Abstract
Voltage-dependent anion-selective channel (VDAC) is a β-barrel protein in the outer mitochondrial membrane that is necessary for metabolite exchange with the cytosol and is proposed to be involved in certain forms of apoptosis. We studied the biogenesis of VDAC in human mitochondria by depleting the components of the mitochondrial import machinery by using RNA interference. Here, we show the importance of the translocase of the outer mitochondrial membrane (TOM) complex in the import of the VDAC precursor. The deletion of Sam50, the central component of the sorting and assembly machinery (SAM), led to both a strong defect in the assembly of VDAC and a reduction in the steady-state level of VDAC. Metaxin 2-depleted mitochondria had reduced levels of metaxin 1 and were deficient in import and assembly of VDAC and Tom40, but not of three matrix-targeted precursors. We also observed a reduction in the levels of metaxin 1 and metaxin 2 in Sam50-depleted mitochondria, implying a connection between these three proteins, although Sam50 and metaxins seemed to be in different complexes. We conclude that the pathway of VDAC biogenesis in human mitochondria involves the TOM complex, Sam50 and metaxins, and that it is evolutionarily conserved.
Introduction
Voltage-dependent anion-selective channel (VDAC) is a mitochondrial outer membrane protein that is predicted to have a β-barrel topology. Three isoforms of VDAC have been identified in mammalian mitochondria (Blachly-Dyson et al, 1994; Rahmani et al, 1998). Besides their known function of enabling metabolite exchange between the cytosol and the mitochondrial intermembrane space, VDAC1 and VDAC2 have also been implicated in certain forms of apoptosis (Shimizu et al, 1999; Cheng et al, 2003).
The biogenesis of VDAC has so far been studied primarily in fungi. These studies have shown that the mitochondrial targeting signal is contained within the sequence of the mature protein and is recognized by the receptors Tom20 and Tom22 of the translocase of the outer mitochondrial membrane (TOM) complex (Hamajima et al, 1988; Krimmer et al, 2001). The precursor of VDAC is then translocated through the pore of the TOM complex, formed by Tom40, and transferred to the sorting and assembly machinery (SAM), which is responsible for the integration of β-barrel proteins into the outer membrane of the mitochondria (Krimmer et al, 2001; Wiedemann et al, 2003; Paschen et al, 2003).
In fungi, the central component of the SAM complex is Sam50 (topogenesis of mitochondrial outer membrane β-barrel proteins, Tob55/outer membrane protein, Omp85). Other components include Sam37 (mitochondrial assembly, Mas37/Tom37), Sam35 (Tob38/Tom38) and, in yeast, mitochondrial distribution and morphology, Mdm10 (Kozjak et al, 2003; Paschen et al, 2003; Wiedemann et al, 2003; Milenkovic et al, 2004; Waizenegger et al, 2004; Ishikawa et al, 2004; Meisinger et al, 2004). The homologue of yeast Sam50 has recently been identified in human mitochondria, where it was shown to be required for incorporation of the Tom40 precursor into the mature TOM complex (Humphries et al, 2005). Sam37 has significant sequence homology to mammalian metaxin 1 (Armstrong et al, 1997). Metaxin 2 is proposed to be the mammalian homologue of fungal Sam35, although direct sequence homology is lacking.
Metaxin 1 is thought to tether metaxin 2 to the outer mitochondrial membrane (Armstrong et al, 1999). In addition, it has been implicated in protein import into mitochondria, and has been shown to be required for tumour necrosis factor α (TNFα)-induced cell death (Armstrong et al, 1997; Abdul et al, 2000; Wang et al, 2001). Until now, no role for metaxin 2 in the import of mitochondrial proteins has been established (Armstrong et al, 1999).
We investigated the biogenesis of VDAC in human mitochondria by using inducible, short hairpin RNA (shRNA)-mediated knockdown of the components of the mitochondrial import machinery. We were able to confirm the importance of the TOM complex in human VDAC import. Furthermore, we established the involvement of human Sam50 in the biogenesis of VDAC. Finally, we present data that suggest significant and conserved roles for metaxin 1 and metaxin 2 in the biogenesis of VDAC.
Results
shRNA-directed knockdown of mitochondrial proteins
HeLa cells were transduced with a lentivirus vector-based system in which the expression of both shRNA and the reporter, green fluorescent protein (GFP), was subject to doxycycline (Dox)-inducible transcriptional regulation (Wiznerowicz & Trono, 2003). Single-cell clones were selected for each shRNA, and after 5–7 days of induction with Dox, messenger RNA and protein levels were determined by using real-time PCR and immunoblot analysis, respectively. Using this approach, we obtained stably transduced cell lines that showed inducible knockdown of Tom40 (termed tom40kd-2), Sam50 (sam50kd-2), Tom70 (tom70kd-1) and metaxin 2 (mtx2kd-2). For control purposes, single-cell clones transduced with an empty lentivirus vector (termed pLV-THM) were included.
The TOM complex is required for VDAC import
The knockdown of Tom40, the central component of the TOM complex, was induced for 5 days rather than the 7 days in tom40kd-2 cells to minimize the effects of a lack of Tom40 on other mitochondrial proteins. The level of the Tom40 protein was reduced in comparison with the non-induced control, as were the levels of TOM receptors, Tom20 and Tom22. The levels of other mitochondrial proteins we tested, including VDAC, were not affected (Fig 1A).
Figure 1.
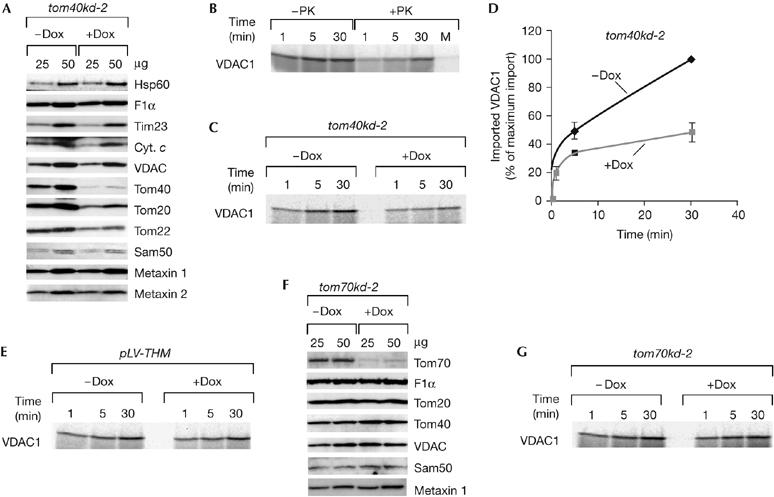
The TOM complex is necessary for VDAC import. (A) Protein levels of mitochondria isolated from tom40kd-2 cells grown for 5 days in the absence (−Dox) or presence (+Dox) of doxycycline. Mitochondria (μg of protein) were analysed by using SDS–PAGE and western blot. (B) 35S-labelled VDAC1 precursor was incubated with isolated HeLa mitochondria for the indicated time periods. The samples were left untreated (−PK) or treated (+PK) with 50 μg/ml protease K upon import and analysed by SDS–PAGE and autoradiography. Mock (M) represents a sample incubated for 30 min, containing no mitochondria. (C,D) Tom40-depleted mitochondria are defective in the import of the VDAC1 precursor. Isolated mitochondria, as in (A), were incubated with 35S-labelled VDAC1 for the indicated time periods, treated with protease K and analysed as in (B). Values on the graph represent the average of three independent import experiments. The longest time point of import into mitochondria from non-induced cells was set to 100%. (E) The VDAC1 precursor was imported, as described in (C), into mitochondria isolated from control pLV-THM cells after 7 days of induction. (F,G) Levels, of proteins in mitochondria isolated from tom70kd-1 after 7 days of induction (F), and the corresponding import of the VDAC1 precursor (G). Cyt. c, cytochrome c; Dox, doxycycline; F1α, ATPase F1 subunit α; Hsp60, heat shock protein 60; pLV-THM, single-cell clones transduced with an empty lentivirus vector; Sam, sorting and assembly machinery; SDS–PAGE, SDS–polyacrylamide gel electrophoresis; Tim, translocase of the inner mitochondrial membrane; Tom, translocase of the outer mitochondrial membrane; VDAC, voltage-dependent anion-selective channel.
After incubation of the 35S-labelled precursor of human VDAC1 with isolated mitochondria, a portion of the protein remained resistant to protease K treatment, whereas there was no protease-resistant protein in a mock control containing no mitochondria (Fig 1B). We observed a significant reduction in the amount of protease-resistant VDAC1 after import into mitochondria in which Tom40 had been depleted (Fig 1C,D). To exclude the possibility that either the presence of Dox in the growth medium or the overexpression of GFP interfered with the ability of mitochondria to import VDAC1, we conducted control experiments by using mitochondria isolated from pLV-THM and tom70kd-1 cells after 7 days of induction with Dox, considering that Tom70 is not involved in the import of VDAC (Hines et al, 1990). The level of the Tom70 protein was significantly reduced in tom70kd-1 mitochondria after Dox treatment, whereas the levels of other proteins we tested were unchanged (Fig 1F). Mitochondria from induced pLV-THM and tom70kd-1 cells showed no defect in the import of VDAC1 precursor (Fig 1E,G); therefore, we conclude that the TOM complex is involved in the import of VDAC into human mitochondria.
Sam50 has a role in VDAC biogenesis
After 7 days of Dox induction in sam50kd-2 cells, Sam50 was virtually undetectable in mitochondria. In addition, the level of Tom40 was reduced. The amount of VDAC, however, was much more affected. Surprisingly, the amounts of both metaxin 1 and metaxin 2 were also markedly reduced after the depletion of Sam50 (Fig 2A). This was not an off-target effect of the shRNA, as the mRNA levels of metaxin 1 and metaxin 2 were not reduced after the induction with Dox (data not shown).
Figure 2.
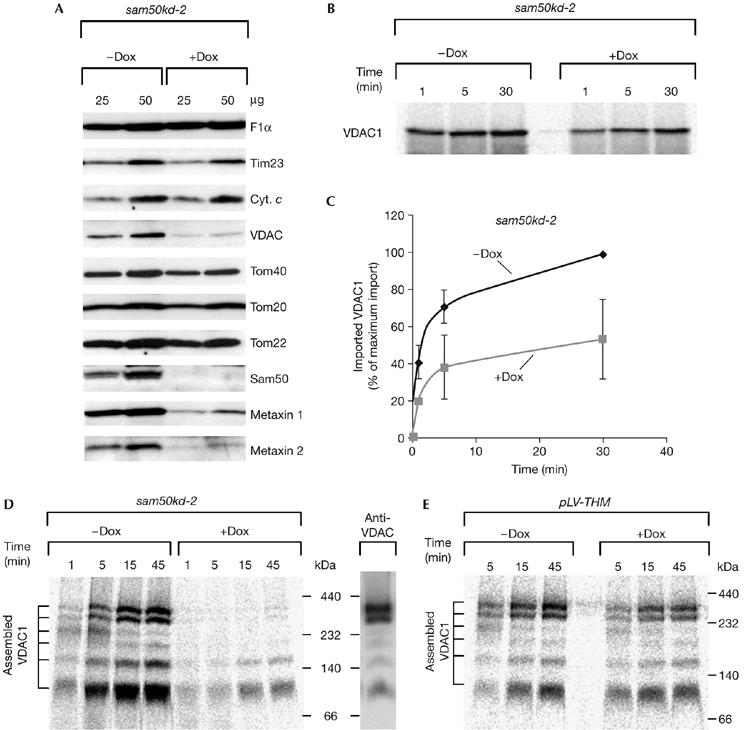
Sam50 is involved in import and assembly of VDAC. (A) Protein levels in sam50kd-2 mitochondria analysed by SDS–PAGE and western blot after 7 days of knockdown induction with doxycycline. (B,C) Mitochondria isolated from induced and non-induced sam50kd-2 cells were incubated with 35S-labelled VDAC1 precursor, treated with 50 μg/ml protease K and analysed by SDS–PAGE. The average of three independent experiments is represented on the graph (C). The longest time point of the import into mitochondria from cells in which Sam50 knockdown was not induced was set to 100%. (D) VDAC1 import into sam50kd-2 mitochondria was carried out as in (B) and protein complexes were analysed by BN-PAGE, autoradiography and western blot. (E) Mitochondria were isolated from control pLV-THM cells after 7 days of doxycycline induction, and import of VDAC1 precursor was carried out as in (B) and (D). BN-PAGE, blue native-polyacrylamide gel electrophoresis; Cyt. c, cytochrome c; Dox, doxycycline; pLV-THM, single-cell clones transduced with an empty lentivirus vector; SAM, sorting and assembly machinery; SDS–PAGE, SDS–polyacrylamide gel electrophoresis; Tim, translocase of the inner mitochondrial membrane; Tom, translocase of the outer mitochondrial membrane; VDAC, voltage-dependent anion-selective channel.
The amount of protease-resistant VDAC1 after import was significantly decreased in mitochondria in which Sam50 had been depleted (Fig 2B,C). When analysed by blue native-polyacrylamide gel electrophoresis (BN-PAGE), we observed that the lack of Sam50 strongly affected the assembly of the VDAC1 precursor into mature complexes (Fig 2D). A control experiment showed that the assembly of VDAC1 was not affected by the induction with Dox in mitochondria isolated from pLV-THM cells (Fig 2E).
We were not able to exclude entirely the possibility that, first, the decreased levels of Tom40 affected the import of VDAC, and, second, the reduction in the amount of metaxins in Sam50-knockdown mitochondria was responsible for the defect in VDAC biogenesis. To clarify this, we isolated mitochondria from the metaxin 2-knockdown cell line, mtx2kd-2, after 7 days of Dox induction. The amounts of metaxin 2 were strongly reduced, whereas Sam50, Tom40 and VDAC protein levels were unchanged. Only the amount of the metaxin 1 protein was reduced (Fig 3A) in spite of the non-reduced level of its corresponding mRNA (data not shown). Even after 15 days of metaxin 2, and consequently metaxin 1, depletion, the amount of Tom40 was not reduced and the amount of VDAC was only moderately affected (Fig 3B). As mentioned above, a reduction in Tom40 of more than 90% did not significantly affect the steady-state level of VDAC (Fig 1A). By contrast, the depletion of Sam50 led to a relatively rapid decrease in the steady-state level of VDAC (Fig 2A). Therefore, the data strongly suggest that Sam50 is necessary for VDAC biogenesis.
Figure 3.
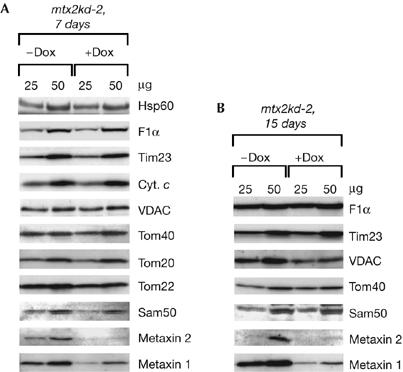
Protein levels in mitochondria depleted of metaxin 2. (A,B) mtx2kd-2 cells were grown in the presence (+Dox) or absence (−Dox) of doxycycline for 7 days (A) or 15 days (B). Mitochondria were isolated and analysed by using SDS–polyacrylamide gel electrophoresis and western blot. Cyt. c, cytochrome c; Dox, doxycycline; Hsp60, heat shock protein 60; Sam, sorting and assembly machinery; Tim, translocase of the inner mitochondrial membrane; Tom, translocase of the outer mitochondrial membrane; VDAC, voltage-dependent anion-selective channel.
Metaxins are required for VDAC import and assembly
Considering that prolonged depletion of metaxins in mitochondria affects VDAC levels, we wanted to explore further the role of these proteins in VDAC biogenesis. In metaxin-depleted mitochondria, the amount of protease-resistant VDAC1 after import was reduced by approximately 50% (Fig 4A,B). Assembly of the VDAC1 precursor into mature complexes was more strongly affected (Fig 4C), resulting in approximately less than 10% of assembled VDAC1 in the mitochondria from induced mtx2kd-2 cells.
Figure 4.
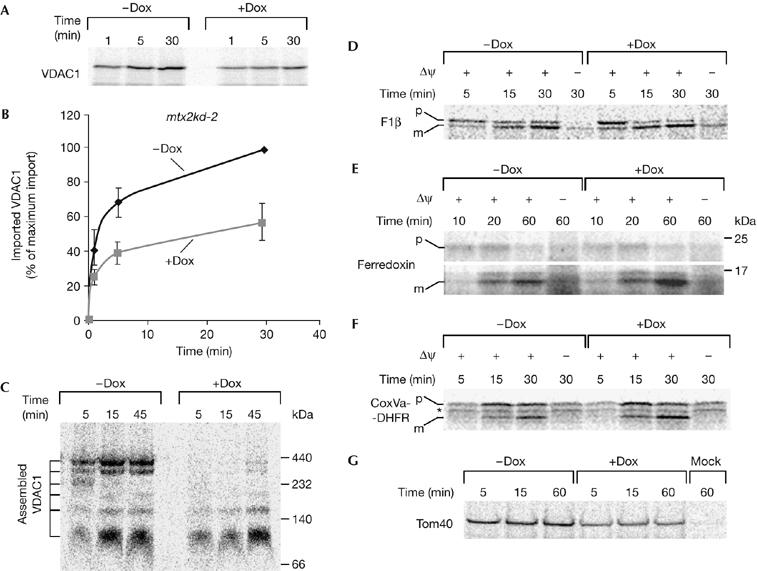
Metaxins are required for VDAC biogenesis, but not for the import of matrix precursors. (A,B) Knockdown of metaxins was induced with doxycycline in mtx2kd-2 cells for 7 days. Mitochondria were isolated and incubated with 35S-labelled VDAC1 precursor for the indicated time periods. After protease K treatment, mitochondria were analysed by SDS–polyacrylamide gel electrophoresis and autoradiography. (B) The graph represents an average of three independent import experiments. The longest time point of import into mitochondria from doxycyline-untreated cells was set to 100%. (C) Import was carried out as in (A), and protein complexes in mitochondria were analysed by blue native-polyacrylamide gel electrophoresis. (D–F) 35S-labelled matrix precursors F1β (D), ferredoxin (E) and CoxVa-DHFR (F) were imported into metaxin 2-depleted mitochondria as in (A). (G) 35S-labelled precursor of outer-membrane β-barrel protein Tom40 was imported in metaxin 2-depleted mitochondria as in (A) for the indicated time periods. Mock represents a sample containing no mitochondria. CoxVa-DHFR, cytochrome oxidase subunit Va presequence fused to full-length human dihydrofolate reductase; Dox, doxycyline; Δψ, membrane potential; m, mature protein; p, precursor; Tom40, major subunit of the translocase of the outer mitochondrial membrane 40; VDAC, voltage-dependent anion-selective channel; *, nonspecific translation product.
It was previously proposed that metaxin 1 is involved in the import of presequence-containing mitochondrial precursors (Armstrong et al, 1997; Abdul et al, 2000). Although similar reports exist about the yeast homologue of metaxin 1, Sam37 (Gratzer et al, 1995), they have been contradicted and it has since been shown that Sam37 is required only for the assembly of β-barrel proteins (Ryan et al, 1999; Wiedemann et al, 2003). We therefore endeavoured to clarify whether the same is true for metaxin 1. We used the following mitochondrial precursors: ATPase F1 subunit β (F1β), ferredoxin and a chimaera consisting of the cytochrome oxidase subunit Va presequence fused to full-length human dihydrofolate reductase (CoxVa-DHFR). Depletion of metaxins had no effect on the import of any of these matrix-targeted precursors, whereas the dissipation of mitochondrial membrane potential (Δψ) by valinomycin did abolish import in a manner independent of metaxin knockdown (Fig 4D–F). Therefore, metaxins are not necessary for the import of these presequence-containing precursors. Conversely, the import of another β-barrel precursor, Tom40, was strongly affected by depletion of metaxins (Fig 4G). We conclude that metaxins have a specific and important role in the import and assembly of VDAC and other β-barrel proteins.
Sam50 and metaxin 2 are present in distinct complexes
To address further the relationship between Sam50 and metaxins, HeLa mitochondria were subjected to BN-PAGE, followed by second dimension SDS–PAGE and western blot (Fig 5A). After a comparison of control mitochondria with Sam50- or metaxin 2-depleted mitochondria (data not shown), we established that Sam50 is found in a protein complex of more than 200 kDa as reported previously (Humphries et al, 2005), whereas metaxin 2 could be observed only in a high-molecular-weight complex of approximately 600 kDa, distinct from the Sam50-containing complex. Most of the metaxin 1 protein migrated at less than 60 kDa, as already reported (Abdul et al, 2000), but a portion was found migrating in the high-molecular-weight range of more than 600 kDa with a smear down to 200 kDa (Fig 5A). Similar observations could be made after analysing mitochondria by gel filtration chromatography (Fig 5B). We conclude that metaxin 1 and metaxin 2 are present together in an approximately 600 kDa complex that does not include Sam50.
Figure 5.
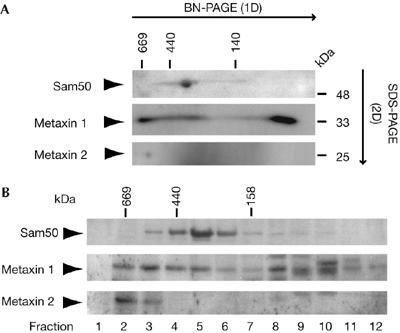
Analysis of Sam50 and metaxin complexes by two-dimensional electrophoresis and gel filtration. (A) Isolated mitochondria (100 μg of protein) were solubilized in 1% digitonin buffer and subjected to blue native-polyacrylamide gel electrophoresis (BN-PAGE) in the first dimension (1D) and SDS–PAGE in the second dimension (2D), followed by western blot with antibodies against Sam50, metaxin 1 and metaxin 2. (B) Isolated mitochondria (400 μg of protein) were solubilized in 1% digitonin buffer and subjected to gel filtration chromatography. Proteins were precipitated by trichloroacetic acid and analysed by SDS–PAGE and western blot. SAM, sorting and assembly machinery; SDS–PAGE, SDS–polyacrylamide gel electrophoresis.
Discussion
The import of proteins into mitochondria has been studied primarily in fungi owing to the relative ease of genetic manipulation of these organisms. In human cells, RNA interference has been used to deplete proteins either in a transient way or by producing non-inducible knockdown cell lines. To avoid problems that accompany such approaches, we used an inducible shRNA-knockdown system (Wiznerowicz & Trono, 2003). This is the first report of it being systematically applied to the knockdown of mitochondrial import proteins. The cell lines that we obtained can be used to facilitate further studies of the import routes of other proteins targeted to human mitochondria.
In this study, we were able to establish the involvement of the human TOM complex in the VDAC import, which is similar to what has already been shown in yeast (Krimmer et al, 2001). Our experiments further suggest that human Sam50, already known to be required for the assembly of one β-barrel protein, Tom40 (Humphries et al, 2005), is also involved in the assembly of VDAC. In addition, in yeast, β-barrel proteins require Sam37 and Sam35 for membrane integration (Wiedemann et al, 2003; Milenkovic et al, 2004; Waizenegger et al, 2004; Ishikawa et al, 2004). We have shown that metaxin 1 and metaxin 2, their presumed homologues in humans, are also necessary for the biogenesis of two β-barrel proteins, VDAC and Tom40, but are not required for the import of three matrix-targeted precursors. This contradicts previous reports, in which metaxin 1 was shown, by using antibody blocking, to be required for in vitro import of ferredoxin (Armstrong et al, 1997), and its overexpression in vivo led to a decrease in the import of several mitochondrial precursors (Abdul et al, 2000). However, our findings are in agreement with the observation that antibodies against metaxin 2 do not influence the import of ferredoxin (Armstrong et al, 1999). Our system has the advantage of selectively depleting metaxin 1 and metaxin 2 without inadvertently affecting other components of the import machinery.
Although metaxins seem to perform functions similar to their yeast counterparts, important differences exist between the mammalian and the yeast SAM complex components. Metaxin 1 is reported to interact with metaxin 2, tethering it to the outer membrane (Armstrong et al, 1999), whereas in yeast Sam35 interacts directly with Sam50 (Waizenegger et al, 2004). Furthermore, depletion of Sam35 leads to a reduction in Sam50 levels in fungi (Waizenegger et al, 2004), whereas this is not the case with metaxin 2 and human Sam50 (Fig 3). Interestingly, we have observed that the depletion of Sam50 affects the levels of metaxin 1 and metaxin 2 (Fig 2A). One explanation is that these proteins are substrates of Sam50, as are Tom40 and VDAC. This, however, would contradict the current model in which Sam50 is required only for the biogenesis of β-barrel proteins (Kozjak et al, 2003; Paschen et al, 2003; Humphries et al, 2005). A second possibility is that Sam50 and metaxins are functionally connected, similarly to our observation that when Tom40 is depleted so are the other two TOM complex subunits, Tom20 and Tom22 (Fig 1A). However, our data indicate that metaxin 1 and metaxin 2 form a high-molecular-weight complex that is distinct from the Sam50-containing complex (Fig 5). Although we see a small portion of metaxin 1 migrating in a similar molecular weight range as Sam50, it has been reported that antibodies against metaxin 1 were unable to shift the mammalian SAM complex (Humphries et al, 2005). Together, these data would favour the existence of two different complexes in mammalian mitochondria, one containing metaxins and the other containing Sam50, together with a number of so far unidentified proteins.
Methods
Cell culture and generation of shRNA knockdown cell lines. Different shRNA-expressing vectors were constructed by cloning of the shRNAs (Metabion, Martinsried, Germany) into the pLV-THM vector. All constructs were verified by sequencing. The sequences of the targets of the shRNAs are as follows: Tom40, 5′-GGTTGGCAACGGTAACGTTGG-3′; Tom70, 5′-GCATGCTGTTAGCCGATAAAG-3′; Sam50, 5′-GGACATTCACTGAAATCATCT-3′; and Mtx2, 5′-GGGAAGTCAAACGTAAGATGA-3′. Stably transduced, inducible knockdown cell lines were constructed as described previously (Wiznerowicz & Trono, 2003; see also http://tronolab.epfl.ch/). Briefly, HeLa cells were transduced with the lentiviral pLV-tTR-KRAB-Red vector in the presence of Polybrene (Sigma-Aldrich, St Louis, MO, USA). Cells were then sorted for dsRed expression with the MoFlo® Flow Cytometer (Dako Denmark A/S, Glostrup, Denmark) to generate single cell clones. One HeLa cell clone stably expressing the tTR-KRAB-Red construct was infected with shRNA-carrying virus. Cells transduced with the empty vector were used as a control. Single cell clones were obtained by sorting based on the expression of dsRed, but not green fluorescent protein, to exclude repressor-defective cells. Cells were cultivated in RPMI 1640 (GIBCO, Invitrogen, Carlsbad, CA, USA) with 10% FCS (Biochrom Ltd, Cambridge, UK) and penicillin/streptomycin. To induce the expression of shRNAs, cells were treated with 1 μg/ml Dox for 5 or 7 days as indicated. For validation of the knockdown levels, cell clones were induced with doxycyclin (BD Biosciences, San Jose, CA, USA) for 7 days, and RNA was isolated using the RNAeasy Kit (Qiagen, Hilden, Germany). Quantitative real-time PCR was performed with the QuantiTect SYBR Green real-time PCR Kit (Qiagen) according to the manufacturer's protocol. All vectors were obtained from D. Trono, Ecole Polytechnique Fédérale de Lausanne, France.
In vitro transcription and translation. Ferredoxin and Tom40 cDNAs were obtained by PCR from the clones IRATp970G0617D and IRAUp969B0955D6, respectively (RZPD Deutsches Ressourcenzentrum für Genomforschung GmbH, Berlin, Germany). cDNAs encoding human VDAC1, F1β, CoxVa and DHFR were obtained by reverse transcription from the total HeLa mRNA. All were cloned into the pGEM-4Z vector (Promega, Madison, WI, USA). The CoxVa-DHFR construct consisted of the complete DHFR fused to the first 44 amino acids of CoxVa. The proteins were transcribed/translated in vitro from the plasmids in the presence of 35S methionine/cysteine (GE Healthcare, Buckinghamshire, UK) using the TnT Quick Coupled System (Promega).
In vitro import assays, BN-PAGE and gel filtration chromatography. Isolation of mitochondria from cells and in vitro import were carried out as described previously (Pon & Schon, 2001; Humphries et al, 2005). The import reaction was supplemented with 2 mM ATP and 10 mM sodium succinate. Membrane potential was dissipated by the addition of 1 μM valinomycin before import, where indicated. Import was carried out at 25°C, except for F1β, which was carried out at 37°C. Samples were treated with 50 μg/ml of protease K on import. Protease K was inhibited by the addition of 2 mM phenylmethylsulphonyl fluoride (PMSF). Samples for BN-PAGE analysis were lysed in 45 μl of 0.5% digitoning buffer (0.5% digitonin (Sigma) in 20 mM Tris–HCl, 0.1 mM EDTA, 1 mM PMSF, 50 mM NaCl, 10% (v/v) glycerol, pH 7.4), incubated on ice for 15 min, centrifuged for 10 min at 16,100g, mixed with 5 μl of loading dye (5% (w/v) Coomassie brilliant blue G-250, 150 mM Bis-Tris, 500 mM ɛ-amino-n-caproic acid, pH 7.0) and analysed on 6–13% polyacrylamide gradient gels (Schägger & von Jagow, 1991). 35S-labelled proteins were visualized by using a Fuji FLA3000 imaging system and quantified using AIDA Image Analyzer software. For gel filtration chromatography, a Superdex 200 HR 10/30 column (GE Healthcare) was used.
Antibodies. Antibodies against human Tom40 and Sam50 were a gift from N.J. Hoogenraad; against human metaxin 1 a gift from K. Terada; against rat Tom70 (crossreactive with human Tom70) a gift from K. Mihara; and against mouse metaxin 2 (crossreactive with human metaxin 2) a gift from P. Bornstein. Antibodies against Tom20, Tim23, cytochrome c and F1α antibodies were purchased from BD Biosciences, Tom22 from GeneTex (San Antonio, TX, USA), VDAC from Abcam (Cambridge, UK) and Hsp60 from Stressgen Bioreagents (Ann Arbor, MI, USA).
Acknowledgments
We thank M.T. Ryan for experimental advice, R. Winter for help with gel filtration chromatography, A. Henry for critically reading the manuscript and A. Klein for technical assistance. This work was supported by Alexander von Humboldt Fellowship to V.K.-P. and by the Deutsche Forschungsgemeinschaft (DFG) SPP1131 to T.R.
References
- Abdul KM, Terada K, Yano M, Ryan MT, Streimann I, Hoogenraad NJ, Mori M (2000) Functional analysis of human metaxin in mitochondrial protein import in cultured cells and its relationship with the Tom complex. Biochem Biophys Res Commun 276: 1028–1034 [DOI] [PubMed] [Google Scholar]
- Armstrong LC, Komiya T, Bergman BE, Mihara K, Bornstein P (1997) Metaxin is a component of a preprotein import complex in the outer membrane of the mammalian mitochondrion. J Biol Chem 272: 6510–6518 [DOI] [PubMed] [Google Scholar]
- Armstrong LC, Saenz AJ, Bornstein P (1999) Metaxin 1 interacts with metaxin 2, a novel related protein associated with the mammalian mitochondrial outer membrane. J Cell Biochem 74: 11–22 [PubMed] [Google Scholar]
- Blachly-Dyson E, Baldini A, Litt M, McCabe ERB, Forte M (1994) Human genes encoding the voltage-dependent anion channel (VDAC) of the outer mitochondrial membrane: mapping and identification of two new isoforms. Genomics 20: 62–67 [DOI] [PubMed] [Google Scholar]
- Cheng EHY, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ (2003) VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science 301: 513–517 [DOI] [PubMed] [Google Scholar]
- Gratzer S, Lithgow T, Bauer RE, Lamping E, Paltauf F, Kohlwein SD, Haucke V, Junne T, Schatz G, Horst M (1995) Mas37p, a novel receptor subunit for protein import into mitochondria. J Cell Biol 129: 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamajima S, Sakaguchi M, Mihara K, Ono S, Sato R (1988) Both amino- and carboxy-terminal portions are required for insertion of yeast porin into the outer mitochondrial membrane. J Biochem 104: 362–367 [DOI] [PubMed] [Google Scholar]
- Hines V, Brandt A, Griffiths G, Horstmann H, Brutsch H, Schatz G (1990) Protein import into yeast mitochondria is accelerated by the outer membrane protein MAS70. EMBO J 9: 3191–3200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries AD, Streimann IC, Stojanovski D, Johnston AJ, Yano M, Hoogenraad NJ, Ryan MT (2005) Dissection of the mitochondrial import and assembly pathway for human Tom40. J Biol Chem 280: 11535–11543 [DOI] [PubMed] [Google Scholar]
- Ishikawa D, Yamamoto H, Tamura Y, Moritoh K, Endo T (2004) Two novel proteins in the mitochondrial outer membrane mediate β-barrel protein assembly. J Cell Biol 166: 621–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozjak V, Wiedemann N, Milenkovic D, Lohaus C, Meyer HE, Guiard B, Meisinger C, Pfanner N (2003) An essential role of Sam50 in the protein sorting and assembly machinery of the mitochondrial outer membrane. J Biol Chem 278: 48520–48523 [DOI] [PubMed] [Google Scholar]
- Krimmer T et al. (2001) Biogenesis of porin of the outer mitochondrial membrane involves an import pathway via receptors and the general import pore of the TOM complex. J Cell Biol 152: 289–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisinger C, Rissler M, Chacinska A, Szklarz LKS, Milenkovic D, Kozjak V, Schonfisch B, Lohaus C, Meyer HE, Yaffe MP (2004) The mitochondrial morphology protein Mdm10 functions in assembly of the preprotein translocase of the outer membrane. Dev Cell 7: 61–71 [DOI] [PubMed] [Google Scholar]
- Milenkovic D, Kozjak V, Wiedemann N, Lohaus C, Meyer HE, Guiard B, Pfanner N, Meisinger C (2004) Sam35 of the mitochondrial protein sorting and assembly machinery is a peripheral outer membrane protein essential for cell viability. J Biol Chem 279: 22781–22785 [DOI] [PubMed] [Google Scholar]
- Paschen SA, Waizenegger T, Stan T, Preuss M, Cyrklaff M, Hell K, Rapaport D, Neupert W (2003) Evolutionary conservation of biogenesis of β-barrel membrane proteins. Nature 426: 862–866 [DOI] [PubMed] [Google Scholar]
- Pon LA, Schon EA (2001) Mitochondria. Academic Press, London, UK [Google Scholar]
- Rahmani Z, Maunoury C, Siddiqui A (1998) Isolation of a novel human voltage-dependent anion channel gene. Eur J Hum Genet 6: 337–340 [DOI] [PubMed] [Google Scholar]
- Ryan MT, Müller H, Pfanner N (1999) Functional staging of ADP/ATP carrier translocation across the outer mitochondrial membrane. J Biol Chem 274: 20619–20627 [DOI] [PubMed] [Google Scholar]
- Schägger H, von Jagow G (1991) Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem 199: 223–231 [DOI] [PubMed] [Google Scholar]
- Shimizu S, Narita M, Tsujimoto Y (1999) Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399: 483–487 [DOI] [PubMed] [Google Scholar]
- Waizenegger T, Habib SJ, Lech M, Mokranjac D, Paschen SA, Hell K, Neupert W, Rapaport D (2004) Tob38, a novel essential component in the biogenesis of β-barrel proteins of mitochondria. EMBO Rep 5: 704–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ono K, Kim SO, Kravchenko V, Lin SC, Han J (2001) Metaxin is required for tumor necrosis factor-induced cell death. EMBO Rep 2: 628–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann N, Kozjak V, Chacinska A, Schonfisch B, Rospert S, Ryan MT, Pfanner N, Meisinger C (2003) Machinery for protein sorting and assembly in the mitochondrial outer membrane. Nature 424: 565–571 [DOI] [PubMed] [Google Scholar]
- Wiznerowicz M, Trono D (2003) Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. J Virol 77: 8957–8961 [DOI] [PMC free article] [PubMed] [Google Scholar]


