Abstract
Calbindin-D28k, a calcium binding protein that is thought to act as a facilitator of calcium diffusion in intestine and kidney, is known to be regulated by vitamin D in these tissues. Calbindin-D28k is also present in pancreatic β cells, but its function in these cells is not known. To determine a role for calbindin-D28k in the β cell, rat calbindin-D28k was overexpressed in the pancreatic β cell line RIN 1046-38 by transfection of calbindin in expression vector, and changes in insulin mRNA were examined. Five transfected RIN cell clones were found to overexpress calbindin 6- to 35-fold as determined by radioimmunoassay. Northern blot analysis revealed increases in abundance in calbindin mRNA (>20-fold for most clones). Overexpressed calbindin was functional because it was capable of buffering calcium in response to a rapid calcium influx induced by 1 and 5 μM calcium ionophore. In cells transfected with calbindin, there was a marked increase in the expression of insulin mRNA (>20-fold for most clones compared with vector transfected cells). Besides an increase in insulin mRNA, calbindin overexpression was also associated with an increase in insulin content and release (a 5.8-fold increase in insulin release was noted for clone C10, and a 54-fold increase was noted for clone C2). To begin to address the mechanism whereby overexpression of calbindin results in increased insulin gene expression, calbindin-overexpressing clones were transiently transfected with plasmids incorporating various regions of the rat insulin I (rInsI) promoter linked to the chloramphenicol acetyltransferase coding sequence. Transient transfection with reporter plasmids bearing the regulatory sequences of the rInsI promoter (−345/+1) or five copies of the Far-FLAT minienhancer (−247/−198) from the rInsI promoter suggests that increased insulin mRNA in calbindin transfected cells is due, at least in part, to enhanced insulin gene transcription. These studies provide the first direct evidence (to our knowledge) for a role for calbindin in β cell function.
Calbindin-D28k belongs to a family of high affinity calcium-binding proteins that includes calmodulin, parvalbumin, troponin C, and S100 protein (1). Calbindin-D28k is present in the highest concentrations in avian intestine and in avian and mammalian kidney, brain, and pancreas (2, 3). In intestine and kidney, calbindin is regulated by 1,25-dihydroxyvitamin D3. However, in brain calbindin is not influenced by vitamin D status. Various functions have been proposed for calbindin based on its calcium-binding properties, i.e., facilitation of the diffusional flux of calcium in the intestinal enterocyte (4), protection of neurons against excitatory calcium toxicity (5), and action as a mobile calcium buffer restricting evoked calcium signals in nerve synapses and hair cells (6, 7). In the pancreas, early autoradiographic and immunocytochemical studies have localized 1,25-dihydroxyvitamin D3 receptors (8) and calbindin (9), respectively, to the β cells. It had been suggested that vitamin D may be acting in the pancreatic β cell by modulating intracellular calcium, perhaps by mechanisms involving calbindin. However, in spite of these early findings, the role of 1,25-dihydroxyvitamin D3 and calbindin in β cell function is not yet clear (10).
It is well known that calcium plays an important role in β cell stimulus–secretion coupling and is regulated by glucose and other secretagogues. Uptake and metabolism of glucose by the β cell result in the closure of ATP regulated K+ channels, causing membrane depolarization and opening of voltage-gated L-type Ca2+ channels which results in transient rises in the intracellular free calcium concentration ([Ca2+]i; refs. 11–13). Glucose induced insulin secretion has been reported to occur in a pulsatile and oscillatory manner coincident with intracellular calcium oscillations (14). The β cell can also be affected by activation of receptors coupled to effector systems such as the phospholipase system or adenylate cyclase (11, 12). Activation of protein kinase C has also been reported to modulate insulin secretion (11, 15, 16). Thus stimulus secretion coupling in the β cell is a result of a complex calcium-mediated transduction pathway that is not yet clearly understood. It has been suggested that calcium-binding proteins such as calmodulin (17) and calcyclin (18) are important mediators of this stimulus–secretion response in the β cell following the rise in [Ca2+]i.
In previous studies, we found that treatment of the rat insulinoma cell line RIN 1046-38 with 2 mM butyrate induced calbindin-D28k mRNA and protein levels in accord with the induction by butyrate of insulin content and secretion, suggesting a possible role for calbindin as a modulator of the insulin secretory process (10). To further investigate the role of calbindin in β cell function, we used a eukaryotic expression vector to overexpress calbindin in RIN cells and studied its effects on insulin expression and transcription.
MATERIALS AND METHODS
Plasmids and Probes.
Calbindin-D28k cDNA was isolated by PCR from cDNA prepared from rat renal distal tubular mRNA, and the identity of the calbindin insert was confirmed by sequencing as described (19). The calbindin cDNA insert was cloned into the plasmid pREP4 (Invitrogen) to create an expression plasmid designated pREP4-CB28 as described (19). The chimeric plasmids pFOX-CAT.RIP (−85 to +1), pFOX-CAT (−345 to +1), pFOX-CAT.RIP (−85 to +1).5 FF, and pFOX-CAT were kindly provided by M. German (University of California, San Francisco) and have been described previously (20). Briefly, region −85 to +1 represents the minimal rat insulin I (rInsI) promoter, −345 to +1 represents all the known positive and negative regulatory sequences of the insulin promoter, and (−85 to +1).5 FF represents five copies of the glucose-responsive region of the insulin promoter, the Far-Flat (FF) region, ligated to rInsI promoter (20). pFOX-CAT is the promoterless parent vector. All constructs contain the chloramphenicol acetyltransferase (CAT) gene as the reporter.
The mouse calbindin-D28k cDNA used for Northern blot analysis was a 1.2-kb insert from the EcoRI site of pIBI76 (21). The mouse insulin cDNA, a 0.68-kb insert present in the RsaI–EcoRI site of pGEM, was obtained from S. Efrat (Albert Einstein College of Medicine, Bronx, NY). The cDNA for 18S rRNA was a gift of R. Guntaka (University of Missouri, Columbia, MO). Human GLUT1 cDNA (phGLUT1) and rat GLUT2 cDNA (prGLUT2–1) were obtained from Graeme Bell (University of Chicago, Chicago).
Stable Transfection of RIN 1046-38 Cells and Clonal Selection.
About 50% confluent RIN 1046-38 cells (passage > 50, grown in RPMI 1640 media) in T-75 flasks were used to carry out stable transfection by lipofectin (GIBCO/BRL). Ten micrograms each of pREP 4 (vector alone) and pREP–calbindin-D28k were mixed with 50–75 μl of lipofectin, added to cells in serum-free media, and incubated for 18 h at 37°C. Serum was added the next day. On the third day after transfection, RPMI 1640 medium containing the antibiotic hygromycin (400 μg/ml) was added to the cells. After 4 weeks of hygromycin selection, colonies were handpicked under sterile conditions and propagated in 24-well plates.
Western Blot Analysis and Radioimmunoassay (RIA).
Cell extracts of various clones were prepared by freeze–thawing the cells in 50 mM Tris·HCl buffer (pH 7.8). The supernatant solution obtained after centrifugation at 14,000 × g for 10 min at 4°C was then used for protein estimation using the method of Bradford (22). Five micrograms of protein of each clone was analyzed by Western blot analysis using 125I-labeled protein A as described (25).
Calbindin protein levels were determined by RIA using antiserum against rat renal calbindin and purified rat renal calbindin-D28k as the standard (24).
mRNA Isolation and Northern Blot Analysis.
Total RNA was isolated from various clones by the guanidinium isothiocyanate-phenolchloroform method (25) and poly(A)+ RNA was selected by oligo(dT) cellulose affinity chromatography. Northern blotting was performed as described (23). cDNAs were labeled with [32P]dCTP to a specific activity of 108–109 cpm/μg DNA using a random primed DNA labeling kit (Boehringer Mannheim). After probing with calbindin-D28k or insulin cDNAs, the filters were rehybridized with 18S rRNA cDNA (control probe). A 40-fold range of calbindin-transfected and vector-transfected RIN cell mRNA was used to determine linearity. The relative optical density for each Northern blot probed with calbindin and insulin cDNAs was divided by the relative optical density obtained after probing with 18S rRNA cDNA to normalize for sample variation. Intensities of autoradiographs of varying exposures were quantitated by densitometry using a Dual-Wavelength Flying Spot Scanner (Shimadzu). Efforts were made to expose autoradiograms such that the optical density values would fall within the linear response range. However, when insulin and calbindin mRNAs in certain calbindin-transfected clones were compared with insulin and calbindin mRNA in vector-transfected cells in the same Northern blot, after the longer autoradiographic exposure needed to observe the results in vector-transfected cells, levels of mRNA in the calbindin-transfected cells were found to be beyond the linear range of film sensitivity. Quantitation of mRNA for those calbindin-transfected clones is, therefore, expressed as greater than the maximum observed in the linear range.
Immunocytochemical Analysis for Insulin.
Cells were grown on glass coverslips in RPMI 1640 medium and then fixed in 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. Cells were stained with a guinea pig anti-insulin antibody (1:1000) overnight at 4°C, followed by fluorescein coupled (Fab)2 goat anti-guinea pig antibody at 1:1000 for 2 h at room temperature. Cells were examined with epifluorescence microscopy using a Zeiss Axiophot system and a fluorescein filter set, and were photographed using Kodak EPH film.
Insulin Release and Insulin Content.
The clones under study were grown in RPMI 1640 medium containing 11 mM glucose for 2–4 days. The medium was removed and a fresh serum-free medium containing 11 mM glucose was added. The insulin concentration in the medium was determined by ELISA (26) and by RIA (Insulin 125I RIA kit from Incstar, Stillwater, MN) using a rat insulin standard and guinea pig anti-insulin serum. Insulin release is expressed as nanograms of insulin per 106 cells per hour. For the ELISA, rat insulin was from Linco Research Immunoassay (St. Charles, MO) and guinea pig anti-porcine insulin antibody (M8309) was from Nordisk (Dagsvaert, Denmark). The number of cells was obtained by counting after incubation. Insulin content was determined from the same cultures used for insulin release. For measurement of cellular insulin content, the cells were sonicated in 1 M acetic acid/0.1% BSA. Values for insulin content and release were obtained from four separate experiments using three different cultures of either RIN cells transfected with vector alone or calbindin-transfected clones C2 or C10 per experiment.
Intracellular Ca2+ Measurement.
Intracellular Ca2+ was determined using dual excitation wavelength fluorometry of fura-2 on individual cells subcultured onto coverslips for 18–48 h (27). Fura-2 fluorescence (510-nm emission with alternate 334- and 380-nm excitation by means of a mercury lamp) were imaged with an oil immersion Nikon UV-fluor objective (×40, n.a. 1.4) using an inverted microscope (Zeiss Axiovert). Video frames containing images of fura-2 fluorescence of cells illuminated at 334 and 380 nm were digitized at a resolution of 512 × 512 with 8-bit resolution. Cells of interest were outlined and intensity values recorded throughout the experiment. The [Ca2+]i for each cell was estimated from the ratio of 334- and 380-nm fluorescence intensity. Values from the 28–48 cells monitored for each experiment were then averaged (27). Bromo-A23187 (Molecular Probes) was dissolved at a concentration of 5 mM in dimethyl sulfoxide and diluted in buffer to the indicated concentration.
Transient Transfections and CAT Assays.
Clones were grown in RPMI 1640 medium (GIBCO/BRL) supplemented with 10% fetal bovine serum (GIBCO/BRL) and maintained using 400 μg/ml hygromycin. All transfections were carried out by the DEAE-dextran method (28) using 5 μg of chimeric plasmid and 5 μg of pXGH (human growth hormone plasmid controlled by metallothionein promoter from Nichols Institute Diagnostics, San Juan Capistrano, CA). The positive control was pRSV-CAT (3 μg). Cell extracts were prepared 60–65 h after transfection. CAT assays were performed at constant growth hormone values (Allegro hGH kit from Nichols Institute Diagnostics) using standard protocols (28). Acetylated and nonacetylated forms of chloramphenicol were separated by TLC and visualized by autoradiography. CAT activity was quantitated by densitometric scanning of the autoradiograms.
Statistical Analysis.
Results are presented as the mean ± SE; data were analyzed with Student’s unpaired t test or Dunnett multiple comparisons test.
RESULTS
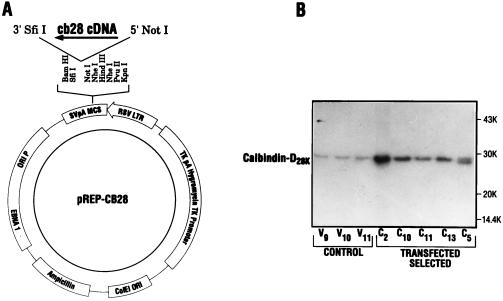
To determine a role for calbindin-D28k in the β cell, rat calbindin-D28k was overexpressed in the rat pancreatic β cell line RIN 1046-38 by transfection of calbindin in the eukaryotic expression vector pREP4 (Fig. 1A). Five positive clones designated C2, C10, C11, C13, and C5 were found to overexpress calbindin as determined by Western blot analysis (Fig. 1B). Levels of calbindin-D28k as measured by RIA in the positive clones overexpressing calbindin are shown in Table 1. The average concentration of calbindin in cells transfected with vector alone was 0.4 ± 0.1 μg/mg protein. Clone C2 expressed the highest level of calbindin (14.2 ± 1.7 μg/mg protein), which represented a 35.5-fold induction in calbindin. Clone C10 expressed the next highest level of calbindin (3.7 ± 0.4 μg/mg protein), followed by C13, C5, and C11. Thus, as shown in Table 1, positive clones were found to overexpress calbindin from 6.3-fold (for C11) to a higher level of overexpression of 35.5-fold for C2.
Figure 1.
Stable transfection and overexpression of calbindin-D28k (CB28) in RIN 1046 cells. (A) pREP-CB28 construct. (B) Western blot analysis of clones transfected with either vector alone (V9, V10, and V11) or pREP-CB28 (C2, C10, C11, C13, and C5). The results shown are representative of results obtained in five separate experiments. (The split band in clone C5 is likely an artifact of proteolysis and was not otherwise seen in several other Western blots of calbindin expression in C5 and other clones.)
Table 1.
Levels of calbindin-D28k in selected transfected RIN cells as determined by RIA
| Clones | Calbindin-D28k, μg/mg protein | Induction, -fold |
|---|---|---|
| Control (transfected with vector alone) | 0.4 ± 0.1 | |
| C2 | 14.2 ± 1.7 | 35.5 |
| C10 | 3.7 ± 0.4 | 9.3 |
| C13 | 3.3 ± 0.1 | 8.3 |
| C5 | 3.2 ± 0.6 | 8.3 |
| C11 | 2.5 ± 0.2 | 6.3 |
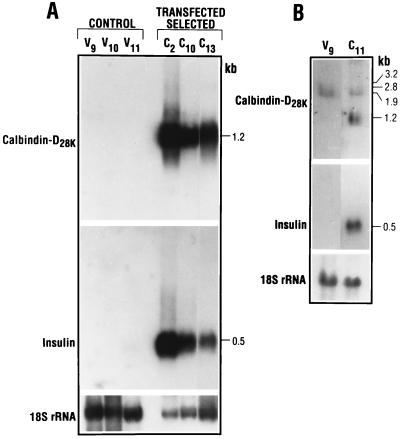
Northern blot analysis indicated an increase in calbindin-D28k mRNA in RIN cells transfected with pREP–calbindin-D28k (Fig. 2). Similar to the results observed for calbindin protein, calbindin-D28k mRNA was most abundant in clone C2. High levels were also found in clones C10 and C13. Note that, as shown in Fig. 2, corresponding increases in insulin mRNA were observed in the different positive clones. Clone C11, which was found to contain the least amount of calbindin protein among the five positive clones, was also found to express lower levels of calbindin mRNA (5.5 ± 1-fold greater than RIN cells transfected with vector alone compared with >30-fold induction of calbindin mRNA observed for clones C2, C10 and C13; Fig. 2B). Although lower levels of calbindin mRNA were induced in C11, insulin mRNA was also induced in this clone (Fig. 2B). Clones C2 and C10 were used for further investigation in this study.
Figure 2.
Northern blot analysis of RIN cell mRNA. (A) Northern analysis of vector-transfected clones, V9, V10, and V11, and calbindin-D28k-transfected clones, C2, C10, and C13. (B) Northern blot analysis of vector-transfected clone V9 and calbindin-D28k-transfected clone C11. With longer exposure, endogenous calbindin-D28k mRNA (1.9 kb) is observed. This mRNA can be distinguished from that of the transfected DNA transcript, which is a shorter mRNA (1.2 kb) because it lacks the 5′ and 3′ untranslated regions. Longer autoradiographic exposure was needed to observe calbindin and insulin mRNA in vector-transfected cells. Thus, the exact induction in most of the calbindin-overexpressing clones was difficult to quantify because mRNA levels were beyond the linear range of film sensitivity. However, it was noted that levels of calbindin and insulin mRNA in C2, C10, and C13 were increased >30-fold compared with levels in vector-transfected cells. Levels of calbindin mRNA were induced 5.5 ± 1-fold, and levels of insulin mRNA were induced 14-fold to >20-fold in clone C11. Results shown are representative of 4–10 separate experiments. Radiographic exposure time for the results shown: calbindin-D28k mRNA (A, 1 day; B, 5 days); insulin mRNA (A, 16 h; B, 3 days).
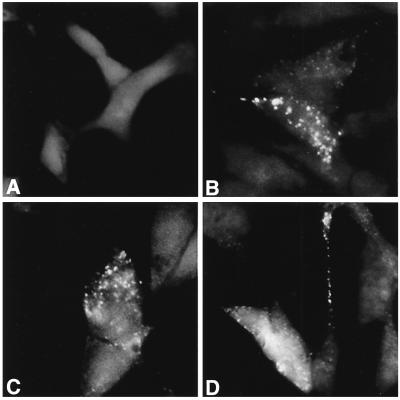
Positive clones were examined immunocytochemically using insulin antibody and were analyzed for insulin content and release. Immunocytochemical studies revealed a marked increase in insulin immunoreactivity in RIN cells overexpressing calbindin (Fig. 3). Approximately 20% of calbindin-transfected cells demonstrated many insulin granules that were never observed in control cells. Quantitative analysis indicated that the insulin content of vector-transfected cells was 4.5 ± 0.5 ng of insulin per 106 cells. The insulin content was 3.4- and 12.3-fold higher in clones C10 and C2, respectively (15.4 ± 3 and 55.5 ± 2.1 ng of insulin per 106 cells in clones C10 and C2, respectively; P < 0.01 compared with vector-transfected control cells). The assay of the concentration of insulin in the media indicated that in clone C2, there was a 54-fold increase in insulin release [control (vector-transfected cells), 0.15 ± 0.03 ng of insulin per 106 cells per hour; C2, 8.16 ± 0.93 ng of insulin per 106 cells per hour; P < 0.01]. For clone C10, which overexpressed calbindin 9-fold, insulin release was increased 5.8-fold (control, 0.15 ± 0.03 ng of insulin per 106 cells per hour; C10, 0.87 ± 0.18 ng of insulin per 106 cells per hour; P < 0.01). Thus transfection and overexpression of calbindin in RIN cells resulted in not only increased insulin mRNA levels but also increased insulin content and release. Calbindin-overexpressing clones appeared to be preferentially releasing rather than storing insulin. It should be noted, however, that multiple factors, which have not been tested in these studies, could be involved in the increased insulin release observed in calbindin-overexpressing RIN cells, for example, increased leakage or increased proteolysis.
Figure 3.
Analysis of insulin distribution by immunocytochemistry with anti-insulin antibody of transfected clones. (A) Vector-transfected clone V9. (B–D) Calbindin-D28k-transfected clone C2. Shown in B–D are C2 cells from the same batch and passage number. Immunocytochemical staining for insulin was similar to what has been occasionally seen in some similarly fixed and stained RIN cell preparations (M. German, personal communication).
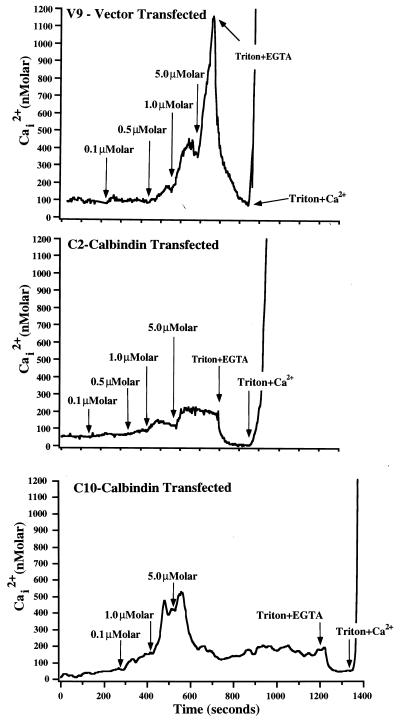
To begin to address the mechanism of the effect of calbindin overexpression, intracellular calcium levels were examined. Overexpression of calbindin did not significantly alter basal [Ca2+]i when compared with control cells [basal [Ca2+]i (mean ± SE) in clones C10 and C2 = 63.3 ± 11 and 78.7 ± 6 nM, respectively; P > 0.5 compared with basal [Ca2+]i in vector-transfected cells (71.4 ± 6 nM)]. However, cells overexpressing calbindin exhibited a much attenuated increase in intracellular Ca2+ when exposed to a divalent cation ionophore, bromo-A23187, which was most apparent at concentrations of 1 and 5 μM ionophore for clone C2 and at 5 μM for clone C10 (Fig. 4). Note that, as shown in Fig. 4, 5 μM ionophore increased [Ca2+]i to nearly 1200 nM in the vector-transfected clones. In C2 cells, this concentration of ionophore increased [Ca2+]i to only about 200 nM, and in C10 cells an increase to 500 nM was observed. These results demonstrate buffering of A23187-induced calcium influx by calbindin.
Figure 4.
Representative recordings of the effects of calcium ionophore bromo-A23187 on intracellular Ca2+ in clones transfected with vector (clone V9) or calbindin-D28k (clones C2 and C10). Intracellular Ca2+ was determined in individual cells by microfluorometry of fura-2. The recordings above are the means calculated from 40–45 cells within one field monitored during the course of the experiment. At the times indicated, cells were exposed sequentially to increasing concentrations of bromo-A23187. At the end of each experiment, cells were exposed to Ca2+-free conditions (0.02% Triton X-100 + 10 mM EGTA) and subsequently to high Ca2+ conditions (0.02% Triton X-100 + 10 mM Ca2+) to calibrate dye fluorescence for low and high Ca2+ concentrations. Similar results were observed in 3–4 additional experiments performed with each of these cell clones.
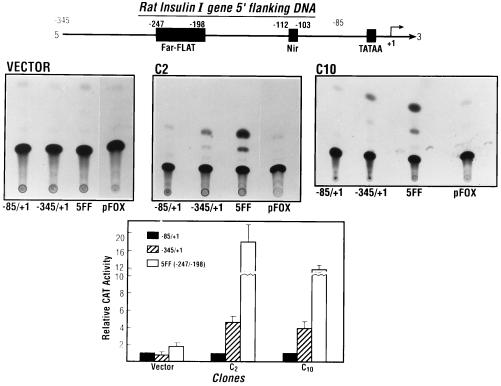
The mechanism of the effect whereby calbindin overexpression results in increased insulin mRNA was also examined using transient transfection studies. Transient transfection with plasmids bearing the regulatory sequences of the rInsI promoter (−345/+1) or five copies of the FF minienhancer (−247/−198) ligated to the minimal rat insulin promoter in calbindin-transfected cells resulted in significant inductions in CAT activity compared with CAT activity observed in cells transfected with vector alone (Fig. 5). Compared with CAT activity observed using the minimal −85/+1 promoter, in vector-transfected cells basal CAT activity was not significantly different using −345/+1 but was 2-fold greater using the construct containing five copies of FF minienhancer. Transfection of C2 and C10 with the construct containing 5 FF resulted in an 18 ± 4-fold and an 11.6 ± 1-fold induction in CAT activity, respectively, under the same conditions (Fig. 5). Transfection of C2 and C10 with plasmids containing −345/+1 of the rInsI promoter resulted in a 4.6 ± 0.7-fold and a 3.9 ± 0.8-fold induction in CAT activity, respectively (Fig. 5). These results suggest that increased insulin mRNA in calbindin-transfected cells may be due to enhanced insulin gene transcription that is mediated in part by elements within the FF region.
Figure 5.
Transient transfection of vector clones and calbindin-D28k-overexpressing clones C2 and C10 with rInsI promoter–CAT gene constructs. (Top) Schematic representation of the rInsI gene 5′ flanking DNA. (Middle) Enhancement of CAT activity using −345/+1 or 5 FF in calbindin-transfected cells. (Bottom) Graphic representation of CAT activity (each bar represents the mean ± SEM of three to five independent transfections). CAT activity using −345/+1 or 5 FF in the calbindin-overexpressing clones C2 or C10 was significantly different from CAT activity in vector-transfected cells (P < 0.05).
Because overexpression of GLUT1 or GLUT2 glucose transporters has been demonstrated to alter insulin secretion by RIN cells (29, 30), we probed Northern blots of poly(A)+ RNA from control and calbindin-overexpressing clones for GLUT1 and GLUT2 mRNA. No GLUT2 mRNA was detected in any of the cells, whereas a weak signal for GLUT1 mRNA was detected in RIN cells. Calbindin overexpressing did not alter the level of GLUT1 mRNA expression (data not shown).
DISCUSSION
This study indicates that elevated levels of calbindin-D28k in the rat β cell line RIN 1046-38 have a stimulatory effect on insulin synthesis. Studies in calcium-absorbing epithelial cells have suggested that calbindin has a role in transcellular calcium transport (4, 31). However, in the β cell, this is not a likely function for calbindin. Although calbindin has been reported to have no effect on calcium uptake (32), it has been suggested that calbindin may also act to buffer large intracellular fluxes of calcium (5–7). In our studies, calcium ionophore treatment at 1 and 5 μM revealed that calbindin-transfected cells could indeed buffer calcium significantly better than vector-transfected cells. Thus the overexpressed calbindin was functional and considered capable of buffering calcium in response to a rapid increase in [Ca2+]i. Knowledge of the concentration of calbindin within the cell, which could be assessed in future studies, and a correlation with changes in intracellular calcium would more stringently support a calcium-buffering role of calbindin in these cells in response to calcium ionophore. It is also possible that an increase in calbindin-stimulated plasma membrane calcium pump activity might influence [Ca2+]i in response to calcium ionophore. However, calbindin overexpression did not affect basal [Ca2+]i in these cells. Similar to our findings, the recent findings of Rhoten and Sergeev (33) show that butyrate treatment of RIN cells [which increases calbindin levels 4-fold (10)] dramatically attenuates the induction in [Ca2+]i in response to glucose, K+ depolarization, Bay K8655, thapsigargin, and ionomycin without affecting basal [Ca2+]i. Chard et al. (6) introduced calbindin into rat sensory neurons and similarly found a decrease in an evoked calcium signal. Calbindin did not affect basal [Ca2+]i in these neurons. The lack of an effect on basal [Ca2+]i in both neurons and RIN cells suggests that basal [Ca2+]i may be determined primarily by other factors, such as Ca2+ ATPase or Na+Ca2+ exchange rather than by calcium-binding proteins. Although overexpressed calbindin in transfected RIN cells results in enhanced calcium buffering in response to calcium ionophore, because basal [Ca2+]i is unaltered, it is not likely that changes in cytosolic free calcium are the cause of the changes in insulin.
Our results instead suggest that an effect on insulin transcription underlies, at least in part, enhanced insulin biosynthesis observed in calbindin-overexpressing RIN cells. In direct support of a role for calbindin in transcription of the insulin gene is the large increase in insulin mRNA in RIN cells overexpressing calbindin which have not been treated with a secretagogue and in which there is no alteration in basal [Ca2+]i (Fig. 2). The calbindin-transfected clones also showed increased CAT activity when either −345/+1, which contains the regulatory sequences of the rInsI promoter, or five copies of the FF miniehancer (−247/−198) were used. The rInsI gene contains two identical 8-bp sequences that are important for the cell-specific function of the insulin promoter, the Nir element (−111/−104), and the Far element (−238/−231; ref. 34). These elements bind a heterodimer of Pan1 and Pan2 helix–loop–helix proteins in association with a β cell-specific helix–loop–helix partner (35–37). Although multiple sequence elements appear to be involved in the response of the insulin promoter to glucose, the FF minienhancer has been reported to confer glucose responsiveness when linked to a heterologous promoter, and at least part of the response involves activation of binding of transcription factors at the Far element (20). It has been suggested that calcium plays a role by regulating these transcription factors (20, 38). It is possible that calbindin may affect the calcium-mediated regulation of the components of the transcription complex. Although its predominant localization is in the cytosol, previous electron microscopic studies have indicated that some calbindin is also localized in the nucleus (39). The authors suggested the possibility that calbindin may function as a nuclear regulator. In this study, we have not addressed whether calbindin overexpression alters glucose responsiveness because insulinoma cell lines have little or no transcriptional response to glucose (40). However, our results do suggest that the increase in insulin transcription as a result of calbindin overexpression is mediated in part by the FF element. It should be noted, however, that the increase in transcription does not fully account for the marked increases observed in insulin mRNA. It is possible that insulin mRNA stabilizing factors may also be playing a role in the increased insulin mRNA levels observed with calbindin overexpression. It will be of interest in future studies to determine whether calbindin can interact with transcription factors that have been reported to bind the FLAT element and to examine stimulated insulin release by transfecting calbindin in cells that respond to glucose, such as βHC cells (41) or primary cultures of rat islet cells.
Studies related to other calcium-binding proteins present in the islet have resulted in different effects on insulin secretion. Overexpression of calmodulin in the β cell of the pancreas in a transgenic mouse model resulted, unlike calbindin overexpression, in decreased insulin mRNA and an insulin secretory defect (42). Ribar et al. (17) have recently reported that the basic mechanism which underlies the effect observed with calmodulin overexpression is defective glucose utilization. Calcyclin, another calcium-binding protein that has sequence homology to S100 protein, has recently been identified in the rat pancreatic islet (18). Calcium dependent insulin release from isolated rat islets was reported to be enhanced approximately 2-fold upon addition of 10−6 M calcyclin to the incubation medium (18). Changes in insulin mRNA were not examined. Calgizzarin, another member of the S100 family, had no effect on calcium-stimulated insulin secretion under the same conditions (18). Hence, of the calcium-binding proteins examined thus far, only calbindin has been reported to have a marked inductive effect on insulin mRNA. Whether overexpression of calbindin in the β cell of a transgenic mouse model will result in increased insulin expression, similar to what was observed in RIN cells but unlike what has been observed in the calmodulin transgenic mouse model, remains to be determined. It is possible that each calcium-binding protein present in the β cell may have a unique role in pancreatic islet physiology based on properties beyond simple bulk buffering of cytosolic calcium.
In RIN cells, besides overexpression of calbindin, transfection and overexpression of the low and high Km glucose transporters, GLUT1 and GLUT2, respectively, have also been reported (29, 30, 43). Overexpression of GLUT1 or GLUT2 was found to increase insulin mRNA (3- to 6-fold) and secretion (2- to 4-fold; refs. 29 and 30). In addition, stable transfection of GLUT2 cDNA was reported to confer a 3-fold increase in glucose-stimulated insulin release in intermediate- but not high-passage RIN cells (43). As noted above, alterations in endogenous GLUT1 or GLUT2 expression did not underlie the calbindin effects described in this report. The concept of regulation of insulin biosynthesis and secretion in β cell lines, which secrete lower levels of insulin than normal β cells and which lose glucose responsiveness with time, by overexpression of proteins such as calbindin and GLUT2 may have relevance for diabetes therapy. Through cellular engineering, it may be possible to produce insulinoma-like cells that have characteristics of the normal β cell. Engineered cells could then be encapsulated and transplanted into diabetic animals to provide a model of treatment to reverse the diabetic state.
Acknowledgments
This work was supported by National Institutes of Health Grants DK-38961 (to S.C.) and DK-31398 to (A.S.P.) as well as by a grant from the Research Service of the Veterans’ Administration to A.S.P.
ABBREVIATIONS
- [Ca2+]i
intracellular calcium concentration
- FF
Far-Flat
- CAT
chloramphenicol acetyltransferase
- RIA
radioimmunoassay
References
- 1.Pochet R, Lawson D E M, Heizmann C W, editors. Advances in Experimental Medicine and Biology. Vol. 269. New York: Plenum; 1990. [DOI] [PubMed] [Google Scholar]
- 2.Christakos S, Gabrielides C, Rhoten W B. Endocr Rev. 1989;10:3–26. doi: 10.1210/edrv-10-1-3. [DOI] [PubMed] [Google Scholar]
- 3.Christakos S. Endocr Rev Monogr. 1995;4:108–110. [Google Scholar]
- 4.Feher J J, Fullmer C S, Wasserman R H. Am J Physiol. 1992;262:C517–C526. doi: 10.1152/ajpcell.1992.262.2.C517. [DOI] [PubMed] [Google Scholar]
- 5.Mattson M P, Rychlik B, Chu C, Christakos S. Neuron. 1991;6:41–51. doi: 10.1016/0896-6273(91)90120-o. [DOI] [PubMed] [Google Scholar]
- 6.Chard P S, Bleakman D, Christakos S, Fullmer C S, Miller R J. J Physiol (London) 1993;472:341–357. doi: 10.1113/jphysiol.1993.sp019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts W M. J Neurosci. 1994;14:3246–3262. doi: 10.1523/JNEUROSCI.14-05-03246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark S A, Stumpf W E, Sar M, DeLuca H F, Tanaka Y. Cell Tissue Res. 1980;209:515–520. doi: 10.1007/BF00234764. [DOI] [PubMed] [Google Scholar]
- 9.Morrissey R L, Bucci T J, Empson R N, Lufkin E G. Proc Soc Exp Biol Med. 1975;149:56–60. doi: 10.3181/00379727-149-38742. [DOI] [PubMed] [Google Scholar]
- 10.Lee S, Clark S A, Gill R K, Christakos S. Endocrinology. 1994;134:1602–1610. doi: 10.1210/endo.134.4.8137721. [DOI] [PubMed] [Google Scholar]
- 11.Berggren P O, Arkhammar P, Islam M S, Juntti-Berggren L, Khan A, Kindmark H, Kohler M, Larsson K, Larsson O, Nilsson T, Sjoholm A, Szecowka J, Zhang Q. Adv Exp Med Biol. 1993;334:25–45. doi: 10.1007/978-1-4615-2910-1_3. [DOI] [PubMed] [Google Scholar]
- 12.Newgard C B, McGarry J D. Annu Rev Biochem. 1995;64:689–719. doi: 10.1146/annurev.bi.64.070195.003353. [DOI] [PubMed] [Google Scholar]
- 13.McDermott A M, Sharp G W. Cell Signalling. 1993;5:229–234. doi: 10.1016/0898-6568(93)90014-d. [DOI] [PubMed] [Google Scholar]
- 14.Gilon P, Shepherd R M, Henquin J C. J Biol Chem. 1993;268:22265–22268. [PubMed] [Google Scholar]
- 15.Yada T, Russo L L, Sharp G W. J Biol Chem. 1989;264:2455–2462. [PubMed] [Google Scholar]
- 16.Arkhammar P, Juntti-Berggren L, Larsson O, Welsh M, Nanberg E, Sjoholm A, Kohler M, Berggren P. J Biol Chem. 1994;269:2743–2749. [PubMed] [Google Scholar]
- 17.Ribar T J, Jan C-R, Augustine G J, Means A R. J Biol Chem. 1995;270:28688–28695. doi: 10.1074/jbc.270.48.28688. [DOI] [PubMed] [Google Scholar]
- 18.Okazaki K, Niki I, Iino S, Kobayashi S, Hidaka H. J Biol Chem. 1994;269:6149–6152. [PubMed] [Google Scholar]
- 19.Pollock A S, Santiesteban H L. J Biol Chem. 1995;270:16291–16301. doi: 10.1074/jbc.270.27.16291. [DOI] [PubMed] [Google Scholar]
- 20.German M S, Wang J. Mol Cell Biol. 1994;14:4067–4075. doi: 10.1128/mcb.14.6.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood T L, Kobayashi Y, Franz G, Varghese S, Christakos S, Tobin A J. DNA. 1988;7:585–594. doi: 10.1089/dna.1988.7.585. [DOI] [PubMed] [Google Scholar]
- 22.Bradford M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 23.Varghese S, Lee S, Huang Y, Christakos S. J Biol Chem. 1988;263:9776–9784. [PubMed] [Google Scholar]
- 24.Sonnenberg J, Pansini A R, Christakos S. Endocrinology. 1984;115:640–648. doi: 10.1210/endo-115-2-640. [DOI] [PubMed] [Google Scholar]
- 25.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 26.Kekow J, Ulrichs K, Muller-Ruchholtz W, Gross W L. Diabetes. 1988;37:321–326. doi: 10.2337/diab.37.3.321. [DOI] [PubMed] [Google Scholar]
- 27.Grynkiewicz G, Poenie M, Tsien R Y. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 28.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current Protocols in Molecular Biology. New York: Wiley; 1992. [Google Scholar]
- 29.Shibasaki M, Shibasaki Y, Asano T, Kajio H, Akanuma Y, Takaku T, Oka Y. FEBS Lett. 1990;270:105–107. doi: 10.1016/0014-5793(90)81244-i. [DOI] [PubMed] [Google Scholar]
- 30.Tiedge M, Hohne M, Lenzen S. Biochem J. 1993;296:113–118. doi: 10.1042/bj2960113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bronner F, Stein W D. Am J Physiol. 1988;255:F558–F562. doi: 10.1152/ajprenal.1988.255.3.F558. [DOI] [PubMed] [Google Scholar]
- 32.Pansu D, Bellaton C, Roche C, Bronner F. Prog Clin Biol Res. 1988;252:115–120. [PubMed] [Google Scholar]
- 33.Rhoten W B, Sergeev I N. Endocrine. 1994;2:989–995. [Google Scholar]
- 34.Karlsson O, Edlund T, Moss J B, Rutter W J, Walker M D. Proc Natl Acad Sci USA. 1987;84:8819–8823. doi: 10.1073/pnas.84.24.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson C, Shen L P, Meister A, Fodor E, Rutter W J. Genes Dev. 1990;4:1035–1043. doi: 10.1101/gad.4.6.1035. [DOI] [PubMed] [Google Scholar]
- 36.Cordle S R, Henderson E, Masuoka H, Weil P A, Stein R. Mol Cell Biol. 1991;11:1734–1738. doi: 10.1128/mcb.11.3.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.German M S, Blanar M A, Nelson C, Moss L G, Rutter W J. Mol Endocrinol. 1991;5:292–299. doi: 10.1210/mend-5-2-292. [DOI] [PubMed] [Google Scholar]
- 38.German M S, Moss L G, Rutter W J. J Biol Chem. 1990;265:22063–22066. [PubMed] [Google Scholar]
- 39.Taylor A N, Inpanbutr N. In: Cellular Calcium and Phosphate Transport in Health and Disease. Bronner F, Peterlik M, editors. New York: Liss; 1988. pp. 109–112. [Google Scholar]
- 40.Hammonds P, Schofield P, Ashcroft S. FEBS Lett. 1987;213:149–154. doi: 10.1016/0014-5793(87)81481-3. [DOI] [PubMed] [Google Scholar]
- 41.Radvanyi F, Christgau S, Baekkeskov S, Jolicoeur C, Hanahan D. Mol Cell Biol. 1993;13:4223–4232. doi: 10.1128/mcb.13.7.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Epstein P N, Overbeek P A, Means A R. Cell. 1989;58:1067–1073. doi: 10.1016/0092-8674(89)90505-9. [DOI] [PubMed] [Google Scholar]
- 43.Feber S, Beltrandel Rio H, Johnson J H, Noel R J, Cassidy L E, Clark S, Becker T C, Hughes S D, Newgard C B. J Biol Chem. 1994;269:11523–11529. [PubMed] [Google Scholar]