Abstract
Supplemental oxygenation and carbon dioxide removal through an intravenous respiratory assist catheter can be used as a means of treating patients with acute respiratory failure. We are beginning development efforts toward a new respiratory assist catheter with an insertional size <25F, which can be inserted percutaneously. In this study, we evaluated fiber bundle rotation as an improved mechanism for active mixing and enhanced gas exchange in intravenous respiratory assist catheters. Using a simple test apparatus of a rotating densely packed bundle of hollow fiber membranes, water and blood gas exchange levels were evaluated at various rotation speeds in a mock vena cava. At 12,000 RPM, maximum CO2 gas exchange rates were 449 and 523 mL/min per m², water and blood, respectively, but the rate of increase with increasing rotation rate diminished beyond 7500 RPM. These levels of gas exchange efficiency are two‐ to threefold greater than achieved in our previous respiratory catheters using balloon pulsation for active mixing. In preliminary hemolysis tests, which monitored plasma‐free hemoglobin levels in vitro over a period of 6 hours, we established that the rotating fiber bundle per se did not cause significant blood hemolysis compared with an intra‐aortic balloon pump. Accordingly, fiber bundle rotation appears to be a potential mechanism for increasing gas exchange and reducing insertional size in respiratory catheters.
Patients with acute and acute‐on‐chronic respiratory failure may benefit from supplemental oxygenation and carbon dioxide removal until their lungs heal.1–4 Mortensen5 first introduced the concept of an intravenous respiratory assist device, in which a bundle of hollow fiber membranes (HFMs) is placed within the vena cava through a peripheral vein (e.g., femoral vein) and connected to an oxygen sweep gas flow. Oxygen diffuses out of the gas permeable HFMs into the blood stream while carbon dioxide diffuses into the lumens of the HFMs and is vented externally by the exiting sweep gas. By providing gas exchange independent of the natural lungs, intravenous respiratory assist reduces the gas exchange load required of the lungs and may offer an advantage over mechanical ventilation, the most common respiratory support method used clinically, by eliminating ventilator induced injury to the lungs. Alternatively, intravenous respiratory assist may allow mechanical ventilation at reduced tidal volumes that can ameliorate ventilatory induced injury.6,7 The intravenous respiratory assist device introduced by Mortensen proceeded onto human clinical testing in the 1990s as the IVOX and demonstrated general feasibility for the concept of an intravenous respiratory assist device.3–5,8,9 Nevertheless, issues with clinical trial design, the size of the IVOX (33.3F to 45F),10 and the IVOX level of gas exchange halted further development of the IVOX.
The development of intravenous respiratory assist devices by other research groups continued beyond the IVOX experience with efforts primarily directed toward improving gas exchange performance and reducing device size. The PENSIL was a long, slender respiratory assist catheter incorporating short blind‐ended HFMs along its length in a "bottle‐brush" configuration.11–14 Our group first introduced "active mixing" into a respiratory assist catheter by incorporating a pulsating balloon concentrically within the device's HFM bundle.15–19 Balloon pulsation increased gas exchange efficiency (exchange per HFM surface area) by driving blood flow across the HFMs at a higher velocity than would otherwise exist in the vena cava.20–23 Balloon pulsation increased gas exchange by up to 200% to 300%, depending on vessel size, blood flow rate, and pulsation rate in in vitro and ex vivo tests,19,21 but more modest increases of only 30% to 40% in gas exchange were observed in animal implantation studies.16,22,23 Subsequently, active mixing using fiber vibration was shown to have some effect on increasing gas exchange of the PENSIL device.11–14 Recently, a respiratory assist catheter was developed (the HIMOX) with an integrated microaxial pump on one end of a sheath‐encapsulated HFM bundle.24–26 The microaxial pump of the HIMOX increases its gas exchange performance by directing blood flow across a packed fiber bundle that would be minimally perfused without the pump.
Despite the development efforts described above, the clinical translation of intravenous respiratory assist devices may be impeded by the insertional size of the devices, which is dictated by the amount of HFM area required to achieve appropriate rates of supplemental gas exchange. Our current respiratory catheter, which is being readied for human clinical trials, requires a 32F introduction size even with the enhanced gas exchange efficiency arising from the pulsating balloon.19–23,27,28 We are beginning development efforts toward a new respiratory assist catheter with an insertional size <25F and that can be inserted percutaneously. In this study, we evaluated fiber bundle rotation as a new mechanism in place of balloon pulsation for active mixing and enhanced gas exchange in intravenous respiratory assist catheters. Previously, enhanced gas exchange from fiber rotation was demonstrated in an intravascular respiratory assist catheter (D‐ILAD), using short HFM sheets stacked around a central catheter and rotated in a screw‐like arrangement.29–32 In addition, fiber bundle rotation was shown to enhance gas exchange in extracorporeal respiratory assist devices.33,34 Here, we used a simple bench test apparatus to evaluate the gas exchange characteristics of a rotating densely packed HFM bundle that could potentially be used in developing smaller percutaneous respiratory assist catheters. We evaluated the effect of the fiber bundle rotational speed on gas exchange efficiency in comparison to balloon pulsation. In addition, preliminary hemolysis studies were performed that indicated that the rotation of the fibers per se does not appear to generate problematic hemolysis.
Materials and methods
Bench Test Apparatus
The bench test device consisted of a fiber bundle of 20 cm length, incorporating 525 microporous HFMs of 300 mm OD and 240 mm ID (x30–240 Celgard, Membrana GmbH, Wuppertal, Germany),35 with a total HFM area of 0.1 m². A mat of fibers was rolled around a stainless steel rod (0.083 inch OD), which acted as the support structure for the bundle, and both ends of the fiber mat were potted in Delron potting fixtures using a two‐part epoxy. The effective insertional diameter of the rotating catheter bundle when potted and compressed was 25F (8.33 mm). The fiber bundle was connected to proximal and distal manifolds, which consisted of bearings and seals. The proximal manifold also housed the connection from the stainless steel rod within the fiber bundle to a stainless steel coupling and subsequent stainless steel drive shaft (1 mm OD) of a motor and controller (Series 2444‐024‐B, MicroMo Electronics Inc, Clearwater, FL). The motor was capable of driving rotation up to 12,000 RPM, as measured through an indicator circuit processing the hall sensor impulses within the motor (Model Cub5, Red Lion Controls, York, PA).
Blood Collection/Treatment
Bovine blood was acquired from a slaughterhouse (the morning before gas exchange testing and the morning of hemolysis testing) and anticoagulated with ACD (anticoagulant citrate dextrose) at a ratio of 9∶1 to eliminate clot formation during testing. Penicillin (500,000 U/mL) and gentamicin (0.1 g/mL) were added to the blood to eliminate potential bacteria growth during testing. Immediately after collection, the blood was filtered with the use of a 40‐µm microaggregate blood transfusion filter (SQ40S, Pall Biomedical, Inc, East Hills, NY).
Gas Exchange Evaluation
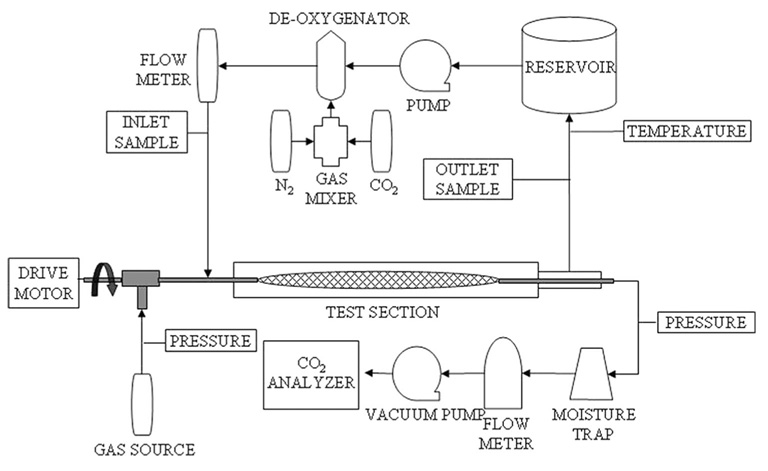
Gas exchange of the rotating fiber bundle was tested within a 7/8‐inch ID (Figure 1) rigid test section with integral spacers that kept the bundle centered during rotation. This test section was filled with either deionized water or slaughterhouse bovine blood (prepared as described above). A sweep gas of pure oxygen (from an external source) was pulled through the inner lumens of the fibers, a moisture trap, thermal mass flowmeter (GR‐116‐A‐PV‐O2, Fathom Technologies, Round Rock, TX), and a sealed vacuum pump (400–3910, Barnant Company, Barrington, IL). The percentage of CO2 in the sweep gas exhaust of the device was measured with the use of a CO2 analyzer (CO2–44B, Physio‐Dyne Instrument Corporation, Quogue, NY) on the positive side of the sealed vacuum pump. Pressure drop (on the sweep gas side) across the device was measured with a differential pressure transducer (143SC, Honeywell International, Inc, Morristown, NJ).
Figure 1.
Schematic of mock vena cava test setup.
Sweep gas flow was set at 3 L/min (as measured by the thermal mass flowmeter). The test fluid (either water or blood) was perfused through the test section at 3 L/min (measured by a rotometer for water and an ultrasonic flow probe (T110, Transonic Systems, Inc, Ithaca, NY) for blood) and 37°C. Liquid side pressure was kept above 100 mm Hg to eliminate microbubble formation. This was necessary for the bench test apparatus only because the sweep gas pressure drop leading to the fiber bundle was small. (In actual application, pneumatic design of an implantable catheter would have sufficient resistance to create a pressure within the fibers opposing microbubble formation and the fibers themselves would have nonporous polymer coatings that would not allow microbubbles.36) Inlet and outlet partial pressures of CO2 and O2 were measured with a blood gas analyzer (ABL505, Radiometer America, Inc, Westlake, OH). Target inlet conditions for water were a PCO2 of 50 mm Hg and a PO2 of 30 mm Hg, while the inlet pCO2 of blood was targeted at 45 mm Hg, according to the Association for the Advancement of Medical Instrumentation (AAMI) standards.37 Total hemoglobin and oxygen saturation were measured for blood experiments with the use of a co‐oximeter (OSM3, Radiometer America, Inc, Westlake, OH). AAMI standards inlet conditions of 65% O2 saturation and 12 g/dL total hemoglobin were used. Gas exchange was evaluated at various rotation rates up to 12,000 RPM. Rotation rates were randomly picked during testing and repeated twice (except for the 12,000 RPM data).
Gas exchange levels (VCO2 and VO2, carbon dioxide and oxygen, respectively) were calculated for the various rotation rates for the water and blood characterization test. VCO2 was calculated from gas side measurements for both types of fluid, using:
| (1) |
where is the flow rate of the sweep gas being pulled through the device and FCO2 is the fraction of CO2 at the exhaust of the catheter. This exchange level was then normalized to our target PCO2inlet of 50 mm Hg by:
| (2) |
to reduce variability associated with small changes (<5 mm Hg) from our 50 mm Hg target of the inlet PCO2 condition.
VO2 for water tests was determined using:
| (3) |
where and are the partial pressures of oxygen after and before the device, respectively, QWATER is the flow rate of water, and is the solubility of oxygen in water at 37°C [0.000317 mL O2/100 mL water per mm Hg].
VO2 for blood tests was determined using:
| (4) |
where QBLOOD is the flow rate of blood and and are the saturation of oxygen in the blood after and before the device, respectively. tHbINLET and tHbOUTLET are the total hemoglobin before and after the device, respectively. and are the partial pressures of oxygen after and before the device, respectively. is the binding capacity of hemoglobin for O2 [1.35 mL/g] and is the solubility of oxygen in blood at 37°C [0.003 mL O2/100 mL blood per mm Hg]. All gas exchange levels were also normalized to the fiber surface area of the catheter.
Evaluation of Hemolysis
Preliminary hemolysis studies were done to establish whether the rotating fibers themselves would cause problematic hemolysis. Red cell damage associated with the bench test apparatus was evaluated in a manner similar to our previously reported data on the balloon‐pulsed respiratory catheter.38 Two identical loops were used to compare the test device with a Datascope intra‐aortic balloon pump, each consisting of a reservoir bag (Affinity Venous Reservoir Bag #321, Medtronic, Inc, Minneapolis, MN), centrifugal blood pump (Biomedicus BioPump BPX‐80 and 540 Bio‐Console, Medtronic, Inc, Minneapolis, MN), pediatric heat exchanger (D1078, Medtronics Electromedics, Minneapolis MN), and a 7/8‐inch rigid test section with integral spacers, all connected using 3/8‐inch laboratory tubing (R‐3603, Tygon, Saint‐Gobain Performance Plastics, Akron, OH). Pressure in the two loops was measured with a pressure transducer. A vacuum (achieved by a Barnant pump) was applied to the sweep gas pathway of the rotational catheter to eliminate possible microbubble formation, and pressure was continually monitored with a pressure transducer.
Protein (as measured by Spotchem EZ SP‐4430, Arkray, Inc, Kyoto, Japan) and hematocrit were measured before testing. A fragility index for the blood was calculated according to Lund et al.38 The blood was slowly added (1.5 L volume) and circulated at 3 L/min (as measured by a T110 Transonic Systems flow probe) and 37°C (as measured using a thermocouple, Type‐T, Cole‐Parmer Instrument Company, Vernon Hills, IL) in a closed loop for 6 hours. The test devices were set at constant rotation and pulsation rates for the duration of the 6‐hour test (rotational catheter @ 7500 RPM, Datascope intra‐aortic balloon pump @ 120 beats per minute). Samples at the outlet of the test device were drawn every half hour for the first 2 hours and then every hour until the 6‐hour time point. Plasma‐free hemoglobin (PFHB) samples were spun at 3800 RPM for 15 minutes (Centrific Model 228, Fisher Scientific International, Inc, Hampton, NH), plasma was siphoned off, then spun for 15 minutes at 10,000 RPM (Galaxy 7, VWR International, West Chester, PA), siphoned off again, respun at 10,000 RPM, and then placed into a cuvette for analysis in the spectrophotometer.
Hematocrit and PFHB were measured for each sample acquired and plotted over the duration of the test. Absorbance values determined at a wave length of 540 nm in the spectrophotometer were converted to PFHB(in mg/dL by):
| (5) |
where 125 mg/dL is the conversion factor for bovine blood and 5 mg/dL is the background reading of nondamaged plasma, using a DI water blank.34,38 The conversion factor was determined through serial dilutions of completely hemolyzed whole bovine blood.
Results
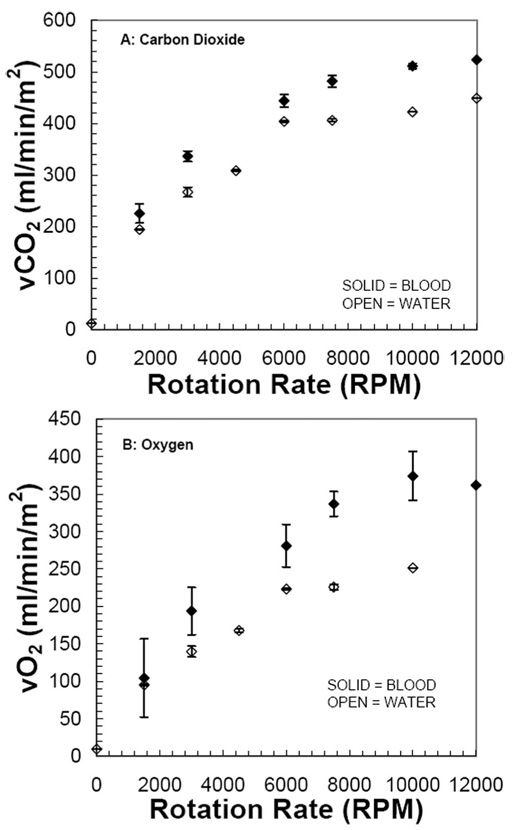
Gas exchange for CO2 and O2 in water and blood increased with increasing rotation rate of the fiber bundle (Figure 2). In the absence of rotation (0 RPM), gas exchange was negligible because of the small fiber bundle size in the test vessel and shunting of flow past the device. The maximum CO2 exchange rates were 449 and 523 mL/min per m² (water and blood, respectively) at 12,000 RPM, whereas the maximum O2 exchange rates were 251 and 374 mL/min per m² (water and blood, respectively) at 10,000 RPM. The rate of increase of gas exchange with increasing rotation rate appeared to diminish or plateau beyond approximately 7500 RPM, and the maximum gas exchange levels were only about 10% higher than those at 7500 RPM.
Hemolysis results are summarized for two separate tests that spanned the range of hemolysis generation seen with our bench test device (Table 1). The two tests were performed using different devices with the same fiber bundle specifications (size and number of fibers, packing densities, fiber bundle size) and with both rotating at 7500 RPM. The control device for both tests was an intra‐aortic balloon pump pulsing at 120 BPM. In the first test (group A: lowest hemolysis), the rate of increase of PFHB was similar between rotating test device and control. In the second test (group B: highest hemolysis), the rate of increase of PFHB was significantly greater for the rotating test device compared with control. Total blood protein was similar in these tests (5.9 versus 6.5 g/dL), as was the fragility index (0.44 ± 0.01 versus 0.38 ± 0.01). As the fiber bundles and rotation speeds were identical in these two tests (as was the in vitro gas exchange, data not shown), comparable hemolysis generation of the test device and control device in group A indicates that rapid fiber rotation per se does not lead to significant hemolysis compared with an intra‐aortic balloon pump but does lead to some other variable component of the test fixture exists that causes the greater hemolysis seen in group B.
Table 1.
Hemolysis Results for Rotating Catheter (Test) and Intra‐aortic Ballon Pump (Control)
| Group | Total Protein (g/dL) | Fragility Index | Hct % | PFHB: Test (mg/dL‐h) | PFHB: Control (mg/dL‐h) |
|---|---|---|---|---|---|
| A | 5.9 | 0.44 | 33 | 40 | 38 |
| B | 6.5 | 0.38 | 37 | 109 | 22 |
Discussion
Intravenous respiratory assist may become a viable treatment for many patients with acute and acute‐on‐chronic lung failures. The clinical implementation of intravenous respiratory assist catheters may be impeded by their relatively large size (32F or greater), which arises from the fiber membrane area required to achieve supplemental gas exchange. We are beginning development efforts toward a new percutaneous respiratory assist catheter (PRAC) with an insertional size <25F and gas exchange comparable to our current balloon‐pulsed catheter.17,19–21,23 The studies reported here evaluated fiber bundle rotation as a new mechanism for enhancing gas exchange in respiratory assist catheters. We fabricated a simple bench test apparatus of a 25F, rotating, rod‐shaped, densely‐packed HFM bundle. Gas exchange increased substantially with increasing bundle rotation rates, ultimately achieving exchange efficiencies of 520 mL/min per m² for CO2 and 370 mL/min per m² for O2. In past, comparable tests of our 32F balloon‐pulsed catheter (membrane area of 0.17 m², maximal gas exchange efficiencies were 280 and 120 mL/min per m² for CO2 and O2, respectively.20 In absolute gas exchange, the rotating 25F PRAC achieved 52 and 37 mL/min for CO2 and O2, respectively, compared with 43 and 31 mL/min for the 32F pulsating balloon catheter.
In this study, we performed gas exchange tests in a simple "mock vena cava" bench flow loop to compare results with a substantial database of gas exchange results for our respiratory catheter, which used a pulsating balloon. The in vitro test system is not meant to mimic the complex environment within the vena cava, in which vessel compliance is one prominent characteristic. Nevertheless, we expect that the active mixing generated by fiber bundle rotation per se will not be substantively affected by vessel compliance. If anything, vessel compliance would be expected to have a greater effect with our pulsating respiratory catheter because of pressure swings during balloon inflation and deflation. When we studied this, however, we did not find any significant differences in gas exchange performance between our pulsating catheter in rigid vessels versus compliant vessels.39 We also tested our pulsating balloon catheter in acute and chronic in vivo tests16,21–23 and found no evidence suggesting obstruction of caval flow. Accordingly, as the rotational fiber bundles studied here take up only 60% of the vessel cross section compared with the pulsating balloon catheter, we would similarly expect no problems associated with obstruction of caval flow.
"Active mixing" is often used when describing the increase in gas exchange caused by imparting or disrupting normal flow fields that would be associated with a passive respiratory assist device (for example, a commercial blood oxygenator). The increase in gas exchange caused by active mixing occurs through a combination of two potential mechanisms: 1) increased relative velocity of blood flow past fiber surfaces; and/or 2) increased mixing that convects oxygenated and decarbonated fluid elements away from the fiber bundle and that convects fresh fluid elements toward the fiber bundle. The former reduces the diffusional boundary layers subjacent to fibers,20,40 whereas the latter maintains a favorable gas tension gradient for diffusion. In this study, active mixing using fiber bundle rotation resulted in approximately a doubling of gas exchange efficiency compared with balloon pulsation. The increase probably is a result of both mechanisms described above. The characteristic velocity (fiber or flow) generated by balloon pulsation at 300 beats per minute (the maximum we were able to achieve) is approximately 2.5 cm/s, assuming a 1‐cm‐diameter balloon, whereas that for fiber bundle rotation at 10,000 RPM is approximately 400 cm/s, assuming an 8‐mm‐diameter fiber bundle. Thus, the relative velocity of fluid elements past fiber surfaces during bundle rotation is likely to be greater than with balloon pulsation. With respect to actual mixing (the second mechanism), we are currently completing flow visualization studies of the rotational fiber bundle prototype. Preliminarily results indicate that the fiber bundle rotation does appear to create Taylor vortices41–44 between the bundle and the vessel wall, which, if confirmed, would increase mixing between the fiber bundle and the flow past the bundle. Taylor vortices are secondary flow patterns with characteristic velocities that are an order of magnitude less than those in the angular rotational direction. Thus, although they do not create shear levels approaching those existing in the rotational direction, they can be very effective for radial mixing compared with diffusion.
Other studies have explored fiber bundle rotation as a mechanism for active mixing and gas exchange enhancement in respiratory assist devices. The D‐ILAD device was an intravascular oxygenator that used short HFM sheets stacked around a central catheter and rotated in a screw‐like arrangement.29–32 The D‐ILAD achieved gas exchange efficiencies of 310 and 208 mL/min per m² for CO2 and O2, respectively. Although lower than the gas exchange efficiency of our device, the D‐ILAD was a larger device (membrane area of 0.29 m²), which may account for the lower efficiency. The D‐ILAD device, however, had a deployed size of approximately 3 cm in diameter. In extracorporeal respiratory assist devices, Svitek et al.34 demonstrated that fiber bundle rotation could be used to enhance gas exchange and provide pumping in a device with a rotating annular fiber bundle. Wu et al.33 reported a similar enhancement of gas exchange for a disk‐shaped rotating fiber bundle. Importantly, these studies also determined that rapidly rotating fibers themselves are not associated with significant hemolysis levels.
In this study, we also performed preliminary hemolysis tests with the principal aim of showing that the rapidly rotating fiber bundle itself does not cause significant hemolysis compared with a control cardiovascular device used clinically. If this were true, then the gas exchange benefits of using a rotating fiber bundle for a percutaneous respiratory catheter would not be worthy of further investigation. Levels of PFHB were compared with an intra‐aortic balloon pump pulsating within an identical circulatory loop as a control.38 The results of the hemolysis tests were variable, even though the test devices had identical fiber bundle specifications and comparable gas exchange (data not shown). In the group A test (lowest hemolysis), the rate of increase of PFHB was comparable between the rotating bundle test device and the control but was significantly greater than the control in the group B test (highest hemolysis). As the devices had identical fiber bundle characteristics (number of fibers, length of fibers, fiber packing density) and were operated at the same rotational speed (7500 RPM), we do not ascribe the hemolysis generated in the second test to the rotating fiber bundle itself. If rotating fibers did cause more hemolysis than control, we would have seen higher hemolysis for the test device in group A. Variability in the blood cannot explain this result because the blood used in the group A tests if anything was more susceptible to hemolysis, as indicated not only by a higher fragility index but also by higher control hemolysis in the group A test. We are not completely surprised by the variability of the hemolysis results for our bench test devices. These devices were fabricated for short‐term bench tests of gas exchange and were not "highly engineered" with regard to rotational components used in blood contacting devices. Nevertheless, the preliminary hemolysis results are important because they indicate that the active mixing generated by using rapidly rotating fibers itself does not appear to cause significant blood damage compared with an intra‐aortic balloon pump, a clinically used cardiovascular device.
In conclusion, fiber bundle rotation appears to be a feasible mechanism for enhancing gas exchange and reducing insertion size for a PRAC. The next step is developing an implantable version of the rotating fiber bundle PRAC. To this end, future work includes 1) designing a deployable "safety cage" that would prevent the rotating fiber bundle from directly contacting vascular endothelium; 2) incorporating and evaluating seal materials and designs, along with bearings, which could be used in a blood contacting application for an acute (7‐ to 10‐day) application; and 3) performing animal implantation tests to evaluate gas exchange performance and device safety in situ.
Figure 2.
Gas exchange results versus rotation rate for blood and water. A, Carbon dioxide normalized to a PCO2 inlet of 50 mm Hg; B, oxygen.
Acknowledgements
The work presented in this publication was made possible by grant HL70051 from the National Institutes of Health, the National Heart, Lung, and Blood Institute, and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute or National Institutes of 372 EASH ET AL. Health. We would like to recognize the University of Pittsburgh's McGowan Institute for Regenerative Medicine for support of this study.
References
- 1.Brunet F, Mira JP, Cerf C, et al. Permissive hypercapnia and intravascular oxygenator in the treatment of patients with ARDS. Artif Organs. 1994;18:826–832. doi: 10.1111/j.1525-1594.1994.tb03331.x. [DOI] [PubMed] [Google Scholar]
- 2.Conrad SA, Eggerstedt JM, Grier LR, et al. Intravenacaval membrane oxygenation and carbon dioxide removal in severe acute respiratory failure. Chest. 1995;107:1689–1697. doi: 10.1378/chest.107.6.1689. [DOI] [PubMed] [Google Scholar]
- 3.Durbin GG., Jr Intravenous oxygenation and CO2 removal device: IVOX. Respir Care. 1992;37:147–153. [Google Scholar]
- 4.Jurmann MJ, Demertzis S, Schaefers HJ, et al. Intravascular oxygenation for advanced respiratory failure. ASAIO J. 1992;38:120–124. [PubMed] [Google Scholar]
- 5.Mortensen JD. Intravascular oxygenator: a new alternative method for augmenting blood gas transfer in patients with acute respiratory failure. Artif Organs. 1992;16:75–82. doi: 10.1111/j.1525-1594.1992.tb00271.x. [DOI] [PubMed] [Google Scholar]
- 6.Fleury B, Murciano D, Talamo C, et al. Work of breathing in patients with chronic obstructive pulmonary disease in acute respiratory failure. Am Rev Respir Dis. 1985;131:822–827. doi: 10.1164/arrd.1985.131.6.822. [DOI] [PubMed] [Google Scholar]
- 7.Weinacker AB, Vaszar LT. Acute respiratory distress syndrome: physiology and new management strategies. Ann Rev Med. 2001;52:221–237. doi: 10.1146/annurev.med.52.1.221. [DOI] [PubMed] [Google Scholar]
- 8.Mira JP, Brunet F, Belghith M, et al. Reduction of ventilator settings allowed by intravenous oxygenator (IVOX) in ARDS patients. Intensive Care Med. 1995;21:11–17. doi: 10.1007/BF02425148. [DOI] [PubMed] [Google Scholar]
- 9.Zwischenberger JB, Tao W, Bidani A. Intravascular membrane oxygenator and carbon dioxide removal devices: a review of performance and improvements. ASAIO J. 1999;45:41–46. doi: 10.1097/00002480-199901000-00010. [DOI] [PubMed] [Google Scholar]
- 10.High KM, Snider MT, Richard R, et al. Clinical trials of an intravenous oxygenator in patients with adult respiratory distress syndrome. Anesthesiology. 1992;77:856–863. doi: 10.1097/00000542-199211000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Baskaran H, Nodelman V, Ultman JS. Gas‐liquid desorption through blind‐ended microporous hollow fibers for an intravascular artificial lung. Ind Eng Chem Res. 1998;37:4142–4151. [Google Scholar]
- 12.High KM, Nicholson T, Richard RB, et al. Effects of blood phase oscillation on gas transfer in a microporous intravascular lung. ASAIO J. 1994;40:M735–M739. doi: 10.1097/00002480-199407000-00096. [DOI] [PubMed] [Google Scholar]
- 13.Nodelman V, Baskaran H, Ultman JS. Enhancement of O2 and CO2 transfer through microporous hollow fibers by pressure cycling. Ann Biomed Eng. 1998;26:1044–1054. doi: 10.1114/1.115. [DOI] [PubMed] [Google Scholar]
- 14.Snider MT, High KM, Richard RB, et al. Small intrapulmonary artery lung prototypes: design, construction, and in vitro water testing. ASAIO J. 1994;40:M533–M539. doi: 10.1097/00002480-199407000-00057. [DOI] [PubMed] [Google Scholar]
- 15.Federspiel WJ, Hewitt T, Hout MS, et al. Recent progress in engineering the Pittsburgh intravenous membrane oxygenator. ASAIO J. 1996;42:M435–M442. doi: 10.1097/00002480-199609000-00026. [DOI] [PubMed] [Google Scholar]
- 16.Macha M, Federspiel WJ, Lund LW, et al. Acute in vivo studies of the Pittsburgh intravenous membrane oxygenator. ASAIO J. 1996;42:M609–M615. doi: 10.1097/00002480-199609000-00060. [DOI] [PubMed] [Google Scholar]
- 17.Federspiel WJ, Hout MS, Hewitt TJ, et al. Development of a low flow resistance intravenous oxygenator. ASAIO J. 1997;43:M725–M730. [PubMed] [Google Scholar]
- 18.Hattler BG, Federspiel WJ. Progress with the development of the intravenous membrane oxygenator. Perfusion. 1999;14:311–315. doi: 10.1177/026765919901400411. [DOI] [PubMed] [Google Scholar]
- 19.Federspiel WJ, Golob JF, Merrill TL, et al. Ex vivo testing of the intravenous membrane oxygenator. ASAIO J. 2000;46:261–267. doi: 10.1097/00002480-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Hattler BG, Federspiel WJ. Gas exchange in the venous system: support for the failing lung. In: Vaslef SN, Anderson RW, editors. The Artificial Lung. Georgetown, TX: Landes Bioscience; 2002. 2002. pp. 133–174. [Google Scholar]
- 21.Hattler BG, Lund LW, Golob J, et al. A respiratory gas exchange catheter: in vitro and in vivo tests in large animals. J Thorac Cardiovasc Surg. 2002;124:520–530. doi: 10.1067/mtc.2002.123811. [DOI] [PubMed] [Google Scholar]
- 22.Golob JF, Federspiel WJ, Merrill TL, et al. Acute in vivo testing of an intravascular respiratory support catheter. ASAIO J. 2001;47:432–437. doi: 10.1097/00002480-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Eash HJ, Frankowski BJ, Litwak K, et al. Acute in vivo testing of a respiratory assist catheter: implants in calves versus sheep. ASAIO J. 2003;49:370–377. doi: 10.1097/01.MAT.0000074991.94234.B6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cattaneo G, Strauss A, Reul H. Compact intra‐ and extracorporeal oxygenator developments. Perfusion. 2004;19:251–255. doi: 10.1191/0267659104pf748oa. [DOI] [PubMed] [Google Scholar]
- 25.Cattaneo GF, Reul H. New fiber configuration for intravenous gas exchange. Int J Artif Organs. 2005;28:244–250. doi: 10.1177/039139880502800309. [DOI] [PubMed] [Google Scholar]
- 26.Cattaneo GFM, Reul H, Schmitz‐Rode T, et al. Intravascular blood oxygenation using hollow fibers in a disk‐shaped configuration: experimental evaluation of the relationship between porosity and performance. ASAIO J. 2006;52:180–185. doi: 10.1097/01.mat.0000204151.56591.28. [DOI] [PubMed] [Google Scholar]
- 27.Eash HJ, Budilarto SG, Hattler BG, et al. Investigating the effects of random balloon pulsation on gas exchange in a respiratory assist catheter. ASAIO J. 2006;52:192–195. doi: 10.1097/01.mat.0000199752.89066.83. [DOI] [PubMed] [Google Scholar]
- 28.Eash HJ, Frankowski BJ, Hattler BG, et al. Evaluation of local gas exchange in a pulsating respiratory support catheter. ASAIO J. 2005;51:152–157. doi: 10.1097/01.MAT.0000153648.11692.D0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makarewicz AJ, Mockros LF, Anderson RW. A pumping intravascular artificial lung with active mixing. ASAIO J. 1993;39:M466–M469. doi: 10.1097/00002480-199307000-00063. [DOI] [PubMed] [Google Scholar]
- 30.Makarewicz AJ, Mockros LF, Anderson RW. A dynamic intravascular artificial lung. ASAIO J. 1994;40:M747–M750. doi: 10.1097/00002480-199407000-00099. [DOI] [PubMed] [Google Scholar]
- 31.Vaslef SN, inventor. Intravascular Lung Assist Device and Method. 5, 037. US patent. 1991:383.
- 32.Vaslef SN, Mockros LF, Anderson RW. Development of an intravascular lung assist device. ASAIO Trans. 1989;35:660–664. doi: 10.1097/00002480-198907000-00160. [DOI] [PubMed] [Google Scholar]
- 33.Wu ZJ, Gartner M, Litwak KN, et al. Progress toward an ambulatory pump‐lung. J Thorac Cardiovasc Surg. 2005;130:973–978. doi: 10.1016/j.jtcvs.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 34.Svitek RG, Frankowski BJ, Federspiel WJ. Evaluation of a pumping assist lung that uses a rotating fiber bundle. ASAIO J. 2005;51:773–780. doi: 10.1097/01.mat.0000178970.00971.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Membranes for oxygenation, apheresis, and i.v. filtration. Membrana; [accessed November 2005]. http://www.membrana.com/oxygenation/products/celgard.htm. [Google Scholar]
- 36.Eash HJ, Jones HM, Hattler BG, Federspiel WJ. Evaluation of plasma resistant hollow fiber membranes for artificial lungs. ASAIO J. 2004;50:491–497. doi: 10.1097/01.mat.0000138078.04558.fe. [DOI] [PubMed] [Google Scholar]
- 37.AAMI standards and recommended practices: Biomedical equipment, Part 1: General safety and design; Equipment for therapy and surgery. American National Standards Institute, Inc; 1996. Cardiovascular implants and artificial organs: Blood‐gas exchangers oxygenators; pp. 633–648. [Google Scholar]
- 38.Lund LW, Hattler BG, Federspiel WJ. A comparative in vitro hemolysis study of a pulsating intravenous artificial lung. ASAIO J. 2002;48:631–635. doi: 10.1097/00002480-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Garcia MY, Hattler BG, Federspiel WJ. Effect of vessel compliance on the in‐vitro performance of a pulsating respiratory support catheter. 2002;124:56–62. doi: 10.1115/1.1428556. [DOI] [PubMed] [Google Scholar]
- 40.Federspiel WJ, Henchir KA. Artificial lungs: Basic principles and current applications. In: Wnek GE, Bowlin GL, editors. Encyclopedia of Biomaterials and Biomedical Engineering. New York, NY: Marcel Dekker, Inc; 2004. pp. 910–921. [Google Scholar]
- 41.Srinivasan R, Jayanti S, Kannan A. Effect of Taylor vortices on mass transfer from a rotating cylinder. AIChE J. 2005;51:2885–2898. [Google Scholar]
- 42.Lewis GS, Swinney HL. Velocity structure functions, scaling, and transitions in high‐Reynolds‐number Couette‐Taylor flow. Phys Rev E. 1999;59:5457–5467. doi: 10.1103/physreve.59.5457. [DOI] [PubMed] [Google Scholar]
- 43.Lathrop DP, Fineberg J, Swinney HL. Turbulent flow between concentric rotating cylinders at large Reynolds number. Phys Rev Lett. 1992;68:1515–1518. doi: 10.1103/PhysRevLett.68.1515. [DOI] [PubMed] [Google Scholar]
- 44.Baier G, Grateful TM, Graham MD, et al. Prediction of mass transfer rates in spatially periodic flows. Chem Eng Sci. 1998;54:343–355. [Google Scholar]