of efficacy by functional assays in vivo. J Immunol. 1974 Oct;113(4):1093–1097. [PubMed] [Google Scholar]
- Waksman B. H. Cellular hypersensitivity and immunity: conceptual changes in last decade. Cell Immunol. 1979 Jan;42(1):155–169. doi: 10.1016/0008-8749(79)90229-6. [DOI] [PubMed] [Google Scholar]


