Abstract
The tubby loci provide a unique opportunity to study adult-onset obesity. Mutation in either mammalian tubby or its homologue in Caenorhabditis elegans, tub-1, results in increased fat storage. Previously, we have shown that TUB-1 interacts with a new Rab GTPase-activating protein, RBG-3, for the regulation of fat storage. To understand further the molecular mechanism of TUB-1, we identified the Rab GTPase downstream of RBG-3. We found that RBG-3 preferentially stimulates the intrinsic GTPase activity of RAB-7 in both human and C. elegans. Importantly, either mutation or RNA interference knockdown in rab-7 reduces stored fat in wild type and tub-1 mutants. In addition, the small GTPase rab-5 and genes that regulate Rab membrane localization and nucleotide recycling are required for the regulation of fat storage, thereby defining a role for endocytic recycling in this process. We propose that TUB-1 controls receptor or sensory molecule degradation in neurons by regulating a RAB-7-mediated endocytic pathway.
Keywords: tubby, Caenorhabditis elegans, RAB-7, fat storage, endocytosis
Introduction
Adult-onset obesity is reaching epidemic proportions worldwide and is a leading cause of type II diabetes, heart disease and hypertension. Mutation in the gene tubby provides one of the few defined models of adult-onset obesity and results in mice with adult-onset obesity, insulin resistance and progressive neurosensory deficits (Carroll et al, 2004). Tubby is also an important candidate gene that influences body weight in humans (Shiri-Sverdlov et al, 2006). Several studies have suggested the following possible roles for tubby: a transcription factor (Boggon et al, 1999; Santagata et al, 2001); an adaptor molecule (Kapeller et al, 1999); or a regulator of vesicular transport (Ikeda et al, 2002; Mukhopadhyay et al, 2005); however, the actual molecular mechanism of tubby is unclear.
The function of tubby as a regulator of fat storage is conserved. Deletion of its homologue in Caenorhabditis elegans, tub-1, leads to increased storage of triglycerides, the main type of fat in worms (Ashrafi et al, 2003; Mukhopadhyay et al, 2005). Therefore, with its amenability for genetic and molecular analysis, C. elegans provides a powerful system for deciphering the molecular mechanism of tubby. We have recently shown that tub-1 interacts with a new Rab GTPase-activating protein (Rab GAP), RBG-3, for the regulation of fat storage (Mukhopadhyay et al, 2005). Here, we show that RBG-3 uses RAB-7 preferentially as a substrate, and that rab-7 is required for normal fat storage in wild type and also tub-1 mutants. In a manner similar to tub-1, we found that rab-7 function is also required for normal chemotaxis. In addition, we identified rab-5 as another important regulator of fat storage. Importantly, involvement of rab-7 and rab-5 indicates that endosomal transport is a functional regulatory component of fat storage. Furthermore, we show that genes that regulate both nucleotide cycling and membrane localization of Rabs are also required for normal fat storage. Together, we propose that in C. elegans TUB-1 regulates fat storage by modulating receptor or sensory molecule degradation through a RAB-7-mediated endocytic mechanism.
Results
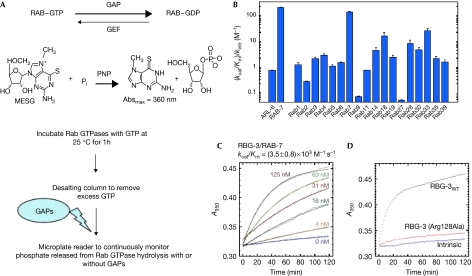
RBG-3 is a GTPase-activating protein for RAB-7
We have shown that TUB-1 functions upstream of a new Rab GAP, RBG-3 (Mukhopadhyay et al, 2005). To explain the downstream signalling mechanism of tub-1, we first profiled the substrate specificity of RBG-3 with respect to a panel of purified mammalian Rab GTPases (Pan et al, 2006), which corresponded to 16 out of the 18 annotated rab genes in the C. elegans database (www.wormbase.org). By using an optical GAP assay that continuously monitors the release of inorganic phosphate resulting from GTP hydrolysis (Fig 1A), we found that the catalytic efficiency (kcat/Km) of RGB-3 relative to the intrinsic rate constant in the absence of RBG-3 (kintr) was highest for Rab7, 5- to 10-fold lower for Rab18 and Rab33, and 10- to 1,000-fold lower for other Rab GTPases (Fig 1B). RBG-3 also showed a 3- to 100-fold higher catalytic efficiency for Rab7 compared with other mammalian Rab GTPases (supplementary Fig S1 online). Thus, RBG-3 shows a preference for human RAB7 as a substrate. When C. elegans RAB-7 was used, we found that RBG-3 could accelerate its GTP hydrolysis (Fig 1B). Kinetic analysis of GTP hydrolysis for RAB-7 in the absence and presence of RBG-3 showed that the catalytic efficiency (kcat/Km) of RBG-3 for RAB-7 was 3,500 M−1 s−1 (Fig 1C). Therefore, we find that RBG-3 has equivalent GAP activity for RAB7 and RAB-7, and significantly weaker activity for other mammalian Rab GTPases.
Figure 1.
Identification of RAB-7 as a preferred substrate of RBG-3. (A) Diagrammatic representation of the GTPase-activating protein (GAP) assay. (B) Profile of catalytic efficiency (kcat/Km) relative to the intrinsic rate constant for GTP hydrolysis of mammalian Rab GTPases, Caenorhabditis elegans RAB-7 and ARL-6. (C) Kinetics of GTP hydrolysis for C. elegans RAB-7 in the absence and presence of the indicated concentrations of RBG-3. Solid lines represent a simultaneously fitted pseudo-first-order Michaelis–Menten model function. (D) Kinetics of GTP hydrolysis for C. elegans RAB-7 in the presence and absence of 250 nM wild-type RBG-3 and the RBG-3(Arg128Ala) mutant. GEF, guanine nucleotide exchange factor; MESG, 2-amino-6-mercapto-7-methylpurine riboside; PNP, purine nucleoside phosphorylase.
The phenotype of tubby mutant mice resembles human syndromes such as Alstrom and Bardet–Biedl (BBS; Naggert et al, 1997). Several loci that lead to BBS have recently been cloned in humans (Beales, 2005; Leroux, 2007) and mutation in ARL6, a small GTPase, was identified as the underlying cause of BBS3 (Fan et al, 2004). Therefore, ARL-6 was considered to be a potential target of RBG-3. When assayed, RBG-3 failed to increase the intrinsic GTPase activity of C. elegans ARL-6 (Fig 1B). These observations suggest that RBG-3 might function as a GAP in a TUB-1/RAB-7 pathway rather than the BBS pathway.
Initially, we suggested that RAB-3 might be a target for RBG-3, on the basis of the following facts: (i) both RAB-3 and TUB-1 are selectively expressed in the neurons (Backberg & Meister, 2004; Mukhopadhyay et al, 2005); (ii) both rab-3 and tub-1 mutants are aldicarb-resistant (Nonet et al, 1997; A.M. & H.A.T., unpublished data); (iii) both rab-3 and tub-1 mutants show behavioural defects (Nonet et al, 1997; Mukhopadhyay et al, 2005); and (iv) mutations result in similar changes in vesicle morphology in neurons (Nonet et al, 1997; Backberg & Meister, 2004). However, on the basis of a GAP assay, we found that RAB-3 is not a substrate for RBG-3. Furthermore, rab-3(y250) or rab-3(y251) (data not shown), and also rab-3 RNA interference (RNAi) worms (Table 1), do not show any defects in fat storage. Therefore, RAB-7 is a preferred substrate of RBG-3 and might be a target of the TUB-1 pathway.
Table 1.
Effect of RNA interference knockdown of different rab and rab-associated protein genes
| CGC name | Sequence name | Alternative name | Effects on growth | Effect on fat storage | |
|---|---|---|---|---|---|
| Wild type | tub-1(nr2004) | ||||
| Rab-1 | C39F7.4 | Rab-1 | S, L, A | ND | ND |
| Rab-2 | F53F10.4 | Unc-108 | E, S, L, A | NC | NC |
| Rab-3 | C18A3.6 | Rab-3, rbl-3 | None | NC | NC |
| Rab-5 | F26H9.6 | Rab-5 | E, L, A | Decrease/ mislocalize | Decrease/ mislocalize |
| Rab-6 | T25G12.4 | Rab-6.2 | None | NC | NC |
| Rab-7 | W03C9.3 | Rab-7 | E | Decrease | Decrease |
| Rab-11 | F53G12.1 | Rab-11.1 | None | NC | NC |
| Rab-14 | K09A9.2 | Rab-14 | None | NC | NC |
| Rab-18 | Y92C3B.3 | Rab-18 | L, A | ND | ND |
| Rab-19 | Y62E10A.9 | Rab-19 | None | NC | NC |
| Rab-27 | Y87G2A.4 | Aex-6 | A | NC | NC |
| Rab-28 | Y11D7A.4 | Rab-28 | None | NC | NC |
| Rab-30 | Y45F3A.2 | Rab-30 | None | NC | NC |
| Rab-33 | F43D9.2 | Rab-33 | None | NC | NC |
| Rab-35 | Y47D3A.25 | Rab-35 | None | NC | NC |
| Rab-39 | D2013.1 | Rab-39 | None | NC | NC |
| — | T08G5.5 | Guanine nucleotide exchange factor | E | Decrease | Decrease |
| — | T05E11.3 | GRP94, endoplasmin, HSP90 | L, A, S, E | Decrease | Decrease |
| — | M57.2 | Protein geranylgeranyltransferase type II, α-subunit | L, E | Decrease | Decrease |
| — | B0280.1 | Protein geranylgeranyltransferase type II, β-subunit | None | NC | NC |
| Obr-1 | Y47D3A.17 | Oxysterol binding protein related | None | NC | NC |
| Obr-2 | F14H8.1 | Oxysterol binding protein related | E | NC | NC |
| Obr-3 | ZK1086.1 | Oxysterol binding protein related | None | NC | NC |
| Obr-4 | C32F10.1 | Oxysterol binding protein related | None | NC | NC |
| A, larval arrest; CGC, Caenorhabditis Genetics Center; E, embryonic lethal; HSP90, heat-shock protein 90; L, larval lethal; NC, no change in fat store; ND, not determined; RNAi, RNA interference; S, sterile. | |||||
| Effect on growth was observed when worms (either wild type or tub-1(nr2004) mutants) were transferred to RNAi at L4 and subsequent generation scored for phenotypes. The rab-1 and rab-18 RNAi effect on fat storage could not be determined owing to the severity of larval lethal/arrest. | |||||
To test whether the observed increase in phosphate production requires the catalytic activity of RBG-3, as would be expected for an authentic RAB-7 GAP, we mutated an arginine residue that constitutes the ‘arginine finger' of RBG-3 to alanine (Arg 128Ala) and determined its effects on the catalytic activity of RAB-7. Rab GAPs with the TRE2/BUB/CDC16 domain have been shown to catalyse GTP hydrolysis by supplying two catalytic residues in trans, an arginine finger and a glutamine finger (Pan et al, 2006). Importantly, this Arg128Ala mutant protein markedly lost its ability to accelerate the hydrolysis of RAB-7 (Fig 1D). The catalytic efficiency of RBG-3(Arg128Ala) decreased to 3 M−1 s−1, which is 1,000-fold lower than that of the wild-type RBG-3. These results show that the catalytic arginine is required for the activity of RBG-3 towards RAB-7.
rab-7 is essential for the regulation of fat storage
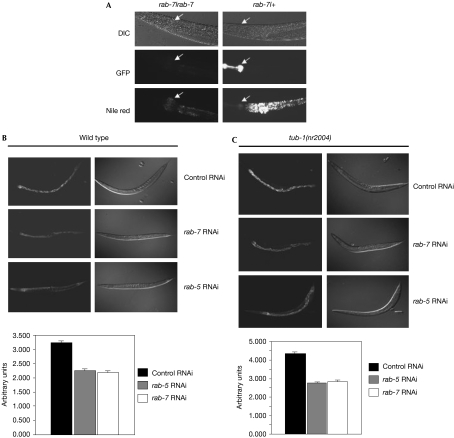
tub-1 and rbg-3 regulate fat storage as shown by Nile Red staining (Mukhopadhyay et al, 2005). Therefore, if rab-7 is a target of the tub-1 pathway, disruption of rab-7 should also affect fat storage. RNAi knockdown of rab-7 or rab-7(ok511) mutants resulted in decreased fat storage in both wild type and tub-1(nr2004) mutants (Fig 2A–C). Thus, RAB-7 is an essential component of the machinery that regulates fat storage in worms.
Figure 2.
Effect of rab-7 and rab-5 RNA interference on wild-type and tub-1(nr2004) fat storage. (A) Homozygous (rab-7/rab-7) or heterozygous (rab-7/+) worms were identified by the presence or absence of GFP expression using the rab-7(ok511) strain (middle panel). Heterozygous worms are wild type with semidominant expression of GFP in the pharynx (indicated by the arrows), whereas homozygous worms lack GFP. The worms were stained with Nile Red to visualize fat storage (bottom panel) and subsequently observed using a rhodamine filter (emission 560–590 nm). (B) Wild-type or (C) tub-1(nr2004) animals were grown on control, rab-7 or rab-5 RNAi and fat staining was visualized using Nile Red (top panel). The corresponding differential interference contrast image is on the right. The Nile Red fluorescence was quantified (bottom panel) using 50 worms. GFP, green fluorescent protein; RNAi, RNA interference.
rab-7 is expressed ubiquitously
tub-1 and rbg-3 are expressed exclusively in the amphid neurons and control fat storage in the intestine (Mukhopadhyay et al, 2005). Therefore, we examined next whether rab-7 was expressed in similar tissues. We expressed the rab-7 gene fused to green fluorescent protein (GFP) in C. elegans. As reported in other systems (Nahm et al, 2003; Verhoeven et al, 2003), RAB-7∷GFP is expressed ubiquitously (Fig 3A), including the amphid neurons where both TUB-1 and RBG-3 are expressed. Expression of the human and mouse homologue of RAB-7 was also found in neurons (Verhoeven et al, 2003; Saxena et al, 2005). Thus, our data show how tissue-specific factors—TUB-1 and RBG-3—regulate fat metabolism by controlling a ubiquitous factor, RAB-7.
Figure 3.
Rab-7 is required for normal chemotaxis. (A) Head region of a rab-7∷gfp-expressing transgenic worm visualized using an FITC filter (left panel) showing expression of GFP. The same worm was dye-filled with DiI and visualized under a rhodamine filter (middle panel) to show the amphid neurons. The two photos were merged (right panel) to show that rab-7 is also expressed in the amphid neurons. (B) Chemotaxis assay of wild-type worms on control RNAi or rab-7 RNAi. Aliquots (1 μl) of benzaldehyde (1:200 diluted), isoamyl alcohol (1:100) or pyrazine (10 mg/ml) were used for the assay. DiI, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine iodade; FITC, fluorescent isothiocyanate; GFP, green fluorescent protein; RNAi, RNA interference.
rab-7 is required for chemotaxis
tub-1(nr2004) mutants are compromised in chemotaxis towards volatile chemoattractants sensed by the AWA and AWC (amphid wing cell A and C, respectively) neurons (Mukhopadhyay et al, 2005). As rab-7 is a potential target of the of tub-1 pathway, we determined whether rab-7-knockdown worms are also defective in chemotaxis. Worms were assayed on pyrazine (10 mg/ml), isoamyl alcohol (1:200) and benzaldehyde (1:100), after being fed rab-7 RNAi or control RNAi bacterial food. Similarly to tub-1 mutants, RNAi of rab-7 resulted in defective chemotaxis to volatile chemoattractants sensed by both the AWA and AWC neurons (Fig 3B). Thus, defects in the TUB-1/RAB-7 pathway result in defective fat storage and defective chemotaxis.
Other RAB proteins in fat storage regulation
To determine the contribution of other C. elegans RABs to fat storage, we used RNAi knockdown of 16 rab genes. Surprisingly, we found that apart from rab-7, only rab-5 significantly affects fat storage. Similar to rab-7, rab-5 knockdown reduced fat storage in both wild type and tub-1(nr2004) mutants (Fig 2B,C). RAB-5 is involved in the early stages of endocytosis, whereas RAB-7 is required for steps leading to fusion with lysosomes (Stein et al, 2003). Therefore, these data suggest that TUB-1 might regulate the degradation of unknown receptors or sensory molecules in neurons, by controlling the endocytic pathway at the level of RAB-7.
Proteins that modify RAB are essential for fat storage
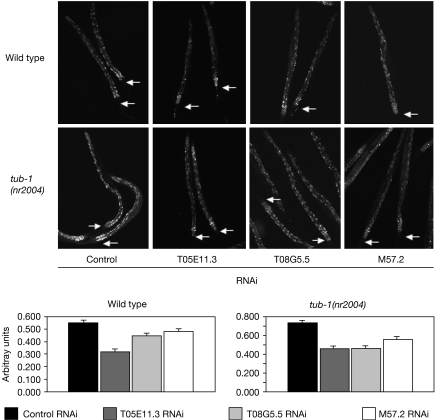
RAB proteins are tightly regulated at the level of membrane association and nucleotide recycling (Stein et al, 2003). The newly synthesized RABs associate with the RAB escort protein (REP), which maintains the complex in a soluble form. This is recognized by RAB geranylgeranyl transferase (RABGGTase) that catalyses the addition of geranylgeranyl groups, facilitating its delivery to the membrane. The RAB–GDP dissociation inhibitor (RAB–GDI) binds to RAB to recycle GDP–RAB to the donor membrane. A heat-shock protein 90 (HSP90) chaperone complex is involved in recognizing the RAB–GDI∷GDP–RAB complex (Sakisaka et al, 2002). We found that several of the RAB regulatory proteins are required for normal fat storage (Fig 4; Table 1). RNAi knockdown of RABGGTase (M57.2), guanine exchange factor (T08G5.5) and HSP90 homologue (T05E11.3) markedly reduced fat storage. We found no defect with the knockdown of Y67D2.1 (REP), B0280.1 (GGTase β-subunit) or oxysterol-binding proteins (obr-1, obr-2, obr-3 and obr-4), whereas the knockdown of Y57G11C.10 (RAB–GDI) was lethal. Together, we found that proteins that mediate correct RAB function are required for normal fat storage.
Figure 4.
Effects of RAB-associated proteins on normal fat storage. Wild-type or tub-1(nr2004) worms were grown on RNAi constructs for T05E11.3 (hsp-82), T08G5.5 (GEF) or M57.2 (GGTase) and fat storage was visualized by using Nile Red. The fluorescence was quantified using 35 worms. Arrows indicate the position of the pharynx. GEF, guanine nucleotide exchange factor; hsp, heat-shock protein; RNAi, RNA interference.
Discussion
Although the tubby gene was cloned a decade ago, the molecular nature of the protein has remained elusive. Using C. elegans, we have recently shown that TUB-1 interacts with RBG-3 to regulate fat storage, implicating vesicular transport in this process (Mukhopadhyay et al, 2005). Here, we have shown that RAB-7 is a downstream target of tubby, thus identifying the endocytic pathway as an important regulator of fat storage.
TUB-1 and RBG-3 are expressed exclusively in the neurons of C. elegans (Mukhopadhyay et al, 2005). However, fat storage occurs only in the intestinal cells of worms. This suggests that there is a neuroendocrine component involved in signalling between the tub-1-expressing neurons and the intestinal cells. The nature of the neuroendocrine signal is largely unknown. However, as the forkhead transcription factor daf-16—the target of the C. elegans insulin-like signalling pathway—fails to suppress the lipid accumulation of tub-1 mutant (Mukhopadhyay et al, 2005), the signal is unlikely to be the C. elegans insulin genes. The endocytic pathway that TUB-1 regulates might be instrumental in secretion of the neuroendocrine signal that is important for the regulation of fat storage.
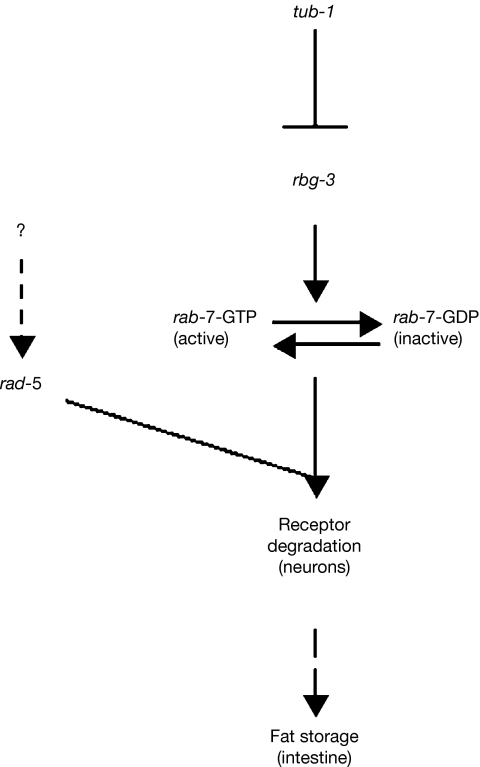
Our findings suggest that, biochemically, a mutation in tub-1 might activate RBG-3 and shift the balance of nucleotide-bound RAB-7 towards the inactive GDP-bound state (Fig 5). The ratio of GTP to GDP-bound RAB-7 might essentially modulate the neuroendocrine signal that is secreted from the neurons to increase fat storage in a tub-1 mutant. However, mutation or RNAi of rab-7 will eliminate both the inactive and active form, resulting in complete abrogation of the neuroendocrine signal and depletion of fat storage in tub-1 mutant and wild-type worms. Similarly, mutations that effectively disrupt the endocytic process, such as depletion of rab-5 (by RNAi), also reduce the fat storage of tub-1, although RAB-5 is not a biochemical target of TUB-1 pathway.
Figure 5.
Mechanism of action of the tub-1 pathway. TUB-1, expressed exclusively in the neurons, regulates RBG-3, a Rab GAP. RBG-3 promotes the GTPase hydrolysis of RAB-7, an important effector of endocytosis. Together, this pathway might regulate the degradation of sensory molecules or receptors in the neurons. Neuroendocrine signals generated in the neurons then regulate fat storage in the intestine. RAB-5 is also an important regulator of endocytosis and fat storage, but is regulated by a mechanism independent of the TUB-1 pathway. Rab GAP, Rab GTPase-activating protein.
We have identified RAB-7 as a target of tub-1 signalling. RAB-7 is proposed to regulate the transport of G-protein-coupled receptors (GPCRs) or other chemosensory receptors from early endosomes to late endosomes and lysosomes (Seachrist & Ferguson, 2003). In one model, which is consistent with our findings, the sensory receptors in C. elegans neurons might need to be periodically replaced with other receptors depending on the sensory inputs, for example, changes in environment. The TUB-1/RBG-3/RAB-7 pathway might regulate recycling and degradation of these receptors in the neurons to control fat storage systemically. Accordingly, Rab5 and Rab7 have recently been found to control endocytic sorting specifically in the neurons (Deinhardt et al, 2006). However, it is still possible that this process might occur in another part of the worm, such as the intestine. Studies on overexpression of rab-7 might help to address this issue; however, overexpression of rab-7 is lethal in C. elegans (A.M. & H.A.T., unpublished data). Our findings are most consistent with a neuronal function for this pathway, as we found that both TUB-1 and RBG-3 are exclusively neuronal, and RBG-3 preferentially uses RAB-7 as a target.
Our study implicates the endocytic pathway in fat storage regulation. We found that apart from rab-7, RNAi knockdown of rab-5 also affects fat storage. RAB5 is involved in segregation of cargo into clathrin-coated vesicles and facilitates early endosome fusion (Stein et al, 2003). We found that some proteins that are important for RAB membrane localization and nucleotide recycling are also required for normal fat storage. The RNAi knockdown of these genes, similar to rab-5 and rab-7, results in varying degrees of embryonic lethality (more than 10%; see Table 1). Therefore, the fat storage phenotype might simply be due to sickness in the treated worms, rather than a specific fat storage defect. To address the specificity of our results, first we fed RNAi bacteria to L1 worms and visualized Nile Red staining in the adults, thus circumventing the embryonic lethality. Second, following RNAi treatment, only healthy-looking worms were used for the Nile Red assays. Our results indicate that the decreased fat storage, as a result of rab-7 or rab-5 RNAi knockdown, is a specific phenotype and not due to a general sickness.
Tubby is an important locus for the regulation of fat storage in humans (Shiri-Sverdlov et al, 2006). Understanding the molecular mechanism of how tubby controls fat storage will help in the evolution of drug targets to adult-onset obesity. Beginning with the identification of RAB7, we have defined early and late endocytic processes in the regulation of fat storage and as a target of tubby. Identifying the proteins that are degraded and recycled is an important next step and might define additional new targets to combat human obesity. Taken together, we have moved one important step forward in understanding the function of tubby in the regulation of fat storage by identifying the vesicular pathway that it regulates.
Methods
Cloning and purification of protein. The cloning and purification of RAB proteins were reported previously (Pan et al, 2006). The GAP domain of RBG-3 (amino acids 7–450) was subcloned into PET24b (Amersham Biosciences, Piscataway, NJ, USA). The RBG-3(Arg128Ala) mutant version was created using the Quickchange Mutagenesis kit (Qiagen-Valencia, CA, USA). BL21(DE3) competent cells transformed with the RBG-3 construct were grown in 2 × YT with 50 mg/l kanamycin at room temperature (23–25°C) to OD600 of 0.3–0.4. Isopropyl-β-D-galactopyranoside was added at a final concentration of 0.05 mM to induce protein expression. After 16–18 h of induction, cells were spun down and resuspended in lysis buffer (50 mM Tris, 100 mM NaCl (pH 8.0), 2 mM MgCl2, 0.1% mercaptoethanol, 0.2 mg/ml lysozyme). Cells were disrupted by sonication and cleared lysates were obtained by centrifugation. The supernatant was loaded on a Ni-NTA agarose column (Qiagen). After the column was washed with a wash buffer (50 mM Tris (pH 8.0), 500 mM NaCl, 20 mM imidazole, 2 mM MgCl2, 0.1% mercaptoethanol), the protein was eluted with elution buffer (250 mM imidazole, 50 mM Tris, 100 mM NaCl (pH 8.0), 2 mM MgCl2, 0.1% mercaptoethanol). RBG-3 protein was further purified by gel filtration on Superdex-75 (Amersham Biosciences).
GAP assay. RAB proteins were first loaded with GTP. RAB (2–3 mg) protein was incubated with 25-fold molar excess of GTP at 25 °C for 1 h in exchange buffer (20 mM HEPES (pH 7.5), 150 mM NaCl, 5 mM EDTA, 1 mM dithiothreitol). Free nucleotides were removed by a D-Salt™ dextran desalting column (Pierce Biotechnology, Rockford, IL, USA) pre-equilibrated with reaction buffer (20 mM HEPES (pH 7.5), 150 mM NaCl). GTP hydrolysis by the RAB GTPases was measured by using the EnzChek Phosphate Assay Kit (Invitrogen, Carlsbad, CA, USA) modified to a 96-well format, which continuously monitors the release of free inorganic phosphate by a spectrophotometric method. Briefly, in each well, a 75 μl solution containing GTP-preloaded RABs was mixed with a 25 μl solution containing enzyme, buffer and GAPs by using a Precision 2000 automated microplate pipetting system (Bio-Tek Instruments Inc., Winooski, VT, USA). The final solution contained 20 mM HEPES (pH 7.5), 150 mM NaCl, 0.15 mM 2-amino-6-mercapto-7-methylpurine riboside (MESG), 7.5 U of purine nucleoside phosphorylase, 10 mM MgCl2, 20 μM GTP-preloaded RABs and the indicated amount of GAPs. The time courses of absorbance change at 360 nm were recorded on a monochromator-based Safire2 microplate detection system (TECAN, Durham, NC, USA). Kinetic data were analysed by simultaneous fitting to the pseudo-first-order Michaelis–Menten equation
where
The catalytic efficiency (kcat/Km) and intrinsic rate constant for GTP hydrolysis (kintr) were treated as global parameters.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
supplementary Information
Acknowledgments
We thank G. Pazour, G. Whitman, S.W. Oh, V. Appleman and members of the Tissenbaum lab for generous help, insights and critical reading of the manuscript. tub-1 mutants were kindly provided by Carl Johnson at Axys Pharmaceuticals. Some strains were kindly provided by T. Stiernagle at the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources. H.A.T. is a William Randolph Hearst Young Investigator. This project was funded in part by a Burroughs Wellcome Career Award in the Biomedical Sciences, an endowment from the William Randolph Hearst Foundation and grants from National Institute on Aging (NIA; AG025891 to H.A.T.) and National Institute of General Medical Sciences (NIGMS; GM56324 to D.G.L.).
References
- Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, Ahringer J, Ruvkun G (2003) Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature 421: 268–272 [DOI] [PubMed] [Google Scholar]
- Backberg M, Meister B (2004) Abnormal cholinergic and GABAergic vascular innervation in the hypothalamic arcuate nucleus of obese tub/tub mice. Synapse 52: 245–257 [DOI] [PubMed] [Google Scholar]
- Beales PL (2005) Lifting the lid on Pandora's box: the Bardet–Biedl syndrome. Curr Opin Genet Dev 15: 315–323 [DOI] [PubMed] [Google Scholar]
- Boggon TJ, Shan WS, Santagata S, Myers SC, Shapiro L (1999) Implication of tubby proteins as transcription factors by structure-based functional analysis. Science 286: 2119–2125 [DOI] [PubMed] [Google Scholar]
- Carroll K, Gomez C, Shapiro L (2004) Tubby proteins: the plot thickens. Nat Rev Mol Cell Biol 5: 55–63 [DOI] [PubMed] [Google Scholar]
- Deinhardt K, Salinas S, Verastegui C, Watson R, Worth D, Hanrahan S, Bucci C, Schiavo G (2006) Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron 52: 293–305 [DOI] [PubMed] [Google Scholar]
- Fan Y et al. (2004) Mutations in a member of the Ras superfamily of small GTP-binding proteins causes Bardet–Biedl syndrome. Nat Genet 36: 989–993 [DOI] [PubMed] [Google Scholar]
- Ikeda A, Zheng QY, Zuberi AR, Johnson KR, Naggert JK, Nishina PM (2002) Microtubule-associated protein 1A is a modifier of tubby hearing (moth1). Nat Genet 30: 401–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapeller R, Moriarty A, Strauss A, Stubdal H, Theriault K, Siebert E, Chickering T, Morgenstern JP, Tartaglia LA, Lillie J (1999) Tyrosine phosphorylation of tub and its association with Src homology 2 domain-containing proteins implicate tub in intracellular signaling by insulin. J Biol Chem 274: 24980–24986 [DOI] [PubMed] [Google Scholar]
- Leroux MR (2007) Taking vesicular transport to the cilium. Cell 129: 1041–1043 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay A, Deplancke B, Walhout AJ, Tissenbaum HA (2005) C. elegans tubby regulates life span and fat storage by two independent mechanisms. Cell Metab 2: 35–42 [DOI] [PubMed] [Google Scholar]
- Naggert J, Harris T, North M (1997) The genetics of obesity. Curr Opin Genet Dev 7: 398–404 [DOI] [PubMed] [Google Scholar]
- Nahm MY, Kim SW, Yun D, Lee SY, Cho MJ, Bahk JD (2003) Molecular and biochemical analyses of OsRab7, a rice Rab7 homolog. Plant Cell Physiol 44: 1341–1349 [DOI] [PubMed] [Google Scholar]
- Nonet ML, Staunton JE, Kilgard MP, Fergestad T, Hartwieg E, Horvitz HR, Jorgensen EM, Meyer BJ (1997) Caenorhabditis elegans rab-3 mutant synapses exhibit impaired function and are partially depleted of vesicles. J Neurosci 17: 8061–8073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Eathiraj S, Munson M, Lambright DG (2006) TBC-domain GAPs for Rab GTPases accelerate GTP hydrolysis by a dual-finger mechanism. Nature 442: 303–306 [DOI] [PubMed] [Google Scholar]
- Sakisaka T, Meerlo T, Matteson J, Plutner H, Balch WE (2002) Rab-αGDI activity is regulated by a Hsp90 chaperone complex. EMBO J 21: 6125–6135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santagata S, Boggon TJ, Baird CL, Gomez CA, Zhao J, Shan WS, Myszka DG, Shapiro L (2001) G-protein signaling through tubby proteins. Science 292: 2041–2050 [DOI] [PubMed] [Google Scholar]
- Saxena S, Bucci C, Weis J, Kruttgen A (2005) The small GTPase Rab7 controls the endosomal trafficking and neuritogenic signaling of the nerve growth factor receptor TrkA. J Neurosci 25: 10930–10940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seachrist JL, Ferguson SS (2003) Regulation of G protein-coupled receptor endocytosis and trafficking by Rab GTPases. Life Sci 74: 225–235 [DOI] [PubMed] [Google Scholar]
- Shiri-Sverdlov R et al. (2006) Identification of TUB as a novel candidate gene influencing body weight in humans. Diabetes 55: 385–389 [DOI] [PubMed] [Google Scholar]
- Stein MP, Dong J, Wandinger-Ness A (2003) Rab proteins and endocytic trafficking: potential targets for therapeutic intervention. Adv Drug Deliv Rev 55: 1421–1437 [DOI] [PubMed] [Google Scholar]
- Verhoeven K et al. (2003) Mutations in the small GTP-ase late endosomal protein RAB7 cause Charcot–Marie–Tooth type 2B neuropathy. Am J Hum Genet 72: 722–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary Information