Abstract
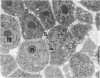
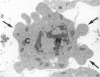
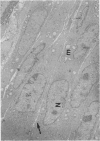
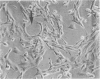
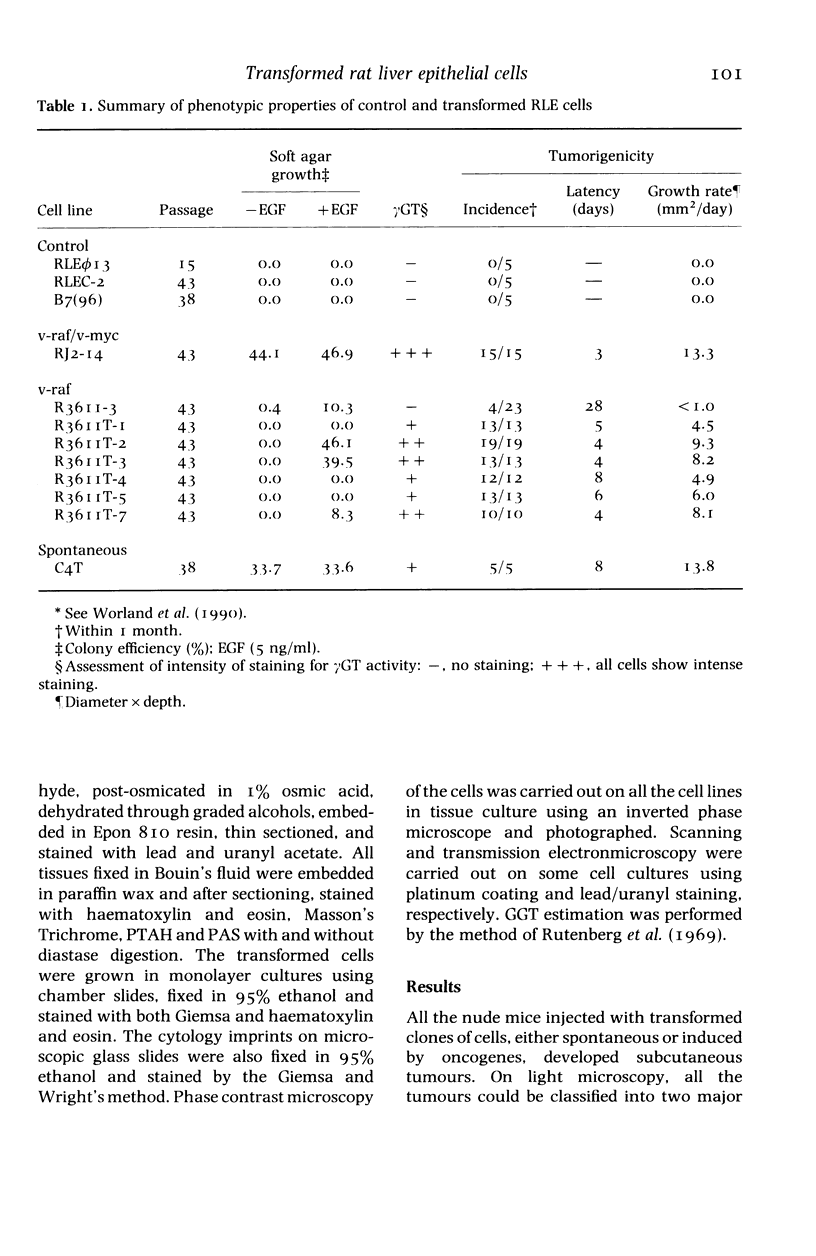
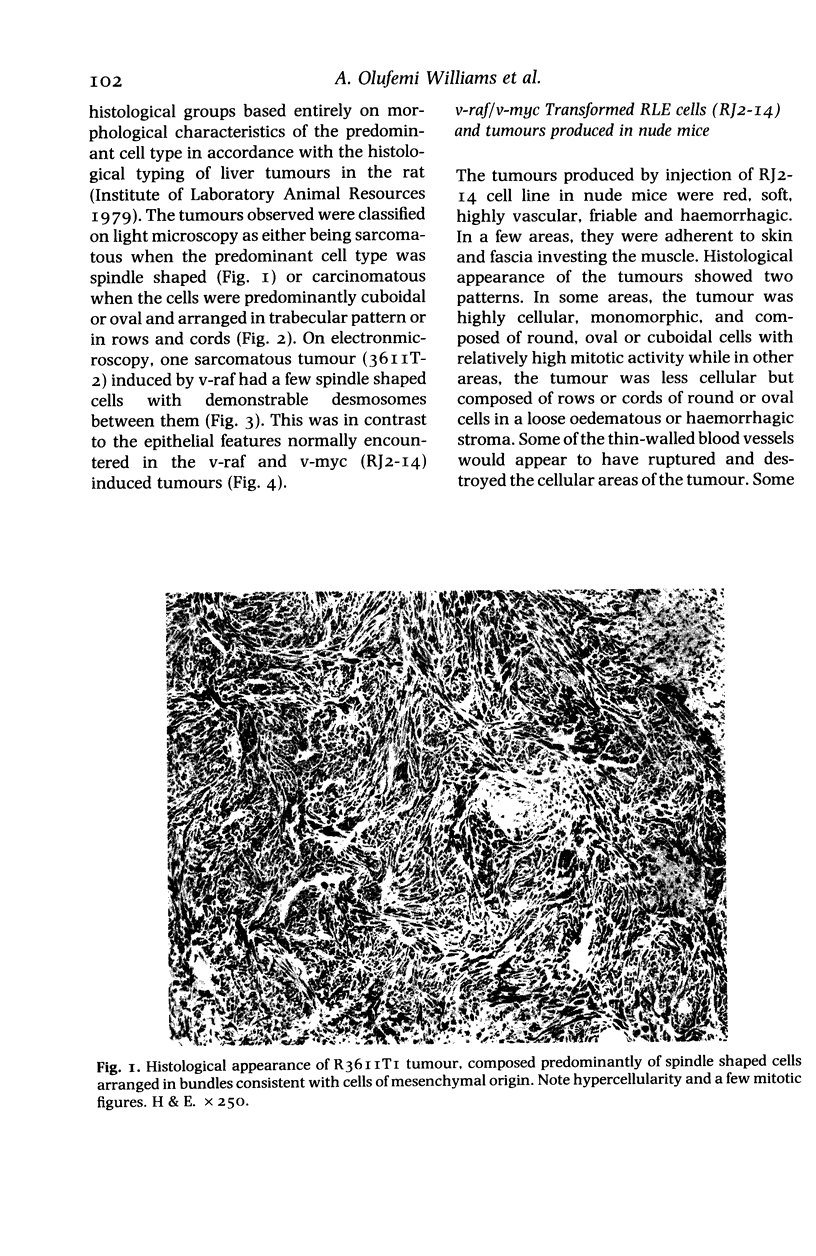
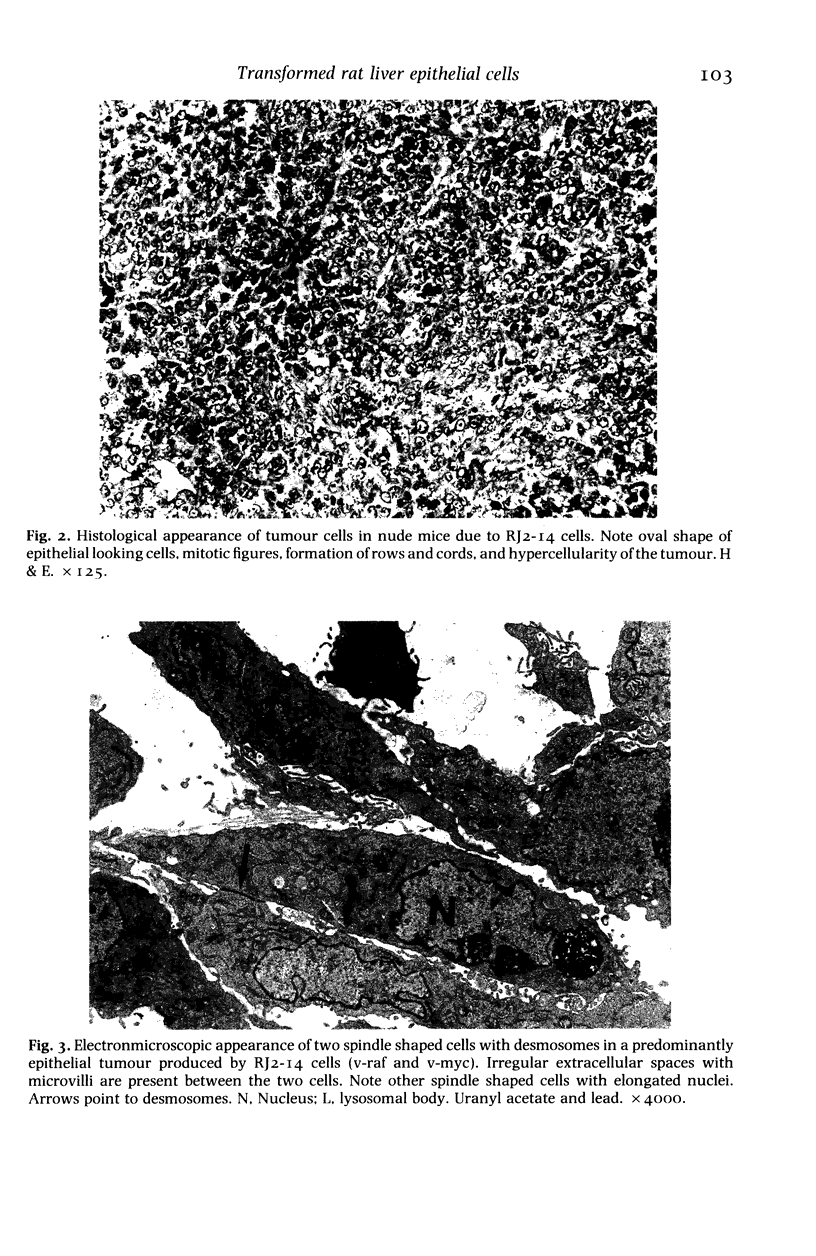
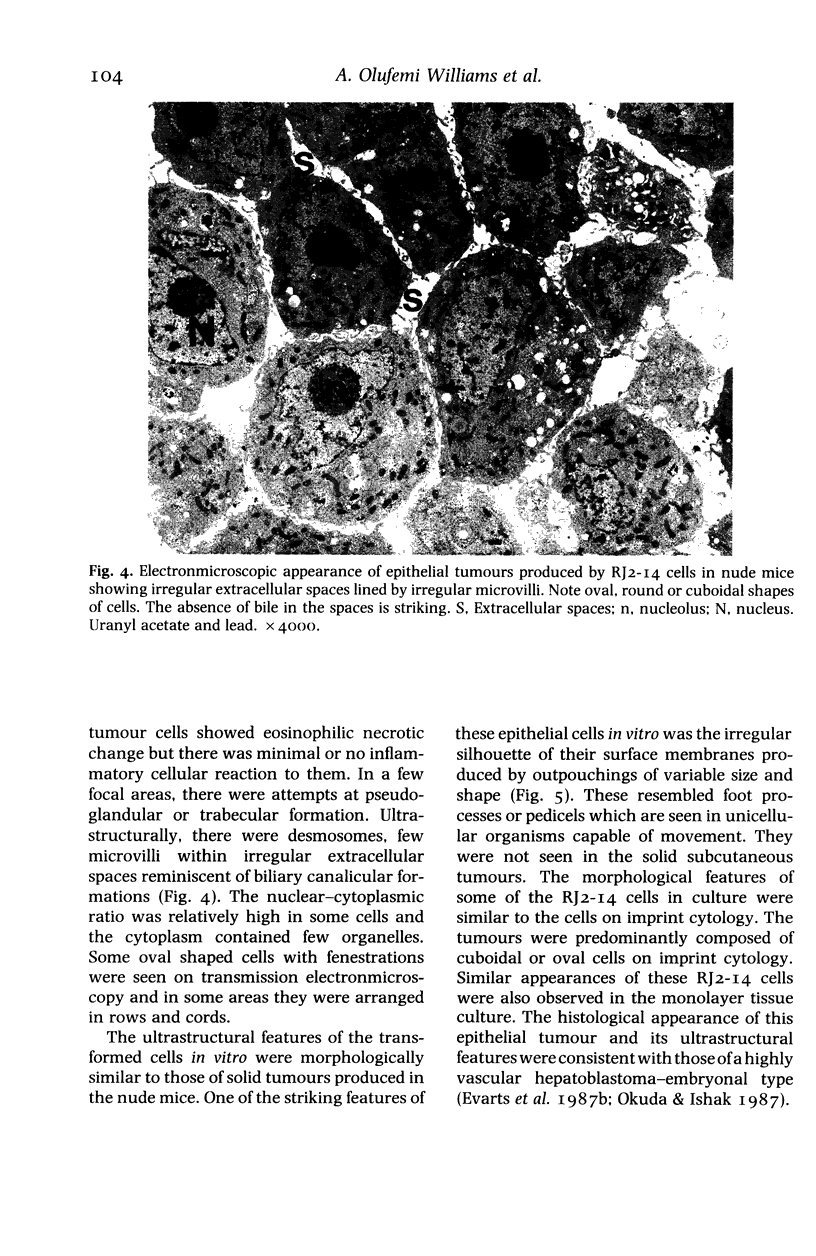
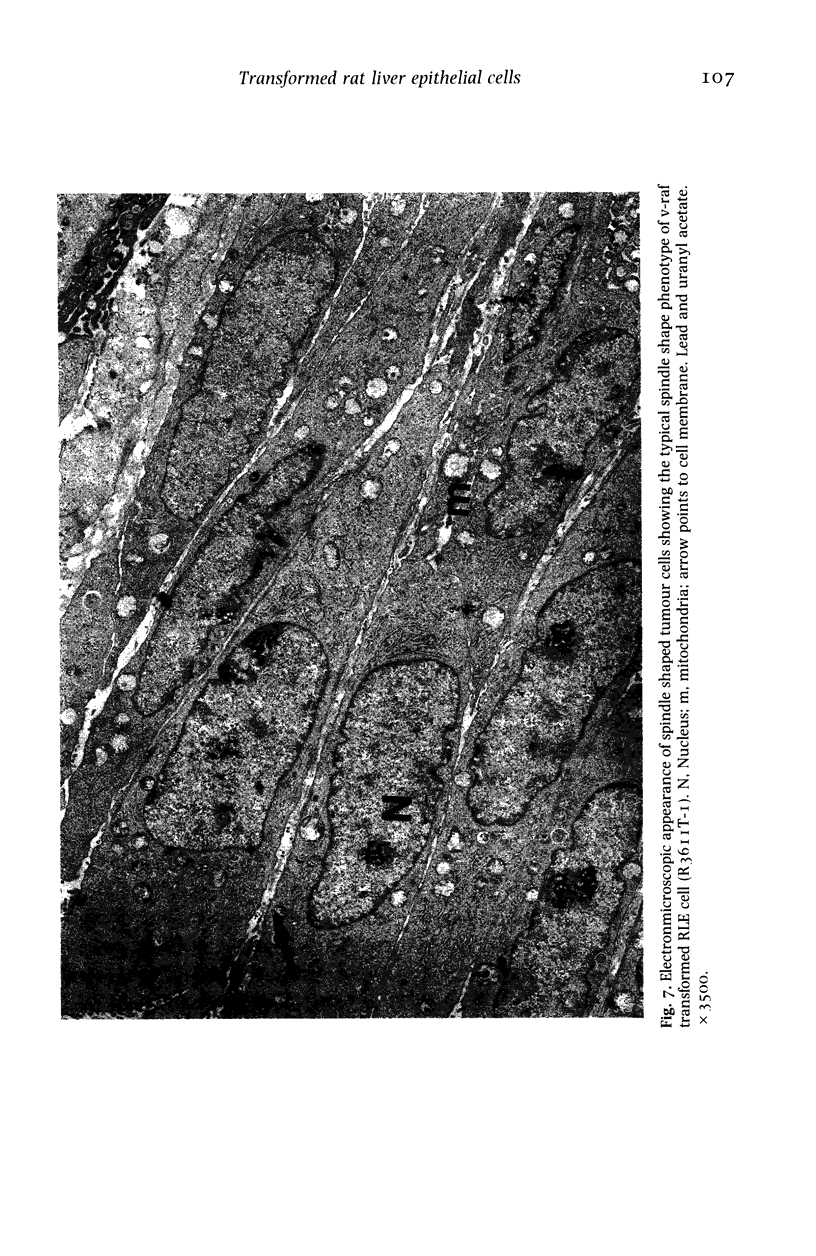
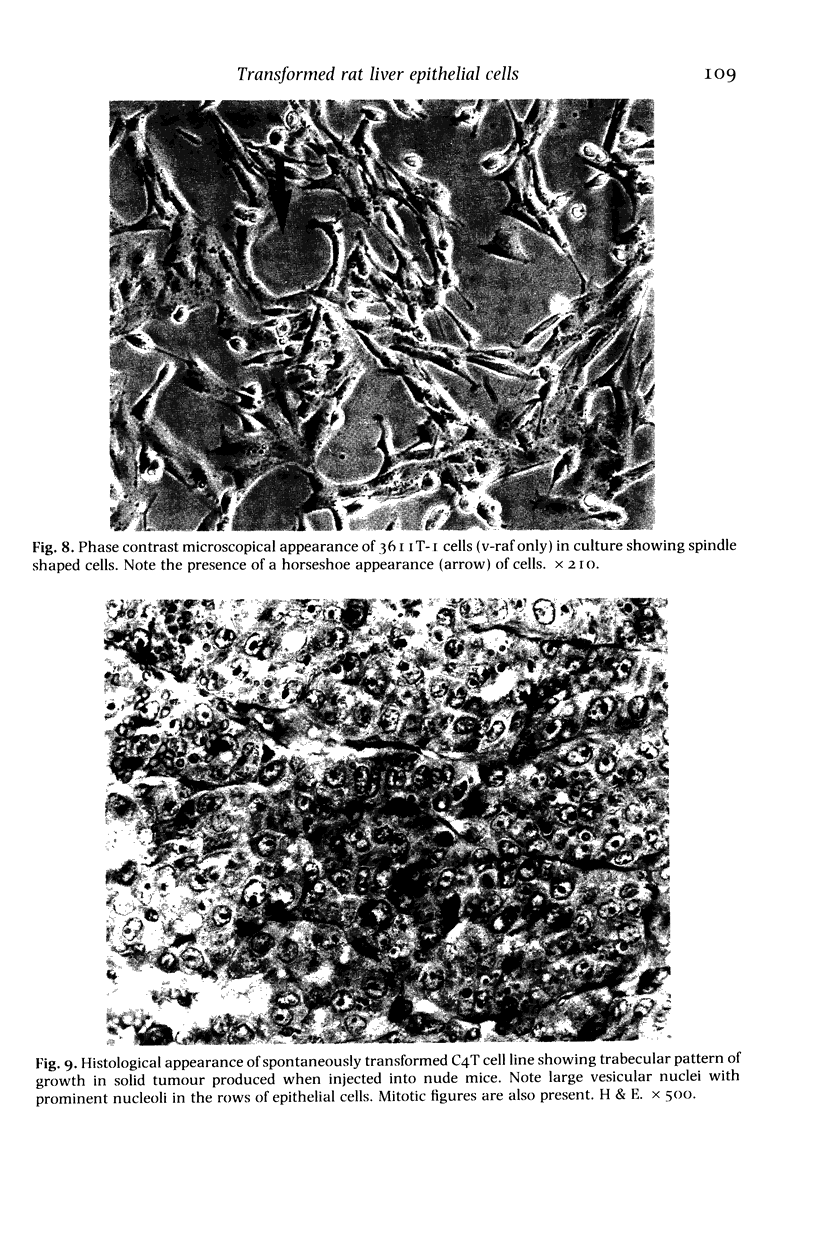
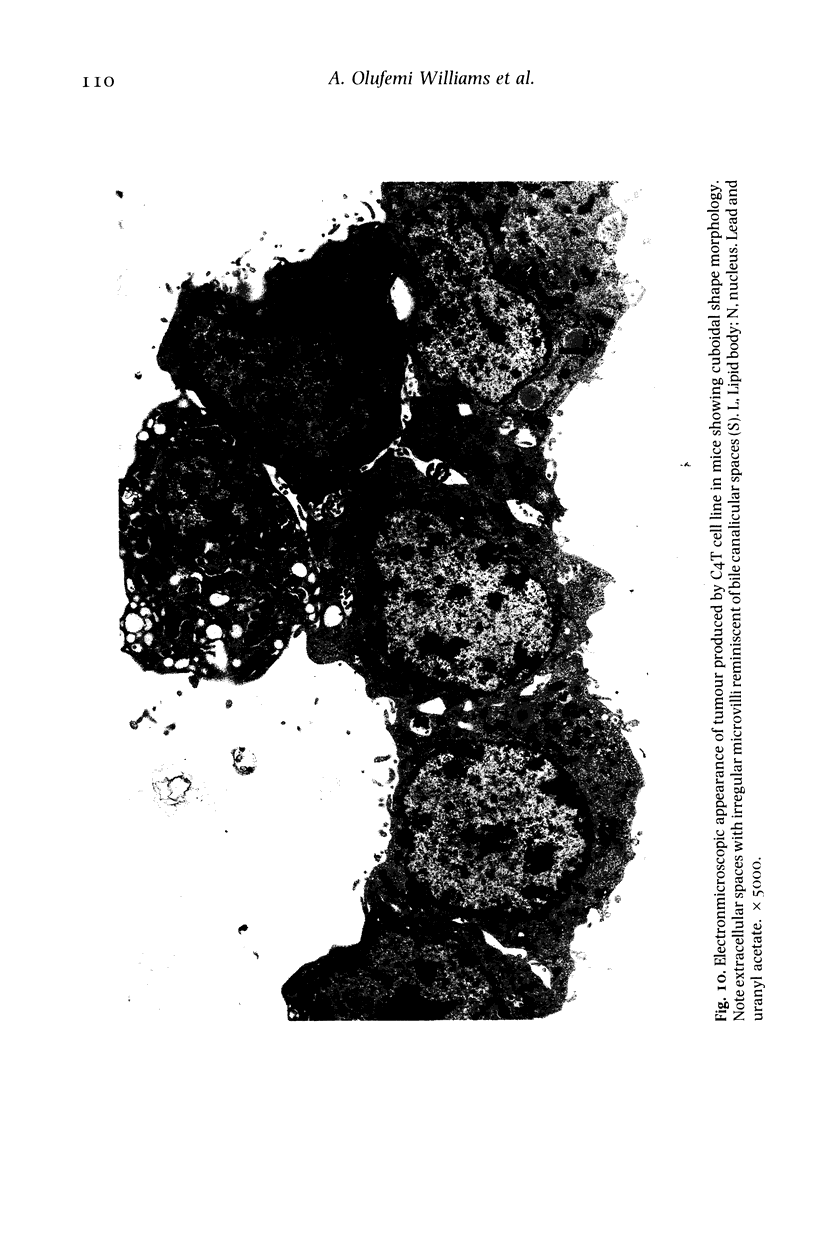
The histological and ultrastructural features of oncogene transformed rat liver epithelial (RLE) cells in culture and spontaneously transformed RLE cells were studied. The tumours produced in nude mice by all the transformed cells were either moderately well differentiated carcinomas or sarcomatous tumours. Epithelial tumours were produced in the RLE cells after transduction of both v-raf and v-myc oncogenes whereas sarcomatous tumours were produced after transduction of v-raf alone. These data suggested that transformation of RLE cells with a single oncogene, v-raf, produced malignant tumours which were consistent with sarcomas while a combination of two oncogenes, v-raf and v-myc, produced an epithelial tumour, consistent with a carcinoma. The effects of these oncogenes on RLE cells indicate that they were able to differentiate and were capable of producing two morphologically distinct tumour types. The possible role of v-myc in switching the sarcomatous lineage to an epithelial tumour lineage is considered to be significant and worthy of further studies. The epithelial tumour produced in RLE cells by combination of v-raf and v-myc is consistent with an embryonal type of hepatoblastoma. The trabecular type of liver cell carcinoma which resulted from spontaneous transformation of RLE cells illustrates the inherent potential of the RLE cell to undergo malignant change and strongly suggests that the RLE cells may be the precursor cells in the development of hepatocellular carcinoma in the rat.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Harris A. W., Pinkert C. A., Corcoran L. M., Alexander W. S., Cory S., Palmiter R. D., Brinster R. L. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985 Dec 12;318(6046):533–538. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- Alexander W. S., Schrader J. W., Adams J. M. Expression of the c-myc oncogene under control of an immunoglobulin enhancer in E mu-myc transgenic mice. Mol Cell Biol. 1987 Apr;7(4):1436–1444. doi: 10.1128/mcb.7.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battifora H. Spindle cell carcinoma: ultrastructural evidence of squamous origin and collagen production by the tumor cells. Cancer. 1976 May;37(5):2275–2282. doi: 10.1002/1097-0142(197605)37:5<2275::aid-cncr2820370518>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Diwan B. A., Ward J. M., Rice J. M. Promotion of malignant 'embryonal' liver tumors by phenobarbital: increased incidence and shortened latency of hepatoblastomas in (DBA/2 x C57BL/6)F1 mice initiated with N-nitrosodiethylamine. Carcinogenesis. 1989 Jul;10(7):1345–1348. doi: 10.1093/carcin/10.7.1345. [DOI] [PubMed] [Google Scholar]
- Garfield S., Huber B. E., Nagy P., Cordingley M. G., Thorgeirsson S. S. Neoplastic transformation and lineage switching of rat liver epithelial cells by retrovirus-associated oncogenes. Mol Carcinog. 1988;1(3):189–195. doi: 10.1002/mc.2940010307. [DOI] [PubMed] [Google Scholar]
- Grisham J. W. Cell types in long-term propagable cultures of rat liver. Ann N Y Acad Sci. 1980;349:128–137. doi: 10.1111/j.1749-6632.1980.tb29521.x. [DOI] [PubMed] [Google Scholar]
- Inaoka Y. Significance of the so-called oval cell proliferation during azo-dye hepatocarcinogenesis. Gan. 1967 Aug;58(4):355–366. [PubMed] [Google Scholar]
- Kuwano H., Sonoda T., Hashimoto H., Enjoji M. Hepatocellular carcinoma with osteoclast-like giant cells. Cancer. 1984 Sep 1;54(5):837–842. doi: 10.1002/1097-0142(19840901)54:5<837::aid-cncr2820540513>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Miyazaki M., Wahid S., Miyano K., Yabe T., Sato J. Expression of gamma-glutamyltranspeptidase in cultures of spontaneously and chemically transformed rat liver cells. Int J Cancer. 1983 Sep 15;32(3):373–377. doi: 10.1002/ijc.2910320318. [DOI] [PubMed] [Google Scholar]
- Nagy P., Evarts R. P., McMahon J. B., Thorgeirsson S. S. Role of TGF-beta in normal differentiation and oncogenesis in rat liver. Mol Carcinog. 1989;2(6):345–354. doi: 10.1002/mc.2940020609. [DOI] [PubMed] [Google Scholar]
- Schuller D. E., Wilson H. E., Smith R. E., Batley F., James A. D. Preoperative reductive chemotherapy for locally advanced carcinoma of the oral cavity, oropharynx, and hypopharynx. Cancer. 1983 Jan 1;51(1):15–19. doi: 10.1002/1097-0142(19830101)51:1<15::aid-cncr2820510105>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Sell S., Leffert H. L. An evaluation of cellular lineages in the pathogenesis of experimental hepatocellular carcinoma. Hepatology. 1982 Jan-Feb;2(1):77–86. doi: 10.1002/hep.1840020113. [DOI] [PubMed] [Google Scholar]
- Sorg B., Schmidt R., Hecker E. Structure/activity relationships of polyfunctional diterpenes of the ingenane type. I. Tumor-promoting activity of homologous, aliphatic 3-esters of ingenol and of delta 7,8-isoingenol-3-tetradecanoate. Carcinogenesis. 1987 Jan;8(1):1–4. doi: 10.1093/carcin/8.1.1. [DOI] [PubMed] [Google Scholar]
- Worland P. J., Hampton L. L., Thorgeirsson S. S., Huggett A. C. Development of an in vitro model of tumor progression using v-raf and v-raf/v-myc transformed rat liver epithelial cells: correlation of tumorigenicity with the downregulation of specific proteins. Mol Carcinog. 1990;3(1):20–29. doi: 10.1002/mc.2940030107. [DOI] [PubMed] [Google Scholar]