Abstract
A respiratory catheter that is inserted through a peripheral vein and placed within the vena cava is being developed for CO2 removal in patients with acute exacerbations of chronic obstructive pulmonary disease (COPD). The catheter uses a rapidly pulsating balloon to enhance gas exchange. In this study, the CO2 removal performance of our catheter was assessed in acute sheep implants and compared with calf implants, primarily because sheep have cardiac outputs (CO) that are more comparable with human CO and lower than calves. Respiratory catheters (25 mL balloon, 0.17 m²) were inserted acutely in sheep (n = 2) and calves (n = 6) through the jugular vein and placed within the vena cava in two positions: spanning the right atrium (RA) and within the inferior vena cava (IVC). The postinsertion CO in the sheep ranged from 4.1 to 7.2 L/min compared with 6.2 to 15.5 L/min for the calves. The maximum CO2 removal rates (vCO2) were 297 ml/min/m² (calf) and 282 ml/min/m² (sheep) in the RA position and 240 ml/min/m² (calf) and 248 ml/min/m² (sheep) in the IVC position. The respective removal rates between animal models were not statistically different (p values > 0 .05 for all data sets). The dependence of the vCO2 on balloon pulsation was also not statistically different between the animal models.
Intravenous respiratory assist (supplemental oxygen supply and carbon dioxide removal) may be a potential means to support patients with acute respiratory failure or with acute exacerbations of chronic respiratory failure (e.g., chronic obstructive pulmonary disease [COPD]).1–4 The technique, under development but not yet in clinical use, involves placing a bundle of hollow fiber membranes (HFMs) into the vena cava through a peripheral vein (e.g., the common femoral vein) and connecting the fibers to an external source of oxygen gas.5 Oxygen flows through the gas permeable HFMs and creates gradients for diffusion of O2 into the blood and CO2 out of blood into the gas stream within the fibers so that the gas exiting the device removes CO2. Blood is partially oxygenated and decarbonated before it reaches the natural lung, and therefore respiratory support is provided independently of the lungs, allowing them to rest and heal, unlike with mechanical ventilation, the therapy most often used to support the failing lung.6,7 Respiratory support with an intravenous catheter is potentially simpler and less complicated than using pumps to bring blood flow outside the body through oxygenators in extracorporeal blood circuits.
Our group has been developing respiratory assist catheters (also known as Hattler Catheters) for supplementing CO2 removal and O2 delivery intravenously in patients with failing lungs.8–10 Our current efforts are focused on developing a respiratory catheter primarily for CO2 removal in patients with acute exacerbation of COPD. These patients offer significant challenges for CO2 removal compared with adequate oxygenation, which can usually be managed using supplemental nasal oxygen.11 COPD patients have inelastic lungs, and they can be difficult to treat using mechanical ventilators. The elevated work required for their breathing can make it difficult to wean COPD patients from mechanical ventilators.7 Accordingly, an intravenous catheter that can supplement CO2 removal in COPD patients with acute exacerbation in lieu of intubation and mechanical ventilation would offer pulmonologists and intensivists an attractive potential method of improved respiratory assist for these patients. Our respiratory assist catheter uses a centrally located balloon within the HFM bundle to promote greater blood convection over the fiber surfaces (enhanced mixing) so as to increase the CO2 gas removal rate of the catheter.12 We have previously reported in bench and in ex vivo animal tests of the respiratory catheter5,10 that increasing the rate of balloon pulsation can increase gas exchange substantially (typically anywhere from 50–200%, depending on test conditions), up until a pulsation frequency is reached, at which time filling and emptying of the balloon becomes limited.5,12,13
In this article, we describe further testing of the gas exchange performance of our respiratory assist catheter using acute sheep implants, and we compare performance with that in acute calf implants performed as part of a larger set of previous animal studies.9 The principal questions the study addressed were (1) does the vCO2 of the catheter differ in sheep compared with calves, (2) does balloon enhancement of gas exchange differ in sheep compared with calves, and (3) how does location of the catheter in the vena cava or size of the pulsating balloon within the catheter affect the above comparisons. The rationale for comparing the respiratory catheter in sheep versus calf implants involves several important issues. Our finding of a reduced dependence of gas exchange on balloon pulsation rate in the calf,9 with a relatively high cardiac output (CO) compared with humans, led us to consider an animal model with a CO more comparable with that in the human. Our ex vivo tests indicate that balloon pulsation enhances gas exchange proportionally more when flow past the catheter is lower.10 The sheep was also the animal model used for preclinical implant tests of the IVOX device, a respiratory catheter that underwent human clinical trials in the early 1990s.14,15 Thus, the sheep implants reported here may provide further comparison of our respiratory catheter with the IVOX, in addition to the previous comparisons we made based on past ex vivo studies.10 Finally, existing animal models of acute respiratory failure have most extensively been developed in sheep,14,16–18 and therefore testing our respiratory catheter in normal sheep is an important prelude to future catheter studies in animal models of respiratory failure.
Methods
Animal Preparation
Our respiratory assist catheter, the Hattler Catheter (HC), was tested in vivo in two male Suffolk cross sheep (59 kg and 76.5 kg) and in six calves (94.5 ± 10.7 kg), the latter as part of a larger set of catheter studies in acute implants. Animal preparation for these studies was performed in the same manner as in our previous experiments;9,10 detailed description can be found in Golob et al.9 Briefly, premedication of 0.5 mg/kg atropine was administered to each sheep. Anesthesia was induced with 10 mg/kg of Brevital (methohexital sodium, Eli Lilly and Co., Indianopolis, IN) and maintained with isoflurane in oxygen and room air (1∶1). Calves used in the previous study were also premedicated with 0.5 mg/kg atropine, anesthetized with 10 mg/kg Brevital, and maintained with isoflurane in oxygen and room air (1∶1). Preparation of the skin of the neck and both femoral triangles was then completed and draped in the normal manner. A fluid filled pressure transducer connected to a pressure and electrocardiograph monitor was used to measure pressure from a 16 gauge polyvinyl chloride catheter in the right femoral artery. Arterial blood gases were also taken from this catheter. A Swan‐Ganz catheter was advanced into the pulmonary artery for CO measurements using a thermal dilution of 5% dextrose solution. Central venous pressure (CVP) measurements were also gathered from this Swan‐Ganz catheter with the port located in the right atrium (RA). Blood gases for CVP were taken from this port on the Swan‐Ganz. Throughout the experiment, the partial pressure of CO2 (pCO2) of the animal was monitored and the ventilator settings adjusted to target a venous pCO2 near 50 mm Hg, as measured using a blood gas analyzer (ABL 505, Radiometer America, Westlake, OH) with blood sampled from the femoral vein. A polypropylene catheter was inserted in the scrotal vein for continuous heparin infusion.
Device Insertion and Operation
Respiratory catheters with 13 ml and 25 ml balloons and a membrane surface area of 0.17 m² were used in these tests. The fiber bundles were 30 cm in length and were fabricated from Celgard (Charlotte, NC) hollow fiber fabric (polypropylene fibers with internal diameter [ID] = 0.024 cm, outside diameter [OD] = 0.030 cm, woven at 54 fibers per inch) wrapped around the central balloon. The devices were inserted through the left jugular vein and advanced so that the fiber bundle was spanning the RA with parts of the bundle in the superior vena cava and in the inferior vena cava (IVC). Once the catheter was in place, gas pathways were connected through an external fitting to supply helium gas to the balloon, 100% O2 to the sweep gas inlet port, and vacuum to the sweep gas exhaust port.
Balloon pulsation was started at 120 beats per minute (bpm) and a sweep gas flowrate of 3.0 L/min was maintained through the fiber bundle by a gas flow controller (GR‐116‐2‐A‐PV, Fathom Technologies, Round Rock, TX) at the vacuum source after insertion of the catheter. The sweep gas flowrate of 3.0 L/min was chosen on the basis of prior work looking at changes in CO2 exchange rate with sweep gas flowrate.19,20 For the CO2 exchange rates accomplished by the respiratory catheter under study here, a sweep gas flowrate of 3.0 L/min results in CO2 exchange that is not significantly limited by the sweep gas flowrate rate (i.e., CO2 exchange rate is within 5% of the maximum possible exchange). A moisture trap was used before the flow controller, and the O2 and CO2 gas fractions (FO2 and FCO2) were measured using a mass spectrometer (MGA 1100, Marquette Electronics, Milwaukee, WI) with sampling port after the flow controller. A pressure transducer (143SC, SenSym Inc., Milpitas, CA) near the exhaust port was used to monitor the sweep gas pressure drop through the device, which generally remains less than 100 mm Hg.
Experimental Protocol
Before device insertion, baseline values for arterial and venous blood gases, CO, total blood chemistries (TBC), plasma free hemoglobin (free Hb), hematocrit, and blood pressure were measured. Blood gases and pressures, as well as hematocrit measurements, were taken every half hour. These parameters were used to monitor the animal's performance throughout the experiment. Free Hb samples were taken every 2 hours following baseline. A TBC measurement was taken at the conclusion of the experiment. Free Hb and TBC samples were used to evaluate the performance of the animal after the conclusion of the experiments and to determine whether there were any abnormalities in the animal. Blood gases and pressure were measured to monitor the animal during the experiment. Venous pCO2 was measured to determine whether ventilator settings adjustments were necessary to maintain a pCO2 of 50 mm Hg.
Insertion into the RA was followed by device pulsation at 120 bpm for a stabilization period of 15 minutes. CO and blood gas measurements were repeated during this period. The device was pulsated at 300, 30, 240, and 120 bpm after the stabilization period. The sweep gas flowrate and the exiting O2 and CO2 gas fractions were recorded at each beat rate. Completion of all beat rates was followed by the helium system for balloon pulsation refill. The gas exchange measurements were then repeated in a different beat rate order (240, 300, 120, and 30 bpm).
The catheter was then advanced entirely into the IVC region, and another measurement of CO was taken. The entire beat rate protocol described above was repeated. After the beat rate protocol, the gas flowrate and exiting gas fractions were recorded without balloon pulsation with the balloon both full and empty. The catheter was then moved back up to the RA location, and, after prior gas exchange was verified at 120 bpm, the gas flowrate and exiting gas fractions were recorded again without balloon pulsation with the balloon both full and empty. The 13 ml balloon catheter was then removed, the CO remeasured, and the 25 ml device was inserted and tested in an identical manner as the 13 ml balloon catheter. Whereas the 13 ml balloon catheter was tested first in the sheep experiments, the 25 ml balloon catheter was tested first in all but one calf experiment.
Final hematologic and hemodynamic data were taken, including blood samples at the conclusion of each animal study. The animal was deeply anesthetized with isoflurane (5%) and then euthanized with 30 ml of supersaturated KCl, intravenously. This method is consistent with the report of the American Veterinary Medical Association's Panel on Euthanasia.21 All animal studies were performed in accordance with standards of the Guide for Care and Use for Laboratory Animals (NIH publication 86‐23, revised 1996) and were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. At necropsy, the placement of the last device tested within the vena cava was noted. Measurements of the lengths and diameters of vena cava segments were also recorded. This information allowed us to verify the approximate location of all catheter devices tested within that animal.
Data Analysis
Our current focus in developing the respiratory support catheter is in its vCO2, which can be measured directly in these animal implants from the sweep gas flowrate and CO2 fraction (FCO2) in the sweep gas exiting the catheter:
| (1) |
We do not measure the vCO2 from blood side measurements before and after the device because this measurement is not as reliable as gas side measurements and requires knowing the precise blood flow rate past the catheter.
Although our ventilation protocol in animal studies targets a venous blood pCO2 of 50 mm Hg, this can only be typically maintained to within ±5 mm Hg during the course of the animal implant. The vCO2 of the respiratory catheter will change proportionally with these changes in venous pCO2, and so to reduce these variations in our reported data we normalized the CO2 exchange to a venous pCO2 of 50 mm Hg using
| (2) |
to correct for these small (<5 mm Hg) deviations in from our target of 50 mm Hg. This normalization is based on measured in the femoral vein.
Oxygen exchange cannot be reliably determined from sweep gas measurements9 but sometimes is estimated using CO and pO2 in the pulmonary artery with device "on" and "off".14,22 This estimate of O2 exchange, however, does not have sufficient accuracy to reliably quantify changes in gas exchange with different balloon pulsation rates. For this reason, we limited our analysis of gas exchange to the CO2 exchange rate determined from the sweep gas measurements as described.
Statistical Analysis
Statistical comparisons were done using two statistical analysis methods (where applicable). The first being a Student's t‐test assuming equal sample variance and the second method being analysis of variance (ANOVA). Both methods of analysis provide p values for a specific set of comparisons. Differences were considered significant for 0.01 ≤ p < 0.05. Differences were considered not statistically significant for p > 0.05, but trending toward statistical significance if 0.05 ≤ p < 0.10. Those cases in which only one method was used is because of limitations in a particular analysis method.
Results
Pre‐ and postinsertion CO measurements for both animal models are shown in Figure 1. CO values before insertion of the 25 ml device for the calves in this study ranged from 8.9 to 13.7 L/min and averaged 10.7 ± 2.2 L/min, whereas those values for the sheep ranged from 7.2 to 7.5 L/min, with a lower average of 7.4 ± 0.2 L/min. The CO ranges for the 13 ml device were 9.6 to 15.3 L/min, with an average of 11.5 ± 3.3 L/min in the calf and 6.4 to 8.5 L/min, with an average of 7.4 ± 1.5 L/min in the sheep. Postinsertion CO values at 120 bpm were 10.7 ± 2.9 (range, 6.3–15.5) L/min and 5.7 ± 2.3 (range,4.1–7.2) L/min for the 25 ml device in the RA location of the calf and sheep, respectively. For the 13 ml device at 120 bpm, average CO values were 10.6 ± 3.3 (range, 8.6–14.3) L/min and 6.3 ± 1.4 (range, 5.3–7.3) L/min for the calf and sheep, respectively.
Figure 1.
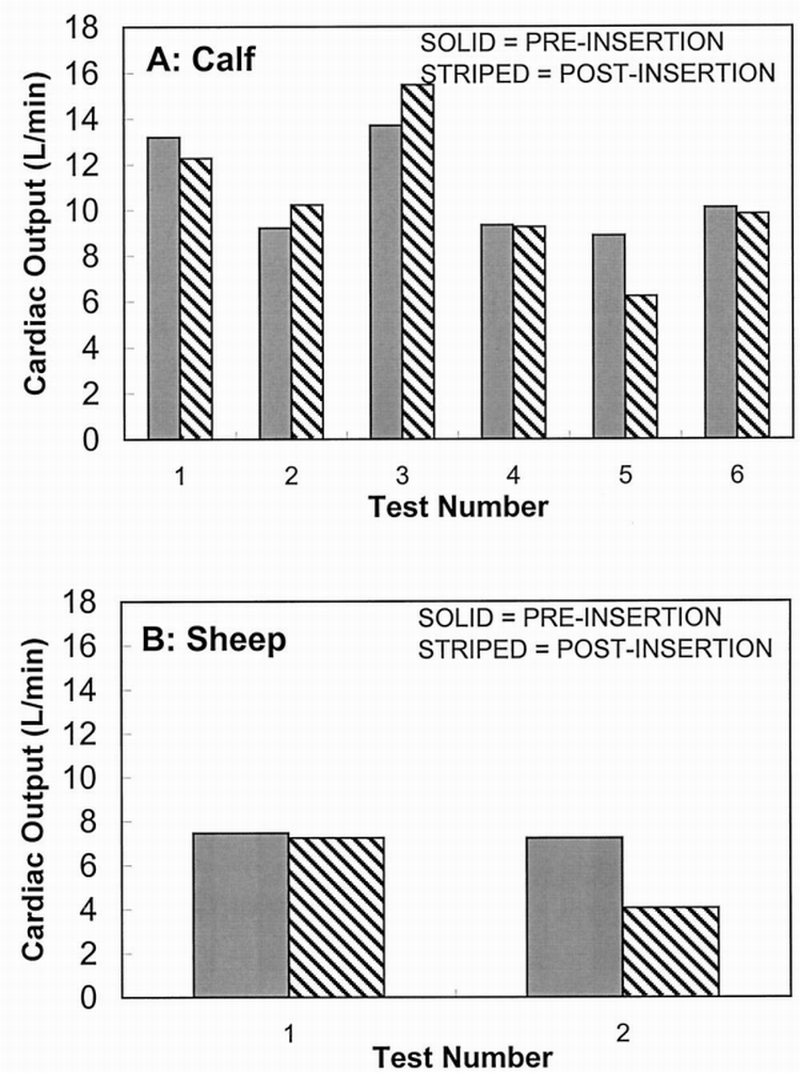
25 ml device cardiac output in the right atrium; pre‐ vs postinsertion at 120 beats per minute.
Figure 2 displays the average CO2 exchange rate (normalized to a venous pCO2 of 50 mm Hg) achieved at maximum balloon pulsation rate in both calf and sheep models for a 25 ml balloon device (Figure 2A) and a 13 ml balloon device (Figure 2B) in the RA and the IVC location (gas exchange values have also been normalized to the surface area of the devices, A = 0.17 m²). The 25 ml calf gas exchange data for RA was statistically significantly higher than IVC, with a Student's t‐test p value of 0.03 (0.04 ANOVA). The 13 ml sheep gas exchange data in the RA compared to the IVC showed a trend toward statistical significance, with a p value of 0.05 for the Student's t‐test but not significant using ANOVA with a p value of 0.25. Gas exchange was not statistically different in the RA versus IVC; Student's t‐test p values were 0.30 (0.14 ANOVA) and 0.65 (0.11 ANOVA) for the 25 ml sheep and 13 ml calf gas exchange data, respectively. The comparison between the two animal models showed no statistical difference when looking at the same device balloon size and same location in the calf and sheep (i.e., a 25 ml device in the RA for sheep vs calf). P values for sheep versus calf were 0.63 (0.53 ANOVA) and 0.79 (0.15 ANOVA) in the RA for 25 ml and 13 ml, respectively; 0.70 (0.98 ANOVA) and 0.90 (0.42 ANOVA) in the IVC for 25 ml and 13 ml, respectively.
Figure 2.
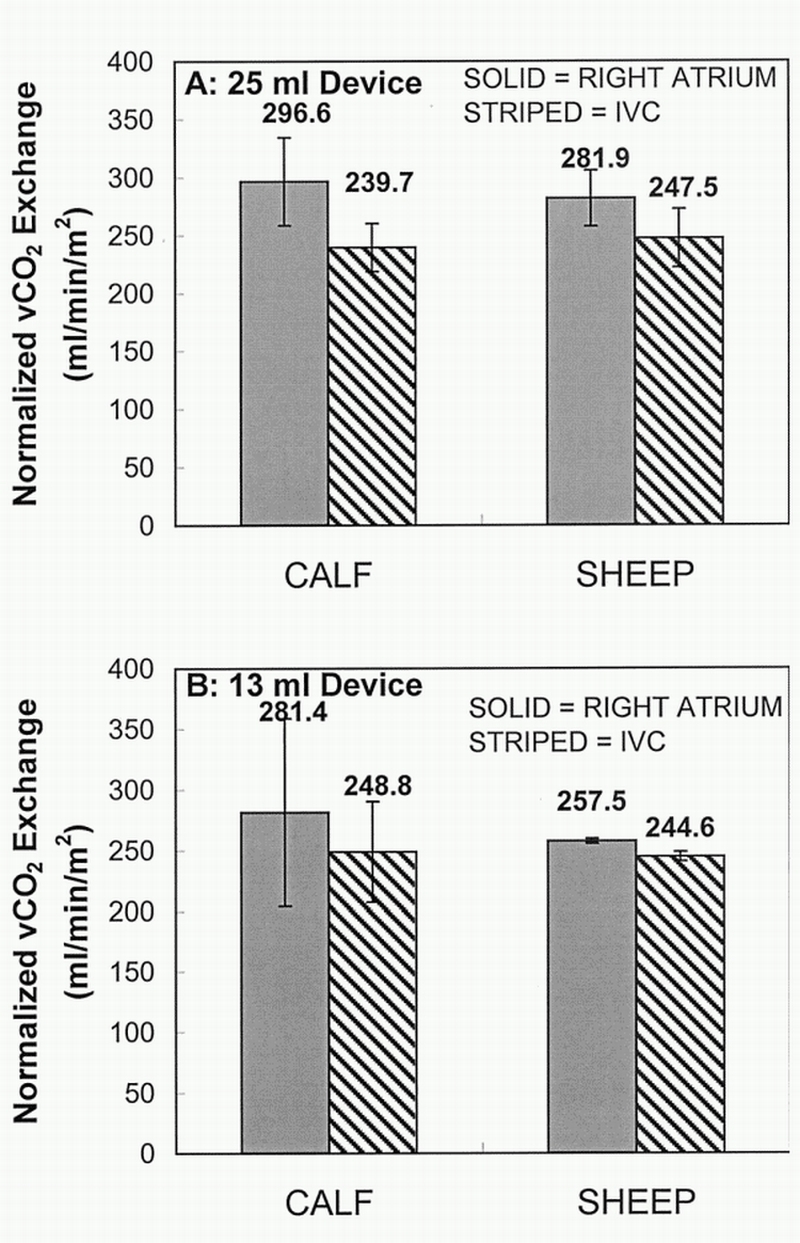
Average maximum CO2 removal rate (vCO2) normalized to a CO2 partial pressure (pCO2) of 50 mm Hg for calf and sheep models. IVC, inferior vena cava.
Figure 3 directly compares average CO2 exchange in the RA location as a function of balloon pulsation for the sheep and calf experiments, whereas Figure 4 makes a similar comparison for the IVC location. In both figures, each animal's CO2 exchange rate at each pulsation rate is normalized to its exchange rate at maximum pulsation (300 bpm). Increase in CO2 exchange from minimum to maximum pulsation ranged from 15 to 35% in the calf experiments for both balloon sizes in both the RA and IVC locations (35 and 32% in RA and 15 and 16% in IVC, 25 mL and 13 mL, respectively). Similarly, the sheep experiments had a CO2 exchange increase range of 23 to 33% (33 and 25% in RA and 23 and 30% in IVC, 25 mL and 13 mL, respectively). There was no statistical difference in gas exchange between balloon size of device in either model (for both the RA and IVC location). Statistical analysis (using both Student's t‐test and ANOVA) showed that all of the above comparisons were not statistically significant, with all p > 0.05 for a given comparison. Beat rate dependence of gas exchange of the 25 ml device is similar to that of the 13 ml device (i.e., there is no statistical difference between the gas exchange data of the two balloon sizes; ANOVA all p > 0.05). At 0 bpm for the 25 ml versus the 13 ml device, only the RA sheep data showed a trend toward statistical significance (p = 0.07 using Student's t‐test). All other data at 0 bpm (comparing the 25 ml with the 13 ml device) was not statistically significant (p = 0.20 for the sheep in IVC, 0.83 and 0.87 for the calf in RA and IVC; all using Student's t‐test).
Figure 3.
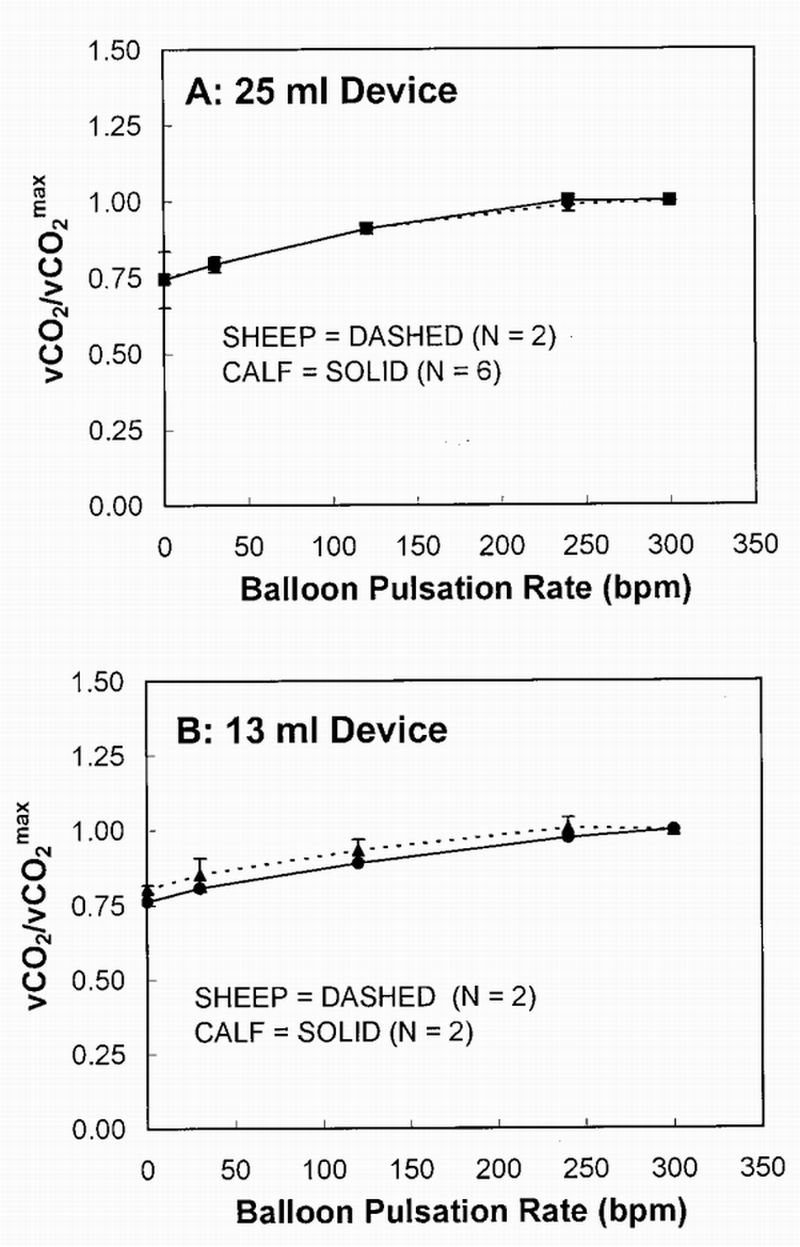
CO2 exchange over maximum CO2 exchange vs balloon pulsation rate in the right atrium location. vCO2, CO2 removal rate.
Figure 4.
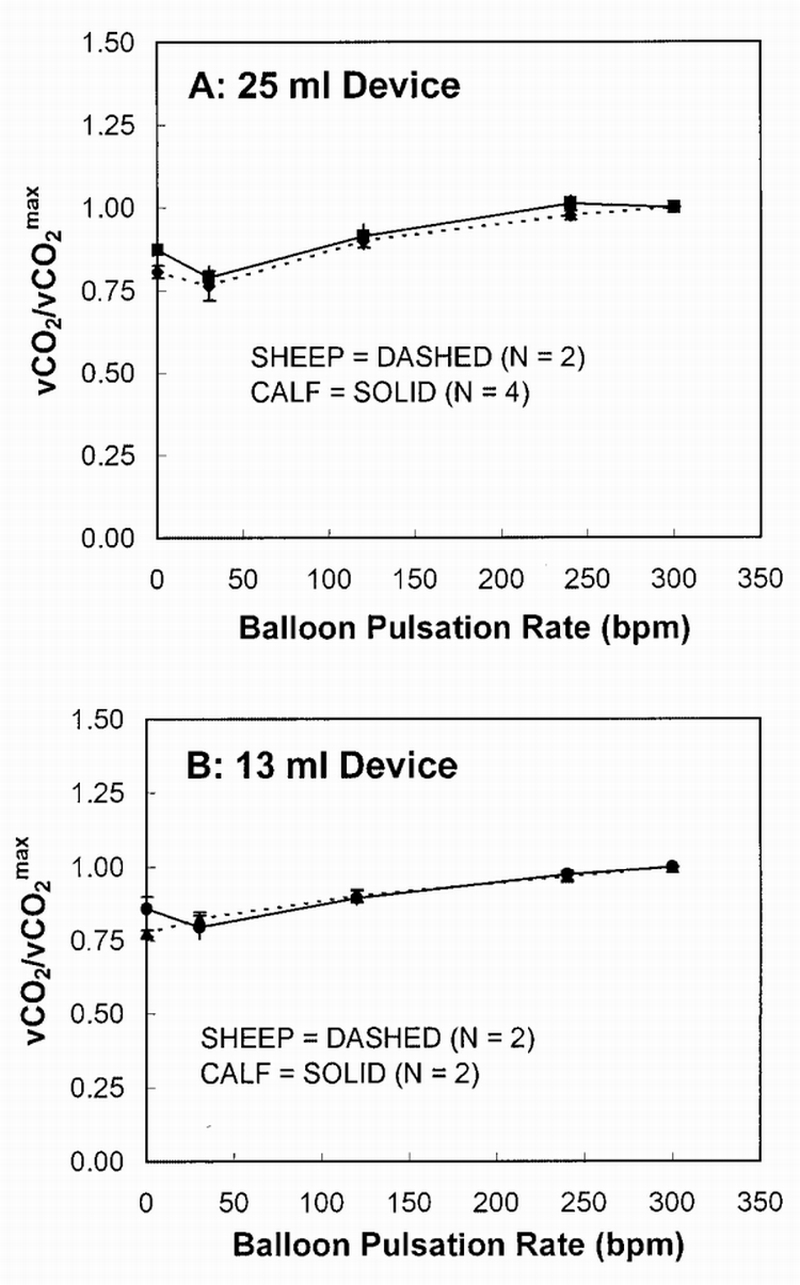
CO2 exchange over maximum CO2 exchange vs balloon pulsation rate in the inferior vena cava location. vCO2, CO2 removal rate.
Free Hb measurements were performed on blood samples taken throughout the experiment. Baseline measurements were compared with results at the termination of the experiment (Figure 5). The average baseline free Hb was 5.25 ± 3.34 mg/dl and 8.94 ± 0.97 mg/dl for the calf and sheep, respectively. Average final free Hb was 6.03 ± 1.49 mg/dl and 10.44 ± 6.63 mg/dl for calf and sheep, respectively. Statistically, there was no significant difference between the baseline and final free Hb measurements for each animal (p = 0.62 calf baseline vs final; p = 0.78 sheep baseline vs final; Student's t‐test and ANOVA resulted in same p values for both comparisons). There was also no significant difference in free Hb between baseline or final values in the two animal models (p = 0.19 baseline calf vs sheep; p = 0.12 final calf vs sheep; Student's t‐test).
Figure 5.
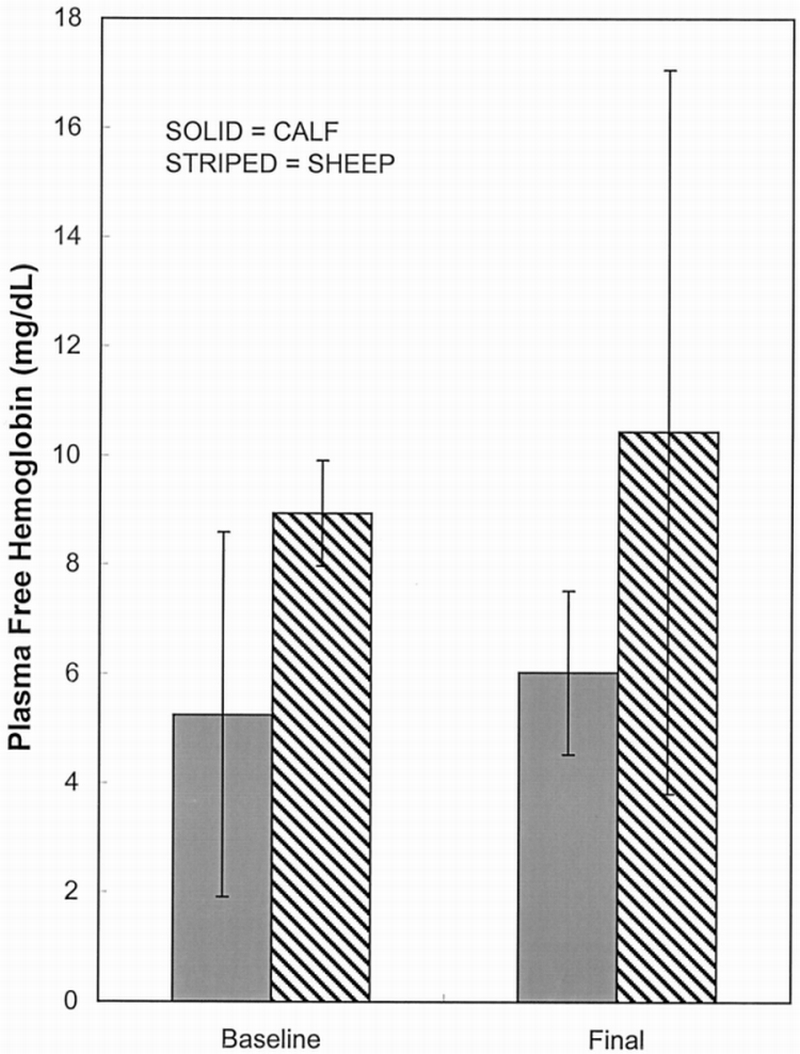
Plasma free hemoglobin data: baseline vs final.
Table 1 shows results of the TBCs done for baseline and final blood samples. These samples were used as a measure of possible abnormalities in the animals and major changes within an animal. In general, results were close to the reference range of an adult specimen. Results within each animal did not change enough to warrant concern by the veterinary surgeon involved in these studies. TBC data was gathered for only one sheep because of difficulties in blood sample shipment associated with post 9/11/01. Comparisons, therefore, cannot be made between the two animal models regarding differences in blood chemistries because of the n = 1 for the sheep model.
Table 1.
Results from Blood Chemistry Samples at Baseline and Final
| Baseline |
Final |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Animal | Hemoglobin (g/dL) | Hematocrit (%) | White Blood Cell (1,000s/mm²) | Platelet Count (1,000s/mm²) | Hemoglobin (g/dL) | Hematocrit (%) | White Blood Cell (1,000s/mm²) | Platelet Count (1,000s/mm²) | |
| Calf 1 | 9.3 | 24.7 | 6.1 | 841 | 10.2 | 27.3 | 13.2 | 584 | |
| Calf 2 | 9.9 | 27.6 | 5.5 | 560 | 9.3 | 25.8 | 9.3 | 628 | |
| Calf 3 | 8.8 | 28 | 5.4 | 474 | 9 | 26.5 | 20.8 | 271 | |
| Calf 4 | 10.6 | 27.8 | 9.2 | 464 | 9 | 26.5 | 11.2 | 316 | |
| Calf 5 | 10.1 | 30.2 | 6.4 | N/A | 10.2 | 25.2 | 6.9 | 355 | |
| Calf 6 | 9.6 | 27.9 | 7.7 | 398 | 9.8 | 27.9 | 11.3 | 276 | |
| Calf reference rangea | 10.0–15.0 | 30.0–46.0 | 4.0–11.0 | 100–800 | |||||
| Sheep 1 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Sheep 2 | 8.1 | 22.1 | 4.3 | 402 | 7.5 | 20.6 | 6 | 289 | |
| Sheep reference rangea | 9.0–14.0 | 23.0–39.0 | 4.0–13.0 | 250–750 | |||||
For adult specimen.
Discussion
Our group is developing a respiratory assist catheter primarily intended for supplementing CO2 removal in patients with acute exacerbation of COPD.8–10 This group of patients suffers principally from CO2 retention because oxygenation can be adequately managed with nasal oxygen.11 This study evaluated and compared the CO2 removal performance of our respiratory assist catheter in acute sheep implants with acute calf implants. Although our prior animal implants were performed in calves,9 a well‐accepted model for cardiovascular device implants in which we have considerable experience,23 the primary rationale for conducting comparative acute implants in sheep versus calves was to study our respiratory catheter in an animal model with a CO closer to average human values. The sheep in these studies had an average CO of 5.7 L/min with the catheter inserted as compared with 10.7 L/min for the calf. CO rather than cardiac index is the appropriate blood flow basis for comparison because it is the magnitude of blood flowrate past the catheter (IVC and SVC pathways) in sheep and calf that can directly affect the gas exchange performance of the catheter.10
Our studies of the catheter in sheep versus calf implants focused on several principal questions. First, we wanted to investigate whether the vCO2 of the catheter differed in sheep compared with calves. Overall, we found that CO2 exchange rates for the respiratory catheter in the calf implants was 5 to 10% greater than in the sheep implants, but these differences did not achieve statistical significance. We were also interested in determining whether our pulsating respiratory catheter when implanted in sheep would exhibit a greater dependence of gas exchange on balloon pulsation rate because of the lower CO in sheep. In initial acute implant tests of our respiratory catheter in calves,9 we found a 13% increase in CO2 exchange (compared with no balloon pulsation) at the maximum balloon pulsation rate of 300 bpm for respiratory catheters. In contrast, in our bench tests of the same respiratory catheters (25 ml balloons) placed within a mock vena cava (7/8 to 1 inch tube) and perfused with 37°C water at 3 L/min, balloon pulsation increased CO2 exchange by 160 to 180% at 300 bpm.5,24
Our initial hypothesis was that the reduced pulsation rate dependence of gas exchange in animal implants may be because of the level of blood flow past the device. The preinsertion CO in our initial acute calf implant tests ranged from 10 to 14 L/min, significantly greater than the average CO of 5 L/min generally reported for humans.25 Von Segesser et al.26,27 and Tao et al.28 showed in bench and animal tests that increasing the blood flowrate past the IVOX respiratory catheter (a passive respiratory catheter with no balloon) increased gas exchange, a not unexpected result because greater flow velocities past fiber surfaces will decrease the diffusional boundary layers that dictate gas exchange.5 If lower blood flow past the catheter in the absence of balloon pulsation results in lower gas exchange, the potential exists for balloon pulsation to generate a greater enhancement of gas exchange in a lower blood flow environment. Our previous ex vivo studies in calves10 support this hypothesis. For example, balloon pulsation at 180 bpm (the maximum pulsation rate that could be achieved with the 40 ml balloons used in the ex vivo studies) increased gas exchange by 135% at 1 L/min, but the increase was only 43% at 4.5 L/min. Nevertheless, in this study, we found no significant difference in the effect of balloon pulsation on gas exchange in the sheep implants compared with the calf implants despite the lower CO in the sheep.
The comparisons of the vCO2 and the extent of balloon enhanced gas exchange in sheep versus calves were also not significantly affected by the location of the catheter in the vena cava (IVC vs RA positions) or the balloon size (13 vs 25 ml) used in the catheter. There are several reasons why we may not have seen a difference in the effect of balloon pulsation on gas exchange in the sheep compared with the calf. First, we cannot directly measure the blood flowrate past the catheter in the vena cava, and, therefore, we use CO only as an index of relative blood flowrate differences between the sheep and the calf. This index may be too indirect or may not be appropriate if there are other significant differences in venous hemodynamics between the sheep and calf, for example in the fractions of CO returning through the IVC versus SVC or the effect of venous collateral veins bypassing these pathways. Another factor may be that the blood flowrate past the respiratory catheter in the sheep was indeed smaller than in the calf, but, because of the smaller cross‐sectional area of the sheep vena cava, the average blood flow velocities past the catheter are comparable in the sheep and calf (IVC OD measurements were 21.8 and 25.7 mm for the sheep and calf, respectively, and SVC OD measurements were 16.9 and 19.7 mm for the sheep and calf, respectively). This smaller vasculature in the sheep might account for the drop in CO for sheep number 2 in Figure 1. The decrease in CO was most likely associated with some drop in venous return because of flow resistance in the vena cava by the device (a drop in CO was almost always seen in calf experiments in which a 40 ml device with larger OD was inserted). The similar gas exchange rates in the absence of balloon pulsation in the sheep and calf may suggest that the catheter was in similar blood flow environments in the vena cava of these animals. An interesting series of tests are those performed by Mihaljevic et al.,29 who lowered CO within the same animal by infusing dopamine, nitroglycerin, and nor‐adrenaline to reduce cardiac contractility. In pilot studies, we infused esmolol (250 mg bolus, drip at 10 ml/hr of 5000 mg esmolol/500 ml NaCl) at the end of a calf experiment but were unsuccessful at significantly lowering CO over a long enough period to study gas exchange and the effect of balloon pulsation.
The effect of balloon pulsation on gas exchange in the sheep and calf implants differs from that seen in bench and in ex vivo tests using the mock vena cava10,24 in which balloon pulsation generally has a much larger impact on gas exchange of the catheter.24 There are several possible reasons worth discussing. The flow environment in vivo most likely differs from the flow environment in our mock vena cava test section. For example, flow patterns in the vena cava are influenced by the presence of bidirectional blood flow (SVC vs IVC directions) past the catheter30,31 and veins draining into the vena cava distributed along its length. A bench test of the respiratory catheter that attempts to incorporate a more realistic venous flow environment for the catheter would not be feasible because in vivo flow patterns would be difficult to quantify and mimic, and variability most likely exists among animals even of the same species. We also do not think that cavitation results from the balloon pulsation of the catheter in vivo. In some of our animal experiments, we measured pressure oscillations in the vena cava immediately adjacent to the fiber bundle using a high fidelity Millar pressure sensor (MPC‐500, Millar Instruments, Inc., Houston, TX). Pressure oscillations caused by balloon pulsation were nearly undetectable (less than ∼ 1–2 mm Hg) because of the large overall compliance of the venous system. Blood cavitation requires more significant negative pressure swings, and, if it were to occur, it would most likely enhance mass transfer because of local mixing associated with bubble formation and collapse.32 We found less balloon enhanced gas exchange in vivo than in bench tests.
The gas exchange studies in sheep reported here were also important because the IVOX respiratory catheter, which underwent human clinical trials in the early 1990s, used sheep for its animal implant studies.14,15 Our respiratory catheter with a 25 ml balloon had a CO2 exchange rate of 282 and 247 ml/min/m² in the RA and IVC positions, respectively, in the sheep. This compares favorably with the CO2 exchange rate of 191 ml/min/m² reported by Zwischenberger et al.22 for the IVOX catheter in acute sheep implants. Our goal for CO2 removal in COPD patients with acute exacerbation is 75 ml/min, a level that we expect will avoid intubation and mechanical ventilation of these patients. The 0.17 m² respiratory catheters tested here achieved from 43 to 48 ml/min of CO2 removal in the sheep. On the basis of these studies, we are developing a full‐size version of our respiratory catheter with a surface area of 0.3 m²2 that can be percutaneously inserted in patients for treatment of COPD with acute exacerbation.
Acknowledgments
This work was supported by the US Army Medical Research Development, Acquisition, and Logistics Command under contract No. DAMD 17‐98‐1‐8638 and the National Institutes of Health (NIH), Heart, Lung, and Blood Institute grant No. HL70051. The views, opinions, or findings contained in this report are those of the authors and should not be construed as an official position, policy, or decision of the Department of the Army or NIH unless so designated by other documentation. The authors thank the University of Pittsburgh's McGowan Institute for Regenerative Medicine for its support.
References
- 1.Conrad SA, Eggerstedt JM, Grier LR, Morris VF, Romero MD. Intravenacaval membrane oxygenation and carbon dioxide removal in severe acute respiratory failure. Chest. 1975;107:1689–1697. doi: 10.1378/chest.107.6.1689. [DOI] [PubMed] [Google Scholar]
- 2.Brunet F, Mira JP, Cerf C, et al. Permissive hypercapnia and intravascular oxygenator in the treatment of patients with ARDS. Artif Organs. 1994;18:826–832. doi: 10.1111/j.1525-1594.1994.tb03331.x. [DOI] [PubMed] [Google Scholar]
- 3.Durbin CG., Jr Intravenous oxygenation and CO2 removal device: IVOX. Respir Care. 1992;37:147–153. [Google Scholar]
- 4.Jurmann MJ, Demertzis S, Schaefers HJ, Wahlers T, Haverich A. Intravascular oxygenation for advanced respiratory failure. ASAIO J. 1992;38:120–124. [PubMed] [Google Scholar]
- 5.Hattler BG, Federspiel WJ. Gas exchange in the venous system: support for the failing lung. In: Vaslef SN, Anderson RW, editors. The Artificial Lung. Georgetown, TX: Landes Bioscience; 2002. pp. 133–174. [Google Scholar]
- 6.Weinacker AB, Vaszar LT. Acute respiratory distress syndrome: physiology and new management strategies. Annu Rev Med. 2001;52:221–237. doi: 10.1146/annurev.med.52.1.221. [DOI] [PubMed] [Google Scholar]
- 7.Fleury B, Murciano D, Talamo C, Aubier M, Pariente R, Milic‐Emili J. Work of breathing in patients with chronic obstructive pulmonary disease in acute respiratory failure. Am Rev Respir Dis. 1985;131:822–827. doi: 10.1164/arrd.1985.131.6.822. [DOI] [PubMed] [Google Scholar]
- 8.Federspiel WJ, Hout MS, Hewitt TJ, et al. Development of a low flow resistance intravenous oxygenator. ASAIO J. 1997;43:M725–M730. [PubMed] [Google Scholar]
- 9.Golob JF, Federspiel WJ, Merrill TL, et al. Acute in‐vivo testing of an intravascular respiratory support catheter. ASAIO J. 2001;47:432–437. doi: 10.1097/00002480-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Federspiel WJ, Golob JF, Merrill TL, et al. Ex vivo testing of the intravenous membrane oxygenator. ASAIO J. 2000;46:261–267. doi: 10.1097/00002480-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Rosen RL, Bone RC. Treatment of acute exacerbations in chronic obstructive pulmonary disease. Obstruct Lung Dis. 1990;74:691–700. doi: 10.1016/s0025-7125(16)30545-4. [DOI] [PubMed] [Google Scholar]
- 12.Hewitt TJ, Hattler BG, Federspiel WJ. A mathematical model of gas exchange in an intravenous membrane oxygenator. Ann Biomed Eng. 1998;26:166–178. doi: 10.1114/1.53. [DOI] [PubMed] [Google Scholar]
- 13.Federspiel WJ, Hewitt T, Hout MS, et al. Recent progress in engineering the Pittsburgh intravenous membrane oxygenator. ASAIO J. 1996;42:M435–M442. doi: 10.1097/00002480-199609000-00026. [DOI] [PubMed] [Google Scholar]
- 14.Cox CS, Jr, Zwischenberger JB, Traber LD, Traber DL, Herndon DN. Use of an intravascular oxygenator/carbon dioxide removal device in an ovine smoke inhalation injury model. ASAIO Trans. 1991;37:M411–M413. [PubMed] [Google Scholar]
- 15.Zwischenberger JB, Nguyen TT, Tao W, et al. IVOX with gradual permissive hypercapnia: a new management technique for respiratory failure. J Surg Res. 1994;57:99–105. doi: 10.1006/jsre.1994.1117. [DOI] [PubMed] [Google Scholar]
- 16.Tao W, Bidani A, Cardenas VJ, Jr, Niranjan SC, Zwischenberger JB. Strategies to reduce surface area requirements for carbon dioxide removal for an intravenacaval gas exchange device. ASAIO J. 1995;41:M567–M572. doi: 10.1097/00002480-199507000-00075. [DOI] [PubMed] [Google Scholar]
- 17.Tao W, Schroeder T, Bidani A, et al. Improved gas exchange performance of the intravascular oxygenator by active blood mixing. ASAIO J. 1994;40:M527–M532. doi: 10.1097/00002480-199407000-00056. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen TT, Zwischenberger JB, Tao W, et al. Significant enhancement of carbon dioxide removal by a new prototype IVOX. ASAIO J. 1993;39:M719–M724. [PubMed] [Google Scholar]
- 19.Federspiel WJ, Hattler BG. Sweep gas flowrate and CO2 exchange in artificial lungs. Artif Organs. 1996;20:1050–1052. doi: 10.1111/j.1525-1594.1996.tb04593.x. [DOI] [PubMed] [Google Scholar]
- 20.Hout MS, Hattler BG, Federspiel WJ. Validation of a model for flow‐dependent carbon dioxide exchange in artificial lungs. Artif Organs. 2000;24:114–118. doi: 10.1046/j.1525-1594.2000.06465.x. [DOI] [PubMed] [Google Scholar]
- 21.Beaver B, Reed W, Leary S, et al. Report of the AVMA Panel on Euthanasia. J Am Vet Med Assoc. 2001;218:669–696. doi: 10.2460/javma.2001.218.669. [DOI] [PubMed] [Google Scholar]
- 22.Zwischenberger JB, Cox CS, Graves D, Bidani A. Intravascular membrane oxygenation and carbon dioxide removal: a new application for permissive hypercapnia? Thorac Cardiovasc Surg. 1992;40:115–120. doi: 10.1055/s-2007-1020127. [DOI] [PubMed] [Google Scholar]
- 23.Griffith BP, Kormos RL, Borovetz HS, et al. HeartMate II left ventricular assist system: from concept to first clinical use. Ann Thorac Surg. 2001;71:S116–S120. doi: 10.1016/s0003-4975(00)02639-4. [DOI] [PubMed] [Google Scholar]
- 24.Hattler BG, Lund LW, Golob JF, et al. A respiratory gas exchange catheter: In vitro and in vivo tests in large animals. J Thorac Cardiovasc Surg. 2002;124:520–530. doi: 10.1067/mtc.2002.123811. [DOI] [PubMed] [Google Scholar]
- 25.Cooney D. Biomedical Engineering Principles: An Introduction to Fluid, Heat, and Mass Transport Processes. New York: Marcel Dekker; 1976. [Google Scholar]
- 26.Tonz M, von Segesser LK, Leskosek B, Turina MI. Quantitative gas transfer of an intravascular oxygenator. Ann Thorac Surg. 1994;57:146–150. doi: 10.1016/0003-4975(94)90383-2. [DOI] [PubMed] [Google Scholar]
- 27.von Segesser LK, Tkebuchava T, Marty B, Leskosek B, Tevaearai H. Intravascular gas transfer. Membrane surface area and sweeping gas flows are of prime importance. ASAIO J. 1997;43:M457–M459. [PubMed] [Google Scholar]
- 28.Tao W, Zwischenberger JB, Nguyen TT, et al. Performance of an intravenous gas exchanger (IVOX) in a venovenous bypass circuit. Ann Thorac Surg. 1994;57:1484–1490. doi: 10.1016/0003-4975(94)90105-8. [DOI] [PubMed] [Google Scholar]
- 29.Mihaljevic T, von Segesser LK, Tonz M, Leskosek B, Turina MI. Influence of hemodynamics on the performances of intravascular gas exchangers. Ann Thorac Surg. 1995;60:1665–1670. doi: 10.1016/0003-4975(95)00647-8. [DOI] [PubMed] [Google Scholar]
- 30.Berne R, Levy M. Cardiovascular Physiology. St. Louis, MO: Mosby‐Year Book; 1997. [Google Scholar]
- 31.Golob JF, Federspiel WJ, Merrill TL, Frankowski BJ, Hattler BG. Analysis of in‐vivo flow effects on the in‐vitro testing of intravascular oxygenators. ASAIO J. 1999;45:126. [Google Scholar]
- 32.Li H, Ohdaira E, Ide M. Effect of ultrasound on driving force of diffusion dialysis. Japan J Appl Phys. 1997;36:3138–3139. [Google Scholar]


