Abstract
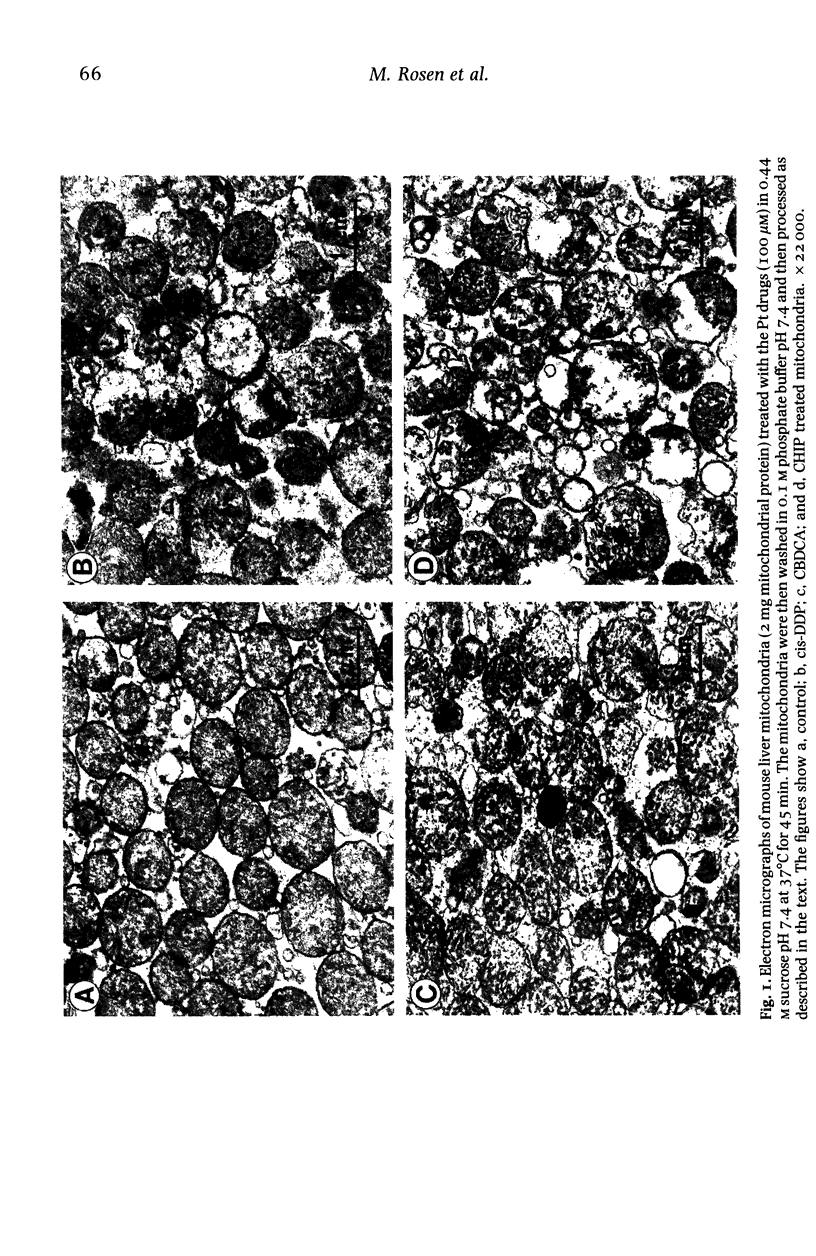
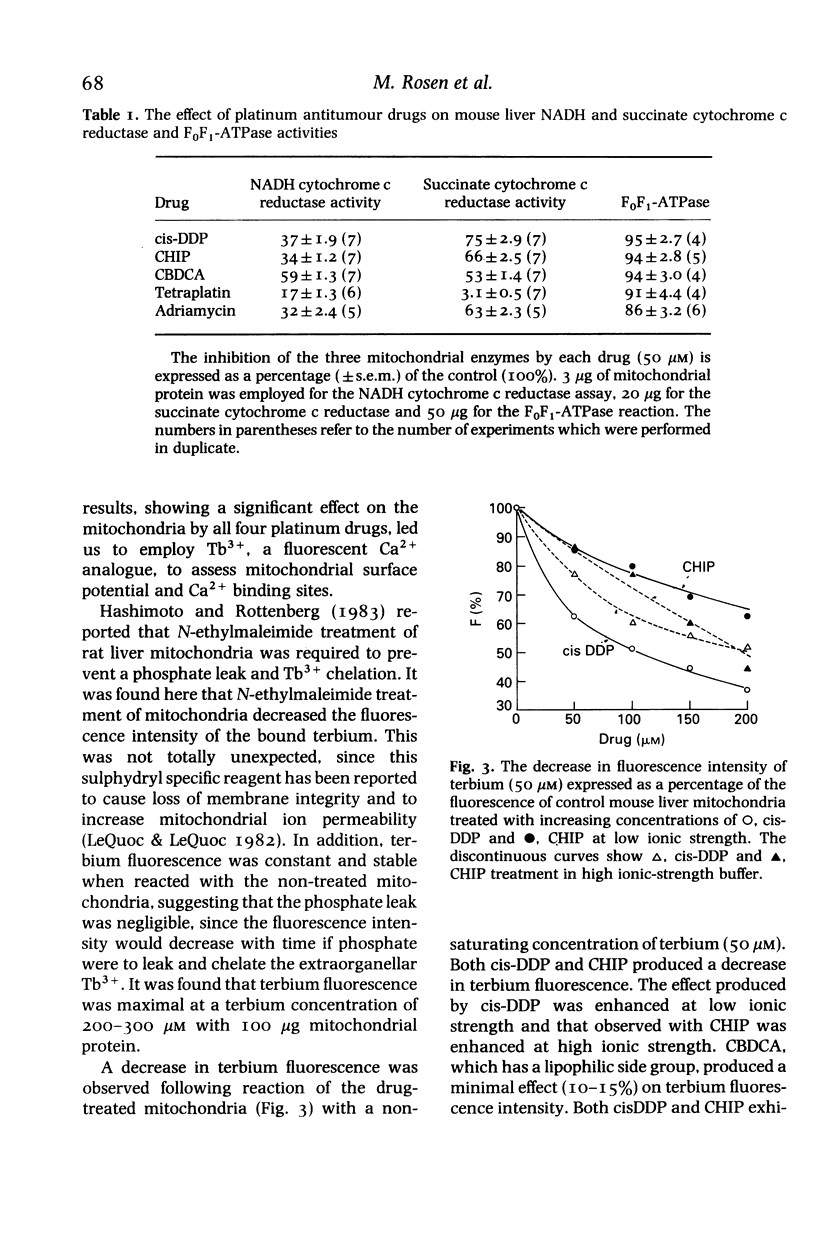
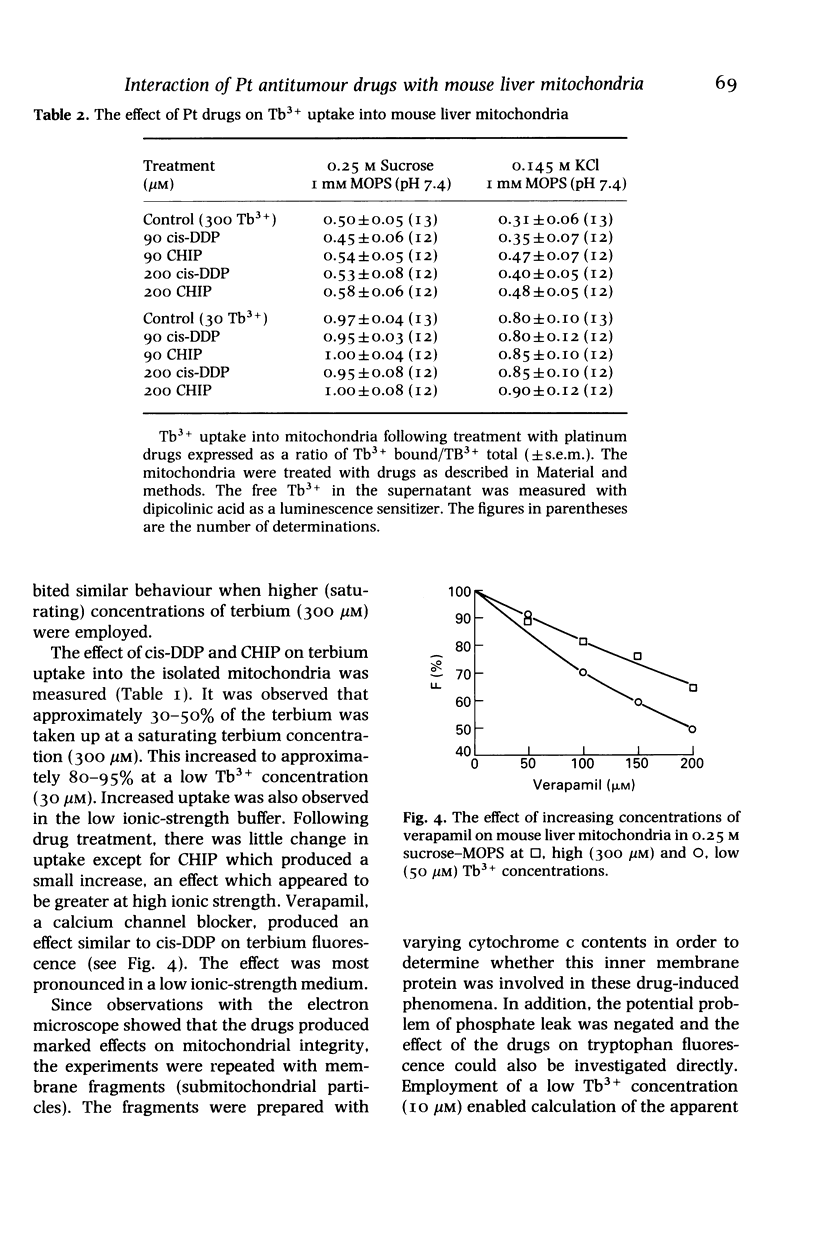
A study was undertaken to determine if cis-DDP and its second generation derivatives produced effects on mouse liver mitochondria, and if any of the observed effects could be correlated with the nephrotoxicity of the drugs. Although changes were observed in mitochondrial morphology, enzyme activity, Ca2+ influx, terbium binding and surface potential, no specific effect was correlated with nephrotoxicity. cis-DDP produced marked changes in mitochondrial morphology; electron probe analysis showed binding of the drug to the mitochondria. Inhibition of complex I and II activity of the respiratory chain and an ionic-strength-dependent effect on Tb3+ (a Ca2+ analogue) fluorescence were observed. The non-nephrotoxic derivatives, CHIP and tetraplatin, also produced significant changes in morphology. Treatment with these derivatives also produced decreases in mitochondrial enzyme activity, but the effect on terbium binding had an ionic-strength dependence which was inverse to that observed with cis-DDP. The tetravalent compounds also had a notable effect on mitochondrial surface potential. Carboplatin had an effect on morphology and Ca2+ influx and it inhibited the respiratory enzymes, although in a manner different from that observed with cis-DDP. Carboplatin had a minimal effect on terbium binding. It is evident that if the platinum drugs enter a cell to exert their action at the nuclear level, they will also depress mitochondrial function. The observed effects did not correlate with nephrotoxicity but, since all four compounds significantly altered mitochondrial structure and function, they may be related to the cytotoxicity of the drug.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barela T. D., Sherry A. D. A simple, one-step fluorometric method for determination of nanomolar concentrations of terbium. Anal Biochem. 1976 Apr;71(2):351–357. doi: 10.1016/s0003-2697(76)80004-8. [DOI] [PubMed] [Google Scholar]
- Bodenner D. L., Dedon P. C., Keng P. C., Katz J. C., Borch R. F. Selective protection against cis-diamminedichloroplatinum(II)-induced toxicity in kidney, gut, and bone marrow by diethyldithiocarbamate. Cancer Res. 1986 Jun;46(6):2751–2755. [PubMed] [Google Scholar]
- Bramwell V. H., Crowther D., O'Malley S., Swindell R., Johnson R., Cooper E. H., Thatcher N., Howell A. Activity of JM9 in advanced ovarian cancer: a phase I-II trial. Cancer Treat Rep. 1985 Apr;69(4):409–416. [PubMed] [Google Scholar]
- Choie D. D., Longnecker D. S., del Campo A. A. Acute and chronic cisplatin nephropathy in rats. Lab Invest. 1981 May;44(5):397–402. [PubMed] [Google Scholar]
- Davis S., Weiss M. J., Wong J. R., Lampidis T. J., Chen L. B. Mitochondrial and plasma membrane potentials cause unusual accumulation and retention of rhodamine 123 by human breast adenocarcinoma-derived MCF-7 cells. J Biol Chem. 1985 Nov 5;260(25):13844–13850. [PubMed] [Google Scholar]
- Ensley J. F., Jacobs J. R., Weaver A., Kinzie J., Crissman J., Kish J. A., Cummings G., Al-Sarraf M. Correlation between response to cisplatinum-combination chemotherapy and subsequent radiotherapy in previously untreated patients with advanced squamous cell cancers of the head and neck. Cancer. 1984 Sep 1;54(5):811–814. doi: 10.1002/1097-0142(19840901)54:5<811::aid-cncr2820540508>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Gordon J. A., Gattone V. H., 2nd Mitochondrial alterations in cisplatin-induced acute renal failure. Am J Physiol. 1986 Jun;250(6 Pt 2):F991–F998. doi: 10.1152/ajprenal.1986.250.6.F991. [DOI] [PubMed] [Google Scholar]
- Gross D. S., Simpkins H. Evidence for two-site binding in the terbium(iii)-nucleic acid interaction. J Biol Chem. 1981 Sep 25;256(18):9593–9598. [PubMed] [Google Scholar]
- Hashimoto K., Rottenberg H. Surface potential in rat liver mitochondria: terbium ion as a phosphorescent probe for surface potential. Biochemistry. 1983 Dec 6;22(25):5738–5745. doi: 10.1021/bi00294a010. [DOI] [PubMed] [Google Scholar]
- Herman T. S., Teicher B. A., Chan V., Collins L. S., Kaufmann M. E., Loh C. Effect of hyperthermia on the action of cis-diamminedichloroplatinum(II), rhodamine 123(2) [tetrachloroplatinum(II)], rhodamine 123, and potassium tetrachloroplatinate in vitro and in vivo. Cancer Res. 1988 May 1;48(9):2335–2341. [PubMed] [Google Scholar]
- Houghton P. J., Bailey F. C., Houghton J. A., Murti K. G., Howbert J. J., Grindey G. B. Evidence for mitochondrial localization of N-(4-methylphenylsulfonyl)-N'-(4-chlorophenyl)urea in human colon adenocarcinoma cells. Cancer Res. 1990 Feb 1;50(3):664–668. [PubMed] [Google Scholar]
- Jones T. W., Chopra S., Kaufman J. S., Flamenbaum W., Trump B. F. Cis-diamminedichloroplatinum (II)-induced acute renal failure in the rat. Correlation of structural and functional alterations. Lab Invest. 1985 Apr;52(4):363–374. [PubMed] [Google Scholar]
- Lê-Quôc K., Lê-Quôc D. Control of the mitochondrial inner membrane permeability by sulfhydryl groups. Arch Biochem Biophys. 1982 Jul;216(2):639–651. doi: 10.1016/0003-9861(82)90254-5. [DOI] [PubMed] [Google Scholar]
- Meijer S., Sleijfer D. T., Mulder N. H., Sluiter W. J., Marrink J., Koops H. S., Brouwers T. M., Oldhoff J., van der Hem G. K., Mandema E. Some effects of combination chemotherapy with cis-platinum on renal function in patients with nonseminomatous testicular carcinoma. Cancer. 1983 Jun 1;51(11):2035–2040. doi: 10.1002/1097-0142(19830601)51:11<2035::aid-cncr2820511113>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Modica-Napolitano J. S., Aprille J. R. Basis for the selective cytotoxicity of rhodamine 123. Cancer Res. 1987 Aug 15;47(16):4361–4365. [PubMed] [Google Scholar]
- Modica-Napolitano J. S., Steele G. D., Jr, Chen L. B. Aberrant mitochondria in two human colon carcinoma cell lines. Cancer Res. 1989 Jun 15;49(12):3369–3373. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recommendations for measurement and presentation of biochemical equilibrium data. Prepared by the Interunion Commission on Biothermodynamics. Biochim Biophys Acta. 1977 Jul 7;461(1):1–14. doi: 10.1016/0005-2728(77)90064-0. [DOI] [PubMed] [Google Scholar]
- Revis N. W., Marusić N. Effects of doxorubicin and its aglycone metabolite on calcium sequestration by rabbit heart, liver, and kidney mitochondria. Life Sci. 1979 Sep 17;25(12):1055–1063. doi: 10.1016/0024-3205(79)90591-5. [DOI] [PubMed] [Google Scholar]
- Simpkins H., Pearlman L. F. Interaction of two second generation platinum antitumor drugs with mouse thymocytes. Cancer Res. 1986 Mar;46(3):1433–1439. [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltshaw E., Evans B. D., Jones A. C., Baker J. W., Calvert A. H. JMS, successor to cisplatin in advanced ovarian carcinoma? Lancet. 1983 Mar 12;1(8324):587–587. doi: 10.1016/s0140-6736(83)92834-9. [DOI] [PubMed] [Google Scholar]




