Abstract
The mechanism of active transport of transfer RNA (tRNA) across membranes is largely unknown. Factors mediating the import of tRNA into the kinetoplast mitochondrion of the protozoon Leishmania tropica are organized into a multiprotein RNA import complex (RIC) at the inner membrane. Here, we present the complete characterization of the identities and functions of the subunits of this complex. The complex contains three mitochondrion- and eight nuclear-encoded subunits; six of the latter are necessary and sufficient for import. Antisense-mediated knockdown of essential subunits resulted in the depletion of mitochondrial tRNAs and inhibition of organellar translation. Functional complexes were reconstituted with recombinant subunits expressed in Escherichia coli. Several essential RIC subunits are identical to specific subunits of respiratory complexes. These findings provide new information on the evolution of tRNA import and the foundation for detailed structural and mechanistic studies.
Keywords: mitochondria, tRNA, import factors, import complex, gene knockdown, reconstitution
Introduction
The import of transfer RNAs into mitochondria has been documented in a large number of species across the phylogenetic scale (for reviews, see Entelis et al, 2001; Bhattacharyya & Adhya, 2004). This process supplies cytosolic tRNAs to replace mutated or deleted organelle-encoded tRNAs for translation of mitochondrial messenger RNAs. However, the mechanism of import seems to have evolved independently in different organisms, resulting in differences in the number and identity of tRNA species that are imported.
Some import factors have been identified in different species. In budding yeast, a single tRNALys isoform is imported into the mitochondria; two soluble proteins—the precursor of the mitochondrially imported isoform of lysyl tRNA synthetase (pre-MSK; Tarassov et al, 1995a) and enolase (Entelis et al, 2006)—are required for this process. Mutational studies also suggested the involvement of components TOM20 (translocase outer membrane 20) and TIM44 (translocase inner membrane 44) of the mitochondrial protein import pore (Tarassov et al, 1995b). In higher plants, different combinations of tRNAs are imported in different species. Recent studies identified the voltage-dependent anion channel (VDAC), TOM20 and TOM40 as tRNA-binding proteins required for the import of tRNA into potato mitochondria in vitro (Salinas et al, 2006). In the kinetoplastid protozoa, which includes the disease-causing Leishmania and Trypanosoma species, more than 24 tRNA species are imported owing to the complete lack of mitochondrial tRNA genes. Early work indicated the in vitro requirement of a small RNA-binding protein on the outer mitochondrial membrane of Leishmania (Adhya et al, 1997), but this protein remains unidentified.
We have previously purified a multiprotein complex (RNA import complex (RIC)) with a molecular mass of approximately 600,000 from the inner mitochondrial membrane of Leishmania tropica, which is functional for the import of tRNA into phospholipid vesicles and human mitochondria in vitro, as well as in the mitochondria of intact human cells (Bhattacharyya et al, 2003; Mahata et al, 2005, 2006). This complex is composed of several import components; two such components have been identified so far: RIC1 and RIC8A are import receptors that bind to distinct subsets of tRNAs (Chatterjee et al, 2006; Goswami et al, 2006). Here, we have attempted to characterize the identities of the remaining subunits of the RIC by using a combination of biochemical and genetic approaches.
Results And Discussion
In kinetoplastid protozoa four macromolecular complexes of the oxidative phosphorylation pathway have been observed by native gel electrophoresis (Horvath et al, 2000a, 2000b; Goswami et al, 2006). On the basis of protein sequencing and enzymatic analyses, and our radiolabelling and western blot data (Figs 1B, 2C,D), three of these have been shown to correspond to complexes V (F1Fo-ATP synthase), IV (cytochrome c oxidase) and III (ubiquinol cytochrome c reductase; UCR). Complex I (NADH dehydrogenase) is absent from these species, although some of the subunits, such as ND1 and ND7, are detectable in mitochondrial extracts (data not shown). We have presented evidence previously that the fourth, and largest complex in L. tropica is identical to the RIC (Chatterjee et al, 2006; Goswami et al, 2006).
Figure 1.
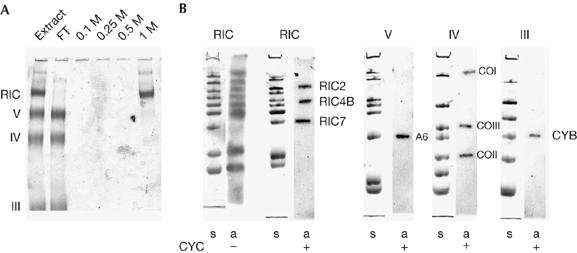
Radiolabelling of RNA import complex subunits. (A) Binding of RIC to a transfer RNA import signal. A Leishmania tropica mitochondrial extract was chromatographed on a tRNATyr D arm affinity column. The column was washed with buffer DB containing the indicated concentrations of KCl. Each fraction was concentrated and run on native BN-PAGE. Mitochondrial complexes are indicated on the left. (B) Inner membrane complexes, labelled with [35S]methionine in the absence (first two lanes from the left; −) or presence (+) of cycloheximide (CYC) and resolved by BN-PAGE were excised, subjected to SDS–polyacrylamide gel electrophoresis and fluorographed. Mitochondrion-encoded subunits are indicated. a, autoradiograph; BN-PAGE, blue native-polyacrylamide gel electrophoresis; CO, cytochrome oxidase; CYB, cytochrome b; FT, flow-through; RIC, RNA import complex; s, Coomassie stain.
Figure 2.
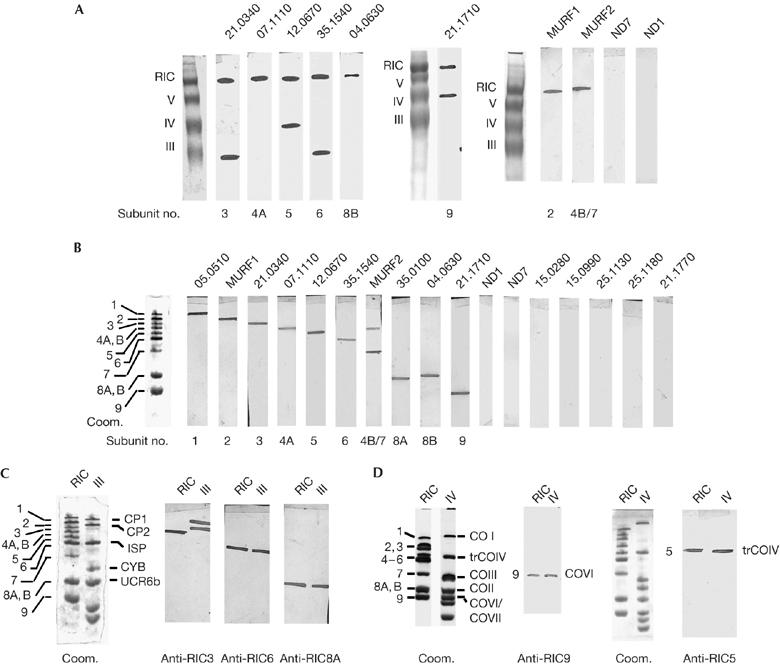
Identification of RNA import complex. (A) First-dimension blots of complexes were probed with antibodies against recombinant proteins encoded by the indicated genes. Each six-digit number is the nuclear-encoded gene ID in the Leishmania major sequence database (see Table 1 and supplementary Table S1 online); the others (MURF1, MURF2, ND1 and ND7) are mitochondrion-encoded. (B) Two-dimensional blots of RIC probed with the indicated antibodies. Subunit numbers are indicated alongside the Coomassie-stained profile on the left. (C,D) Comparative two-dimensional blots of the RIC and complex III (C) and of the RIC and complex IV (D) probed with antibodies against the indicated RIC subunits. Individual subunits of complexes III, IV and V were identified by western blotting and radiolabelling. CO, cytochrome oxidase; Coom., Coomassie stain; CP, core protein; CYB, cytochrome b; ISP, iron sulphur protein; MURF, maxicircle unidentified reading frame; ND, NADH dehydrogenase subunit; RIC, RNA import complex; trCOIV, trypanosome cytochrome oxidase subunit IV; UCR6b, ubiquinol cytochrome c reductase subunit 6b.
The solubilized mixture of complexes was chromatographed on a column containing, as immobilized ligand, an oligoribo nucleotide corresponding to the D arm of Leishmania tRNATyr containing an import signal (Bhattacharyya et al, 2000). Complexes III, IV and V flowed through the column, whereas the retained RIC was eluted at a high salt concentration (Fig 1A). The specific affinity of this complex for tRNA provides confirmation of its identity.
The starting point for determining the identities of the subunits of the RIC was the sequencing of the bands of the affinity-purified complex by mass spectrometry. Each band yielded several tryptic peptides that matched open reading frames (ORFs) in the L. major database. A total of 122 nuclear-encoded ORFs were recovered from 9 bands (supplementary Table S1 online). Candidate proteins were selected for further study on the basis of their molecular masses, which were close to the experimental value, and high or maximum peptide coverage (supplementary information online).
To detect any mitochondrion-encoded components of the RIC, the resistance of organellar, but not cytosolic, protein synthesis to cycloheximide was used. Mitochondria were isolated from cycloheximide-treated cells and inner membrane complexes were resolved by blue native-polyacrylamide gel electrophoresis (BN-PAGE). The individual bands were excised and resolved into their constituent subunits by SDS–polyacrylamide gel electrophoresis (SDS–PAGE), and labelled species were detected by autoradiography. This experiment showed the presence of known mitochondrion-encoded subunits in complexes III, IV and V. Three bands of the RIC (bands 2, 4 and 7) were similarly labelled (Fig 1B), indicating the presence of multiple mitochondrion-encoded subunits.
An antibody against each candidate protein was used to probe western blots of mitochondrial complexes resolved by BN-PAGE (first-dimension blots). Antibodies against proteins encoded by the nuclear genes LmjF05.0510 (RIC1; Goswami et al, 2006), LmjF35.0100 (RIC8A; Chatterjee et al, 2006) and LmjF21.0340, LmjF07.1110, LmjF12.0670, LmjF35.1540, LmjF04.0630 and LmjF21.1710 (Fig 2A) all reacted with the RIC. Antibodies against mitochondrion-encoded maxicircle unidentified reading frame 1 (MURF1) and MURF2 similarly reacted with the RIC, whereas NADH dehydrogenase subunit 1 (ND1) and ND7 antibodies did not (Fig 2A).
All antibodies except one that reacted with the RIC on first-dimension blots, also reacted with a single species on the second-dimension blots (Fig 2B), thus identifying the principal or sole component of each band (Table 1). The exception was anti-MURF2, which gave two bands at positions corresponding to RIC4 and RIC7, respectively. The same two species were detected in total or mitochondrial cellular extracts (data not shown). The larger protein corresponds to the full-length MURF2 of 355 aa residues (Simpson et al, 1998). No other gene homologous to MURF2 is present in the mitochondrial genome; the smaller species is probably derived from MURF2 by internal initiation or proteolytic processing. Thus, band 4 contains two proteins: one nuclear-encoded and the other mitochondrion-encoded (RIC4A and RIC4B, respectively). Similarly, band 8 contains two nuclear-encoded proteins (RIC8A and RIC8B) of nearly identical size that are distinguishable by immunochemistry. By contrast, antibodies against other candidate nuclear-encoded proteins such as the F1-ATP synthase γ-subunit (LmjF21.1770), ribonucleoprotein p18 (LmjF15.0280), succinate dehydrogenase FeS protein (LmjF15.0990) and cytochrome oxidase subunit VII (LmjF25.1130) were not detected to localize to any RIC band (Fig 2B).
Table 1.
Identities of RNA import complex subunits
| Subunit no. | Size (kDa) | Mole ratio* | Gene identity‡ | Gene copy no. | Sequence/structural similarity with known proteins§ | Complex with which shared | Role in import |
|---|---|---|---|---|---|---|---|
| 1 | 62 | 1 | LmjF05.0510 | 2 | F1-ATP synthase subunit α | V | Essential; type I tRNA receptor; tRNA-dependent ATPase; type II tRNA activator |
| 2 | 56 | 2 | MURF1 | 1 | None | None | Not essential# |
| 3 | 47 | 1 | LmjF21.0340 | 1∥ | Core proteins (complex III) | None¶ | Not essential |
| 4A | 43 | 1 | LmjF07.1110 | 1 | None | None | Essential |
| 4B | 43 | 0.5 | MURF2 | 1 | None | None | Not essential# |
| 5 | 40 | 1 | LmjF12.0670 | 1 | None | IV | Not essential |
| 6 | 35 | 3 | LmjF35.1540 | 1 | Complex III iron- sulphur protein | III | Essential |
| 7 | 28 | 1 | MURF2 | 1 | None | None | Not essential# |
| 8A | 21 | 2 | LmjF35.0100 | 1 | Complex III subunit 6b | III | Essential; type II tRNA receptor; negative regulator of type I tRNA |
| 8B | 21 | 1 | LmjF04.0630 | 1 | None | None | Essential |
| 9 | 19 | 3 | LmjF21.1710 | 1 | Complex IV subunit VI | IV | Essential |
| *Stoichiometry determination described in the supplementary information online. ‡Nuclear-encoded genes (italics) designated as in the Leisgmania major database; mitochondrion-encoded genes (bold italics) as in the kinteoplastid U-insertion/deletion editing database. §BLAST and SWISS MODEL. ∥Multigene family of M16 metalloproteases. ¶Other homologues in complex III. #By in vitro import assay. MURF, maxicircle unidentified reading frame. |
Of the ten antibodies that reacted with the RIC on the western blots, four—including the two against mitochondrion-encoded proteins—were specific to this complex, whereas each of the remaining six—all nuclear-encoded—decorated a second mitochondrial complex. These included RIC1/F1α (Goswami et al, 2006), RIC8A/UCR6b (Chatterjee et al, 2006), RIC3, RIC5, RIC6 and RIC9 (Fig 2A). The last four proteins—RIC1, RIC6, RIC8A and RIC9—are structurally homologous to known respiratory components (Chatterjee et al, 2006; Goswami et al, 2006; supplementary Figs S1–S4 online). RIC5 (LmjF12.0670) is a trypanosomatid-specific protein, termed trCOIV (Maslov et al, 2002), which is shared between the RIC and complex IV (Fig 2A,D). These proteins are encoded by single genes (subunits 5, 6, 8A and 9) or by two identical copies of the same gene (subunit 1; Table 1), and are therefore likely to be bifunctional. The sixth protein, encoded by LmjF21.0340 (RIC3, 467 aa residues), is a member of the M16 family of metalloproteases that include mitochondrial processing peptidase (MPP), and subunits CP1 and CP2 of complex III. An antibody against RIC3 crossreacted with four proteins in total promastigote extracts (Fig 3B); of these, CP1 and CP2 are present in complex III, whereas RIC3 is specific to the import complex (Fig 2C).
Figure 3.
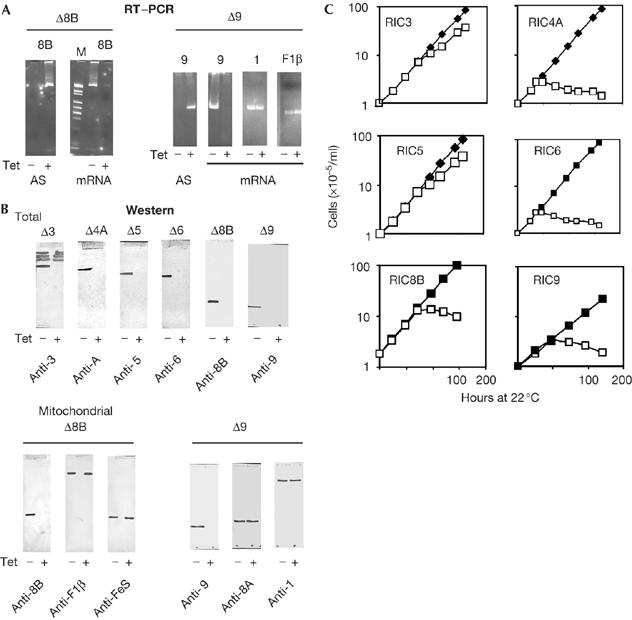
Knockdown of RNA import complex subunits. Leishmania tropica 13-90 hosts were transfected with the appropriate antisense vector, and transformed clones were cultured in the absence (−) or presence (+) of tetracycline. Each knockdown line is represented as Δx, where x is the RIC subunit number. In (A) and (B), tetracycline induction times were 72, 36, 72, 36, 48 and 48 h—that is, the initial point of cell growth cessation (C) for subunits 3, 4A, 5, 6, 8B and 9, respectively. (A) Messenger RNA (mRNA) and antisense (AS) RNA were assayed by reverse transcription–PCR (RT–PCR) using primers specific for the subunit numbered at the top of each panel. Two typical examples (knockdown of RIC8B and RIC9) are shown. (B) Western blots of total (upper panel) or mitochondrial (lower panel) proteins of indicated knockdown lines with antibodies against the indicated subunits. (C) Growth of knockdown lines in the absence (filled squares) or presence (open squares) of tetracycline. F1β, F1Fo-ATP synthase β-subunit; FeS, complex II iron-sulphur protein; M, molecular weight markers; RIC, RNA import complex; Tet, tetracycline.
The subunit stoichiometry was determined by quantification of Coomassie-stained bands and of 35S-labelled subunits in the absence and presence of cycloheximide (Table 1; supplementary Table S2 online). The RIC is composed of 16–17 polypeptides with a total estimated mass of 580 kDa, which is close to previous estimates of approximately 600 kDa on the basis of sedimentation and electrophoretic mobility (Bhattacharyya et al, 2003).
To determine the role, if any, of the detected RIC components in import in vivo, it was necessary to knock down each one by using inducible antisense RNA (Goswami et al, 2006). This resulted in efficient and specific depletion of the targeted mRNA and protein (Fig 3A,B). On induction of antisense RNA corresponding to RIC3 (LmjF21.0340), out of the four M16 metalloprotease family members with sequence similarities and immunological crossreactivity, only the target protein was depleted (Fig 3B), attesting to the specificity of the targeting procedure.
Knockdown of RIC1 (Goswami et al, 2006), RIC8A (Chatterjee et al, 2006) and RIC4A, RIC6, RIC8B and RIC9 (Fig 3C) caused a cessation of growth within 24–48 h, with rounding off of the spindle-shaped cells and loss of viability. By contrast, depletion of RIC3 and RIC5 led to only a marginal reduction in growth rate (Fig 3C). Knockdown of all essential subunits produced severe respiratory defects, for example, more than 90% of RIC9-knockdown cells became negative for cytochrome c oxidase, and oxygen consumption was reduced from 0.96 to 0.23 fmol/min/cell at 22°C.
The effect of knockdown on the contents of several mitochondrial tRNA species was monitored. Depletion of subunits 1 (Goswami et al, 2006) and 8A (Chatterjee et al, 2006) leads to the loss of mitochondrial tRNAs, whereas knockdown of the F1β subunit, which is not a part of the complex (Fig 2B), does not (Goswami et al, 2006). Loss of subunits 4A, 6, 8B and 9 resulted in the depletion of mitochondrial tRNAs; cytosolic tRNA levels were unaltered (Fig 4A). By contrast, knockdown of RIC3 or RIC5 had no marked effect on the mitochondrial tRNA pool (Fig 4A).
Figure 4.
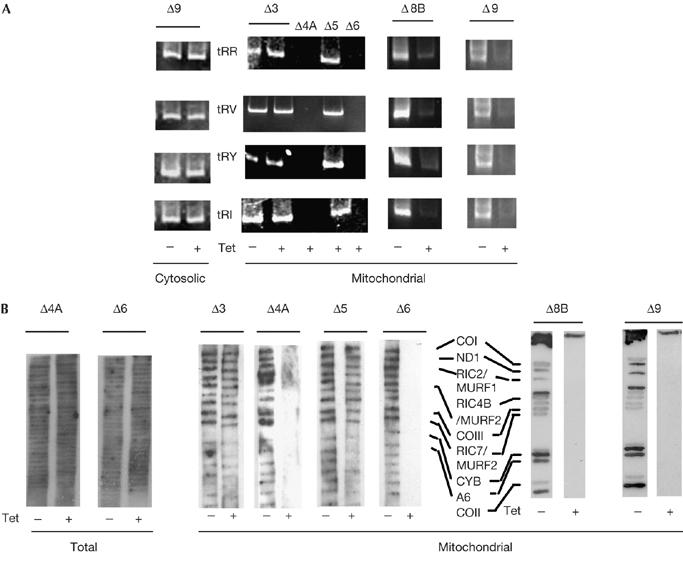
Effect of subunit knockdown on mitochondrial transfer RNAs and translation. (A) Reverse transcription–PCR assays of tRNAs in the cytosolic (102 cell-equivalent) or mitochondrial (103 cell-equivalent) compartments of knockdown cells. (B) Total or mitochondrial proteins in uninduced or knockdown lines [35S]methionine labelled in presence of cycloheximide, resolved by SDS–polyacrylamide gel electrophoresis and fluorographed. CO, cytochrome oxidase; CYB, cytochrome b; MURF, maxicircle unidentified reading frame; ND, NADH dehydrogenase subunit; RIC, RNA import complex; Tet, tetracycline; tRR, tRNAArg; tRV, tRNAVal; tRY, tRNATyr; tRI, tRNAIle.
There was a general inhibition of translation of mitochondrial mRNAs on depletion of the essential subunits; cytosolic translation was unaffected (Fig 4B). Depletion of the non-essential subunits (RIC3 and RIC5) had no marked effect on protein synthesis in either compartment.
To determine whether the identified factors were sufficient for import, in vitro reconstitution was attempted using purified proteins expressed in Escherichia coli. Initially, the eight nuclear-encoded subunits expressed in E. coli were separately re-folded, then mixed together to allow assembly to occur. A major band (R8) of approximately 500 kDa was observed by using BN-PAGE (Fig 5A). ATP-dependent tRNA import directed by the assembled complex into liposomes was observed (Fig 5B). Furthermore, import of tRNATyr, a type I tRNA, was inhibited by low concentrations of tRNAIle, a type II tRNA (Fig 5B). Such an antagonistic action of type II tRNAs on type I tRNA import has been previously observed (Bhattacharyya et al, 2002, 2003). Therefore, the reconstituted complex has the regulatory properties of native RIC. Furthermore, the specific activity of liposome-bound R8 was approximately 75% that of the RIC in intact mitochondria (Fig 5C; supplementary information online).
Figure 5.
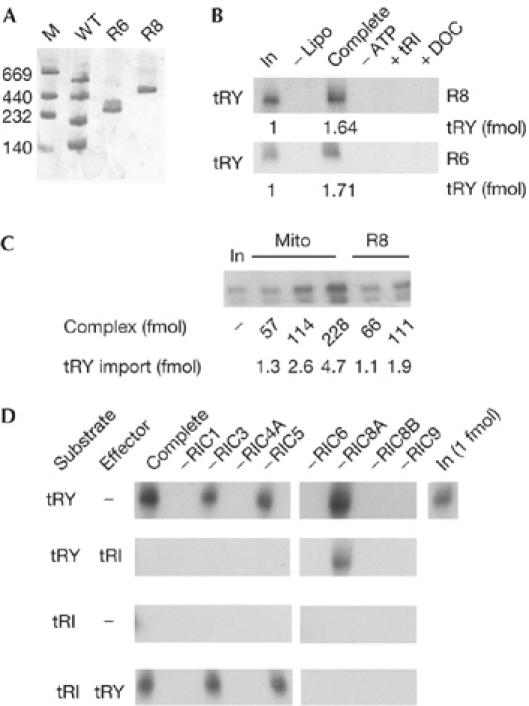
Reconstitution of functional import complexes with recombinant RNA import complex subunits. (A) BN-polyacrylamide gel electrophoresis analysis of complexes reconstituted with six essential (R6, containing subunits 1, 4A, 6, 8A, 8B and 9) or eight (R8, containing the six essential plus subunits 3 and 5) nuclear-encoded subunits. Molecular mass markers (M) of sizes (kDa) indicated on the left. Minor bands in the lanes marked R6 and R8 are possibly dimeric forms. (B) R6- or R8-induced import of tRNATyr into liposomes (Lipo). Sodium deoxycholate (0.5%; DOC) added post-import. (C) Comparison of import by indicated amounts of liposome-bound R8 and mitochondrial (Mito) RIC. (D) Single-omission experiment. Complexes were reconstituted with eight subunits (R8=complete) or with single indicated subunits omitted, and assayed for import into liposomes of substrate with high specific activity (100 fmol) in the absence or presence of effector with low specific activity (10 fmol). In, input substrate, 1 fmol; RIC, RNA import complex; tRI, tRNAIle; tRY, tRNATyr; WT, mitochondrial complexes from normal cells.
Next, complexes lacking individual recombinant subunits were assembled. Omission of RIC1, RIC4A, RIC6, RIC8B and RIC9 resulted in the abolition of import of type I tRNATyr; in the absence of RIC3 and RIC5 there was a small reduction in activity, whereas lack of RIC8A led to a slight stimulation (Fig 5D). In the presence of the type II tRNAIle effector, import by the complete, as well as RIC3- and RIC5-deficient complexes, was inhibited, but import by the RIC8A-deficient complex persisted, indicating the loss of allosteric inhibition as previously reported (Chatterjee et al, 2006). tRNAIle alone was not imported by any of the complexes, but import was induced by the type I tRNATyr effector in complete as well as RIC3- and RIC5-deficient complexes; tRNATyr was ineffective in the absence of RIC1, which positively regulates type II import, or RIC8A, the type II receptor (Fig 5D).
This experiment confirmed the in vivo observations showing the requirement of RIC1, RIC4A, RIC6, RIC8A, RIC8B and RIC9. Furthermore, a complex reconstituted with only these six subunits (R6; Fig 5A) was functional in vitro (Fig 5B), showing them to be sufficient for tRNA import. The import efficiencies of R6 and R8 were nearly identical (Fig 5B).
Our previous work identified RIC1 and RIC8A as allosterically interacting receptors for distinct tRNA subsets (Chatterjee et al, 2006; Goswami et al, 2006). The current work defines the essential subunits and provides leads for future investigations into their individual roles in import.
A significant number of multifunctional, ‘moonlighting', proteins have been recently identified in many systems (for a review, see Jeffery, 2003). The convergence of so many respiratory proteins moonlighting for a single function is unprecedented. Moonlighting proteins have been identified as tRNA import factors in different systems (see above; Tarassov et al, 1995a, 1995b; Entelis et al, 2006; Salinas et al, 2006). The preponderance of multifunctional proteins might be a reflection of the mechanism of emergence of a new cellular function (tRNA import) during evolution.
Methods
Preparation and analysis of mitochondrial complexes. Mitochondrial fractions were isolated from L. tropica promastigotes as described previously (Bhattacharyya et al, 2000). Mitochondria or mitoplasts were extracted using sodium dodecyl maltoside, and complexes were separated by using BN-PAGE, excised and subjected to two-dimensional SDS–PAGE (Goswami et al, 2006). Chromatography of maltoside extracts of mitochondria (5 × 106 cell-equivalent) used an RNA affinity column as described previously (Bhattacharyya et al, 2003).
Protein radiolabelling. Promastigotes were cultured in RPMI1640 medium (Invitrogen, Bethesda, MD, USA) in the presence of a methionine-deficient amino-acid mixture (prepared using the SelectAmine kit, Invitrogen) supplemented with 35S-methionine (1,000 Ci/mmol, 0.4 mCi/ml) for 4 h at 22°C. [35S]-labelled bands were visualized by fluorography.
Peptide analysis and gene retrieval. Tryptic peptides of the RIC subunit bands were sequenced by mass spectrometry at the W.M. Keck Biomedical Mass Spectrometry Laboratory, University of Virginia (VA, USA). Nuclear-encoded ORFs were retrieved from the L. major database (http://www.genedb.org/genedb/leish/index.jsp). Details of peptide analysis and shortlisting are provided in the supplementary information online. Mitochondrial ORFs from L. tarentolae were obtained from Larry Simpson's U-insertion/deletion edited sequence database (http://dna.kdna.ucla.edu/trypanosome/database.html).
Sequence analysis and homology modelling. The L. tropica protein sequences were found to be identical to corresponding sequences in L. major. Selected ORFs were re-subjected to BLAST analysis. Sequences were aligned using ClustalW (www.ebi.ac.uk/clustalw/index.html). Homology models of shared subunits were constructed by SWISS MODEL (www.expasy.ch/swissmod/SWISS-MODEL.html) using the bovine homologue as a template.
Cloning, expression and purification of recombinant protein. Each complete nuclear-encoded ORF was amplified from L. tropica strain UR6 genomic DNA using BamHI (sense) and EcoRI (antisense) linker-primers (supplementary Table S3 online). Expression of glutathione-S-transferase-tagged proteins and purification of recombinant subunits were carried out as described previously (Goswami et al, 2006).
Gene knockdown. In a standardized protocol (details in Goswami et al, 2006), each gene was cloned in the antisense orientation downstream of a tetracycline-regulated T7 RNA polymerase promoter of the expression-cum-targeting vector pGET, and introduced into the expression host L. tropica 13–90. Transformed clones were selected on semisolid agar with 2.5 μg/ml phleomycin. Clonal cultures were induced using 1 μg/ml tetracycline. Cell growth and morphology were monitored and knockdown cells collected at the earliest observed point of growth slowdown—that is, the point of inflection in the growth curve. Cytochrome oxidase and O2 uptake assays were carried out as described previously (Goswami et al, 2006).
RNA analysis. Gene-specific sense or antisense RNA was amplified from cytosolic RNA (105 cell-equivalent) by reverse transcription–PCR using appropriate primer pairs (supplementary Table S3 online), as described previously (Goswami et al, 2006). Cytosolic and mitochondrial tRNAs (from 10–103 cell-equivalent RNA) were similarly amplified.
Reconstitution. Each recombinant protein (100 ng of gel-eluted band in 10 μl of 0.05 M Tris–HCl (pH 8.0), 0.2 M NaCl, 0.1 mM EDTA, 5 mM dithiothreitol (DTT) and 0.2% SDS) was separately diluted fivefold in buffer DB (0.2 M Tris–HCl (pH 7.5), 5 mM MgAc2, 1 mM DTT, 0.1 mM phenylmethylsulphonyl fluoride and 10% glycerol), re-folded on ice for 2 h, and concentrated in a Microcon 10 unit to 30 μl. Indicated combinations of re-folded subunits (15 μl or 50 ng) were combined and adjusted to 120 μl with DB, incubated for 2 h on ice and microconcentrated to a volume of 45 μl. Assembled complexes (10 ng/subunit in 10 μl) were assayed for import of 32P-labelled tRNAs into liposomes by RNase protection (Bhattacharyya et al, 2003). Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Acknowledgments
We thank the W.M. Keck Biomedical Mass Spectrometry Laboratory, University of Virginia (VA, USA), for peptide analysis and T. Chowdhury for technical assistance. This research was supported by Department of Science and Technology grant no. SR/SO/BB-28/2003, Council of Scientific and Industrial Research (CSIR) project no. SMM 003 and CSIR Fellowships to S.M., P.H., S.B. and G.D.
References
- Adhya S, Ghosh T, Das A, Bera SK, Mahapatra S (1997) Role of an RNA binding protein in import of tRNA into Leishmania mitochondria. J Biol Chem 272: 21396–21402 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya SN, Adhya S (2004) The complexity of mitochondrial tRNA import. RNA Biol 1: 84–88 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya SN, Mukherjee S, Adhya S (2000) Mutations in a tRNA import signal define distinct receptors at the two membranes of Leishmania mitochondria. Mol Cell Biol 20: 7410–7417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya SN, Chatterjee S, Adhya S (2002) Mitochondrial RNA import in Leishmania tropica: aptamers homologous to multiple tRNA domains that interact cooperatively or antagonistically at the inner membrane. Mol Cell Biol 22: 4372–4382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya SN, Chatterjee S, Goswami S, Tripathi G, Dey SN, Adhya S (2003) ‘Ping-pong' interactions between mitochondrial tRNA import receptors within a multiprotein complex. Mol Cell Biol 23: 5217–5224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Home P, Mukherjee S, Mahata B, Goswami S, Dhar G, Adhya S (2006) An RNA-binding respiratory component mediates import of type II tRNAs into Leishmania mitochondria. J Biol Chem 281: 25270–25277 [DOI] [PubMed] [Google Scholar]
- Entelis N, Kolesnikova OA, Martin RP, Tarassov IA (2001) RNA delivery into mitochondria. Adv Drug Deliv Rev 49: 199–215 [DOI] [PubMed] [Google Scholar]
- Entelis N, Brandina I, Kamenski P, Krasheninnikov IA, Martin RP, Tarassov I (2006) A glycolytic enzyme, enolase, is recruited as a cofactor of tRNA targeting toward mitochondria in Saccharomyces cerevisiae. Genes Dev 20: 1609–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami S, Dhar G, Mukherjee S, Mahata B, Chatterjee S, Home P, Adhya S (2006) A bi-functional tRNA import receptor from Leishmania mitochondria. Proc Natl Acad Sci USA 103: 8354–8359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath A, Berry EA, Maslov DA (2000a) Translation of the edited mRNA for cytochrome b in trypanosome mitochondria. Science 287: 1639–1640 [DOI] [PubMed] [Google Scholar]
- Horvath A, Kingan TG, Maslov DA (2000b) Detection of the mitochondrially encoded cytochrome c oxidase subunit I in the trypanosomatid protozoan Leishmania tarentolae. J Biol Chem 275: 17160–17165 [DOI] [PubMed] [Google Scholar]
- Jeffery CJ (2003) Moonlighting proteins: old proteins learning new tricks. Trends Genet 19: 415–417 [DOI] [PubMed] [Google Scholar]
- Mahata B, Bhattacharyya SN, Mukherjee S, Adhya S (2005) Correction of translational defects in patient-derived mutant mitochondria by complex-mediated import of a cytoplasmic tRNA. J Biol Chem 280: 5141–5144 [DOI] [PubMed] [Google Scholar]
- Mahata B, Mukherjee S, Mishra S, Bandyopadhyay A, Adhya S (2006) Functional delivery of a cytosolic tRNA into mutant mitochondria of human cells. Science 314: 471–474 [DOI] [PubMed] [Google Scholar]
- Maslov DA, Zikova A, Kyselova I, Lukes J (2002) A putative novel nuclear-encoded subunit of the cytochrome c oxidase complex in trypanosomatids. Mol Biochem Parasitol 125: 113–125 [DOI] [PubMed] [Google Scholar]
- Salinas T, Duchene A-M, Delage L, Nilsson S, Glaser E, Zaepfel M, Marechal-Drouard L (2006) The voltage-dependent anion channel, a major component of the tRNA import machinery in plant mitochondria. Proc Natl Acad Sci USA 103: 18362–18367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L, Wang SH, Thiemann OH, Alfonso JD, Maslov DA, Avila HA (1998) U-insertion/deletion edited sequence database. Nucleic Acids Res 26: 170–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarassov I, Entelis N, Martin RP (1995a) Mitochondrial import of a cytoplasmic lysine-tRNA in yeast is mediated by cooperation of cytoplasmic and mitochondrial lysyl-tRNA synthetases. EMBO J 14: 3461–3471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarassov I, Entelis N, Martin RP (1995b) An intact protein translocating machinery is required for mitochondrial import of a yeast cytoplasmic tRNA. J Mol Biol 245: 315–323 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


