Abstract
Opposing mitochondrial fission and fusion reactions determine the shape and interconnectivity of mitochondria. Dynamin-related protein 1 (Drp1) is an ancient mechanoenzyme that uses GTP hydrolysis to power the constriction and division of mitochondria. Although Drp1-mediated mitochondrial fragmentation is recognized as an early event in the apoptotic programme, acute regulation of Drp1 activity is poorly understood. Here, we identify a crucial phosphorylation site that is conserved in all metazoan Drp1 orthologues. Ser 656 is phosphorylated by cyclic AMP-dependent protein kinase and dephosphorylated by calcineurin, and its phosphorylation state is controlled by sympathetic tone, calcium levels and cell viability. Pseudophosphorylation of Drp1 by mutation of Ser 656 to aspartic acid leads to the elongation of mitochondria and confers resistance to various pro-apoptotic insults. Conversely, the constitutively dephosphorylated Ser656Ala mutant Drp1 promotes mitochondrial fragmentation and increases cell vulnerability. Thus, Drp1 phosphorylation at Ser 656 provides a mechanism for the integration of cAMP and calcium signals in the control of mitochondrial shape, apoptosis and other aspects of mitochondrial function.
Keywords: mitochondria, apoptosis, dynamin-related protein 1, cAMP-dependent protein kinase, calcineurin
Introduction
Mitochondria are dynamic organelles that constantly fuse and divide, and correct regulation of the mitochondrial fission–fusion equilibrium is essential for cellular homoeostasis. Indeed, mutations in optic atrophy 1 (Opa1) and mitofusin 2 (Mfn2)—two mitochondrial GTPases that are necessary for the fusion of the inner and outer membrane of the organelle, respectively—cause neurodegenerative diseases (Alexander et al, 2000; Delettre et al, 2000; Zuchner et al, 2004), and a dominant-negative mutation in the mitochondrial fission enzyme dynamin-related protein 1 (Drp1; also DLP1 for dynamin-like protein 1) was recently implicated in a fatal human birth defect (Waterham et al, 2007).
Drp1 is a member of the dynamin family of large GTPases. Functional domains in Drp1 include the amino-terminal GTPase domain and a carboxy-terminal GTPase effector domain (GED; Fig 1B), the latter participating in intra- and intermolecular interactions, and regulation of GTPase activity (Hoppins et al, 2007). Drp1 is recruited from the cytosolic compartment to mitochondria by adaptor proteins, including the outer mitochondrial transmembrane protein Fis1. In a manner similar to the endocytosis motor dynamin, Drp1 is thought to polymerize into ring- or spiral-shaped superstructures that constrict and eventually sever mitochondria by a GTP hydrolysis-dependent mechanism (Hoppins et al, 2007). While necessary for biogenesis of the organelle, Drp1-dependent mitochondrial fragmentation is also an early and critical event in apoptosis, coinciding roughly with activation of the pro-apoptotic B-cell lymphoma 2 (Bcl2) family member Bax and permeabilization of the outer mitochondrial membrane (Martinou & Youle, 2006).
Figure 1.
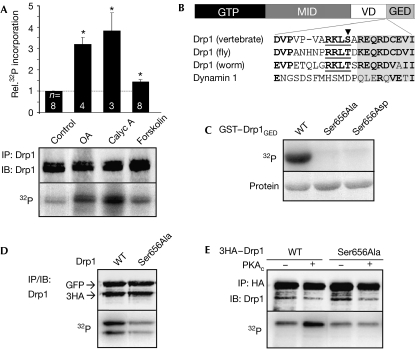
Identification of Drp1 Ser 656 as a PKA phosphorylation site. (A) Endogenous Drp1 was immunoprecipitated (IP) from PC12 cells that had been metabolically labelled with 32PO42−. Cells were treated with okadaic acid (OA, 300 nM, 2 h), calyculin A (Calyc A, 25 nM, 1 h), forskolin (20 μM, 1 h) or vehicle (control) before collection. The bar graph shows 32P incorporation normalized to Drp1 levels and relative to control (mean±s.e.m. of n=3–8 experiments, *P<0.0005 by Student's t-test). (B) Domain diagram of Drp1 and sequence alignment of the boundary between the variable domain (VD) and the GTPase effector domain (GED; PKA consensus underlined). (C) GST–Drp1GED (aa 643–755) fusion proteins were phosphorylated in vitro with PKA and [γ-32P]ATP. (D) GFP and 3 × haemagglutinin (HA)-tagged wild-type (WT) and Ser656Ala Drp1 were expressed in COS cells metabolically labelled with 32P and then immunoprecipitated with a Drp1 antibody. (E) 3 × HA–Drp1 (WT/Ser656Ala) was coexpressed with empty vector (−) or PKAc (catalytic subunit) in COS cells metabolically labelled with 32P and analysed after HA IP. Drp1, dynamin-related protein 1; GFP, green fluorescent protein; GST, glutathione S-transferase; GTP, GTPase domain; IB, immunoblotting; MID, middle domain; PKA, cAMP-dependent protein kinase.
Despite the central importance of Drp1 in mitochondrial function, post-translational regulation of this membrane-remodelling enzyme is not well understood. Previous work suggested a role for sumoylation and ubiquitination in the morphogenetic activity of Drp1 (Harder et al, 2004; Nakamura et al, 2006; Wasiak et al, 2007), and a recent report identified a cyclin-dependent kinase (Cdk) site in Drp1, phosphorylation of which might be responsible for a transient breakdown of the mitochondrial reticulum during mitosis (Taguchi et al, 2007).
Here, we identify a conserved phosphorylation site in Drp1, which is targeted by a second messenger-dependent kinase and phosphatase, cyclic AMP-dependent protein kinase (PKA) and calcineurin (also called protein phosphatase 2B; PP2B). Drp1 mutants with dephosphorylation- and phosphorylation-mimicking substitutions exert opposite effects on mitochondrial morphology and apoptotic sensitivity. Our results indicate that cAMP could mediate survival, in part, through inhibition of Drp1 and that stimulation of the mitochondria-restructuring activity of Drp1 by Ser 656 dephosphorylation is important for programmed cell death.
Results
Drp1 Ser 656 is a major PKA phosphorylation site
We immunoprecipitated endogenous Drp1 from rat phaeochromocytoma PC12 cells that had been metabolically labelled with 32PO42−. Basal 32P incorporation was enhanced severalfold by inhibition of PP1 and PP2A family serine/threonine phosphatases with calyculin A and okadaic acid. Stimulation of cAMP production by forskolin led to a smaller but statistically significant increase in Drp1 phosphorylation (Fig 1A). As cAMP–PKA signalling was implicated in mitochondrial dynamics (Alto et al, 2002; Muller et al, 2005), we scanned the Drp1 sequence for consensus PKA phosphorylation sites. Ser 656 (numbering of rat Drp1 splice variant 1) at the N-terminal border of the GTPase effector domain is highly conserved among metazoan Drp1 orthologues (Fig 1B). All evolutionary substitutions preserve the PKA consensus motif (R-[R/K]- × -[S/T]).
In vitro phosphorylation of wild-type and Ser 656 mutant GST–Drp1643–755 fusion proteins showed that Ser 656 is the only PKA site within this domain (Fig 1C). As shown by transient expression of epitope-tagged complementary DNAs in COS cells, Ser 656 mutation decreased metabolic 32P incorporation into Drp1 to approximately half (52±6%, n=6; Fig 1D). Coexpression of wild-type Drp1 with the PKA catalytic subunit led to a marked enhancement of Drp1 phosphorylation (Fig 1E; 170±19%, n=6), whereas the Ser656Ala mutation blunted this effect (71±6% incorporation compared with wild-type Drp1 without PKAc). Thus, Ser 656 is a major PKA phosphorylation site conserved in evolution.
Drp1 phosphorylation inhibits mitochondrial fission
Drp1 assembles into dimers, tetramers and higher order oligomers, and oligomerization accelerates GTP hydrolysis (Hoppins et al, 2007). Chemical crosslinking of cell extracts expressing epitope-tagged Drp1 showed that neither the dephospho-mimetic Ser656Ala mutation nor the phospho-mimetic Ser656Asp mutation affects Drp1 oligomerization (supplementary Fig 1A online). GTP-agarose pull-down assays from similar extracts indicated that Ser 656 phosphorylation has no qualitative influence on GTP binding (supplementary Fig 1B online). Similarly, [γ-32P]GTP hydrolysis assays carried out with recombinant Drp1 suggested that Ser 656 phosphorylation does not modulate the intrinsic GTPase activity of Drp1 (supplementary Fig 1C online).
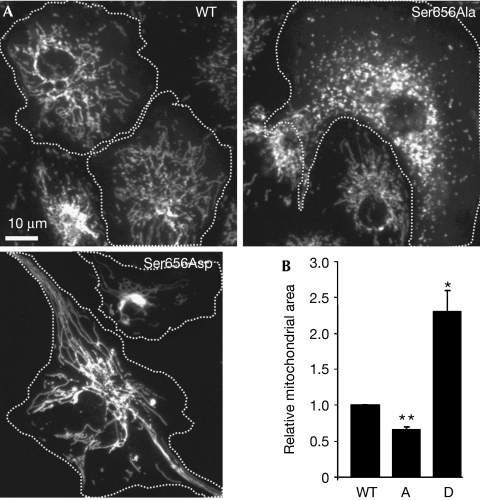
To analyse the effect of Ser 656 phosphorylation on the mitochondria-restructuring activity of Drp1, we combined Ser 656 mutant Drp1 expression with RNA interference (RNAi)-mediated silencing of the endogenous protein. To this end, plasmids were constructed that coexpressed a Drp1-directed short hairpin (sh) RNA and green fluorescent protein (GFP)-tagged Drp1, which was rendered RNAi resistant by silent mutations in the shRNA target sequence (supplementary Fig 2A online). CV1 fibroblasts were transiently transfected to substitute endogenous Drp1 with GFP-tagged wild-type and mutant Drp1, and changes in mitochondrial shape were assessed by digital morphometry of epifluorescence images. Compared with cells substituted with wild-type GFP–Drp1, expression of the phosphomimetic mutant resulted in marked elongation and often perinuclear clustering of mitochondria (Fig 2A,B), presumably because of unbalanced mitochondrial fusion. The opposite phenotype—an abundance of short and dispersed mitochondria—was associated with Drp1 Ser656Ala, indicating that this mutant is hypermorphic (Fig 2A,B). These bidirectional effects are best explained by phosphorylation at Ser 656 inhibiting the scission activity of Drp1.
Figure 2.
Effects of phosphorylation site mutant Drp1 on mitochondrial morphology. (A) Epifluorescence micrographs of immunofluorescently labelled mitochondria from CV1 fibroblasts in which endogenous Drp1 was replaced with wild-type (WT), Ser656Ala and Ser656Asp GFP–Drp1 (transfected cells are outlined). (B) Quantification of the mitochondrial area in CV1 cells substituted with WT, Ser656Ala (A) and Ser656Asp (D) GFP–Drp1 by computer-aided image analysis (mean±s.e.m. of six experiments with 60–80 cells per condition each, normalized to WT). *P<0.01, **P<5 × 10−4 by Student's t-test. Drp1, dynamin-related protein 1; GFP, green fluorescent protein.
To confirm these results using different methods in a different cell type, clonal PC12 cell lines were generated in which endogenous Drp1 was stably replaced with wild-type and Ser 656 mutant GFP–Drp1 (supplementary Fig 2B online). Measuring mitochondrial profiles in transmission electron micrographs of ultrathin sections, we detected a significant increase in the cross-sectional area and length (major axis) of mitochondria in Drp1 Ser656Asp- and a decrease in Ser656Ala-expressing PC12 cells compared with wild type (supplementary Fig 3C online). Cells harbouring the hypermorphic (Ser656Ala) mutant had ultrastructurally normal mitochondria, whereas expression of the hypomorphic (Ser656Asp) Drp1 frequently resulted in swollen mitochondria with christae in various stages of dissolution. In addition, we sometimes noted separation of inner and outer mitochondrial membranes in Drp1 Ser656Asp-harbouring cells (supplementary Fig 3A,B online). Therefore, inhibition of mitochondrial division by prolonged expression of Drp1 Ser656Asp leads to mitochondrial dysfunction. The slower growth rate and increased acidification of the media that we observed in these Drp1-inhibited cells (data not shown) is consistent with decreased ATP production and a switch from oxidative to glycolytic metabolism.
Sympathetic activity promotes Drp1 phosphorylation
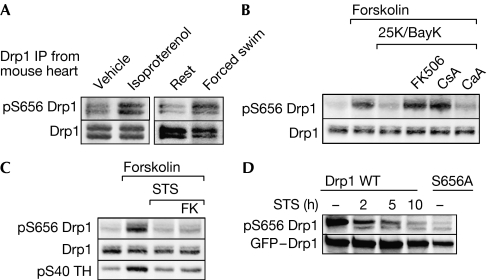
A phosphorylation-specific Drp1 antibody was produced to track Drp1 activity in vivo. We focused on cardiac muscle as a prototypical system for adrenergic regulation of PKA activity. Intraperitoneal injection of the β-adrenergic agonist isoproterenol resulted in reproducible increases in Ser 656 phosphorylation of Drp1 immunoprecipitated from rapidly dissected heart tissue. Mice subjected to a stress and exercise regimen (15 min forced swimming) also showed hyperphosphorylation of heart-derived Drp1 at the PKA site (Fig 3A).
Figure 3.
Regulation of Drp1 Ser 656 phosphorylation in cells and in vivo. (A) Mice received intraperitoneal injections of vehicle or isoproterenol (20 mg/kg; left panel) or were subjected to 15 min of forced swimming or rest (right panel). Drp1 was immunoprecipitated from rapidly dissected heart muscle and analysed for Ser 656 phosphorylation (pS656 Drp1) with a phosphospecific antibody. Multiple bands most likely correspond to splice variants. (B) PC12 cells were preincubated for 15 min with the phosphatase inhibitors FK506 (1 μM), cyclosporin A (CsA, 1 μM) or calyculin A (CaA, 20 nM) and then treated for 60 min with forskolin (10 μM) to activate PKA and 25 mM K+/0.1 μM BayK8644 (25K/BayK) to activate L-type Ca2+ channels. Total lysates were probed for phospho-Ser 656 and total Drp1. (C) PC12 cells preincubated for 15 min with FK506 (FK, 1 μM) or vehicle were treated for 60 min with forskolin (10 μM)±staurosporine (STS, 2 μM), followed by immunoblotting of total lysates as indicated. (D) PC12 cells stably expressing GFP–Drp1 WT or Ser656Ala were incubated with staurosporine (1 μM) for the indicated times (hours), and total lysates were analysed for Drp1 Ser 656 phosphorylation. Data are representative of at least three independent experiments. Drp1, dynamin-related protein 1; GFP, green fluorescent protein; PKA, cAMP-dependent protein kinase, pS40 TH, phospho-Ser 40 tyrosine hydroxylase.
Calcium-dependent dephosphorylation of Drp1 by PP2B
Next, we sought to identify the phosphatase that activates Drp1 through dephosphorylation of Ser 656. Unexpectedly, given that the PP1/PP2A inhibitors calyculin A and okadaic acid increased overall 32P incorporation into Drp1 endogenous to PC12 cells (Fig 1A), neither inhibitor enhanced Drp1 Ser 656 phosphorylation (data not shown). We conclude that PP1/PP2A target sites other than Ser 656. Implicating the calcium-dependent phosphatase calcineurin in the regulation of Drp1, activation of L-type calcium channels by combined membrane depolarization and channel agonist treatment (25 mM KCl, 0.1 μM BayK8466) promoted rapid dephosphorylation of Ser 656 after forskolin pretreatment (Fig 3B). Calcium ionophoresis (2 μM A23187) and calcium release from intracellular stores (10 μM cyclopiazonic acid) also led to Drp1 dephosphorylation (data not shown). Calcium-dependent dephosphorylation was prevented by the calcineurin inhibitors FK506 and cyclosporin A but not by calyculin A (Fig 3B), indicating that calcineurin most likely dephosphorylates Drp1 Ser 656 directly.
Staurosporine antagonizes Drp1 phosphorylation
As PKA is a well-established pro-survival kinase, and because Drp1-mediated mitochondrial fission is important for cytochrome c release from the mitochondria (Martinou & Youle, 2006), we examined the effects of the classical apoptosis inducer staurosporine on Drp1 Ser 656 phosphorylation. Staurosporine blocked forskolin-dependent phosphorylation of endogenous Drp1 apparently by inhibiting PKA activity, as it also prevented tyrosine hydroxylase phosphorylation at Ser 40 (Fig 3C), an established PKA/PP2A site. The staurosporine effect did not involve stimulation of calcineurin activity, as FK506 only marginally increased Drp1 Ser 656 phosphorylation under these conditions (Fig 3C). We also observed staurosporine-induced Ser 656 dephosphorylation at basal cAMP levels (without forskolin), both of stably expressed GFP–Drp1 and endogenous Drp1 (Fig 3D; data not shown).
Drp1 Ser 656 determines apoptotic sensitivity
To examine whether the calcium- or staurosporine-induced dephosphorylation and activation of Drp1 is relevant to programmed cell death, we turned to the PC12 cell lines in which endogenous Drp1 was replaced with wild-type, Ser656Ala mutant and Ser656Asp mutant GFP–Drp1. Two or three independently isolated clones of each Drp1 variant were characterized to control for clonal selection artefacts. Cell morphology, growth rates and spontaneous cell death were comparable in all cell lines except that GFP–Drp1 Ser656Asp-expressing cell lines proliferated at approximately half the rate of other lines, mirroring the phenotype of HeLa cells depleted of Drp1 by RNAi (Benard et al, 2007). In terms of sensitivity to apoptotic stimuli, cell lines expressing wild-type GFP–Drp1 were indistinguishable from GFP-negative cell lines selected in parallel (data not shown).
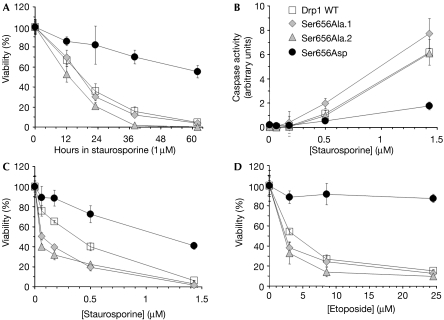
Hypo- and hypermorphic Drp1 mutants showed opposite effects on cell viability in response to several apoptosis inducers. Despite their ultrastructurally abnormal mitochondria, cells expressing the pseudophosphorylated Drp1 hypomorph (Ser656Asp) remained viable in 1 μM staurosporine much longer than wild-type and Drp1 Ser656Ala-expressing cells (Fig 4A). Drp1 Ser656Asp-expressing cells also showed greatly attenuated caspase activation in response to staurosporine (Fig 4B) and showed partial resistance to the topoisomerase inhibitor etoposide, the calcium ionophore A23187 and hydrogen peroxide. Conversely, and in comparison to wild-type Drp1, the constitutively dephosphorylated Ser → Ala mutant conferred increased sensitivity to the same set of insults (shown for two clonal cell lines in Fig 4C,D and supplementary Fig 4A online). One exception was the chemotherapeutic agent doxorubicin, which has pleiotropic effects and promotes cell death by both apoptotic and non-apoptotic mechanisms (Gewirtz, 1999). Doxorubicin killed cells largely indiscriminately of the Drp1 genotype (supplementary Fig 4B online). These experiments indicate that the phosphorylation state of Drp1 at Ser 656 sets the threshold for apoptotic cell death.
Figure 4.
Drp1 Ser 656 determines apoptotic sensitivity. PC12 cells stably substituting wild-type (WT) and Ser 656 mutant GFP–Drp1 for endogenous Drp1 were challenged for the indicated times (A) or for 48 h with the indicated doses of staurosporine or etoposide, followed by caspase activity (B) and viability (A,C,D) assays. Data (mean±s.d. of quadruplicate determinations) are representative of three or more independent experiments. Two to three clonal lines of each Drp1 genotype were analysed (two Drp1 Ser656Ala lines are shown here for comparison). Drp1, dynamin-related protein 1; GFP, green fluorescent protein.
Discussion
Here, we report on the identification of a conserved phosphorylation site in the large GTPase Drp1, which controls the mitochondria-severing activity of the enzyme and mitochondria-dependent cell death. Ser 656 phosphorylation is mediated by PKA in vitro and in intact cells, and occurs in response to sympathetic activity in vivo. Calcium mobilization leads to dephosphorylation of this site, and studies with specific inhibitors have identified calcineurin as the relevant phosphatase. Blocking phosphorylation of Ser 656 by mutation to alanine renders Drp1 hyperactive and sensitizes cells to diverse apoptotic insults, whereas pseudophosphorylation of Drp1 by the Ser656Asp mutation has the opposite effect. These results underscore the pivotal role of Drp1 in apoptosis and provide a molecular mechanism for second messenger regulation of mitochondrial morphogenesis.
It might seem counterintuitive that Drp1 Ser656Asp-expressing PC12 cell lines were significantly protected from apoptotic insults, although their mitochondria showed gross ultrastructural abnormalities. However, our findings parallel the apoptosis resistance, proliferative impairment and bioenergetic defects reported in Drp1-depleted cell lines (Lee et al, 2004; Benard et al, 2007) and thus support the conclusion that Ser 656 phosphorylation inactivates Drp1. As a cancer-derived cell line, PC12 cells are adapted to growth under anaerobic conditions. It remains to be investigated whether Drp1 phosphorylation/inactivation promotes survival in cells such as primary neurons with a lower capacity for glycolytic ATP production.
The identification of Drp1 as a PKA effector is noteworthy in that many growth factors and hormones regulate cellular metabolism by changes in intracellular levels of cAMP. Although the relationship between mitochondrial morphology and bioenergetics is complex (Koopman et al, 2005; Benard et al, 2007), there is some evidence that links cAMP to mitochondrial remodelling (Alto et al, 2002; Muller et al, 2005). With regard to the role of PKA in apoptosis, previous studies have shown that mitochondria-associated PKA activity is critical for cell survival (Harada et al, 1999; Affaitati et al, 2003). Our finding that pseudophosphorylated Drp1 attenuates apoptosis implicates Drp1 as an important survival-promoting substrate of mitochondrial PKA.
The serine/threonine phosphatase calcineurin is one of the main transducers of cytosolic calcium fluxes, with particularly important and well-characterized roles in immune cells and the nervous system (Aramburu et al, 2004). In neurons, ischaemic conditions known to entail massive rises in cytosolic calcium have been shown to fragment mitochondria (Rintoul et al, 2003; Barsoum et al, 2006; Zanelli et al, 2006). To what extent calcineurin-mediated activation of Drp1 is involved in the pathological sequelae of neuronal calcium overload is another question that deserves further study.
The mechanism by which Ser 656 phosphorylation inhibits mitochondrial fragmentation by Drp1 activity is unresolved. Although the phosphorylation site is strategically positioned near the GTPase effector domain, our in vitro studies did not show differences in oligomeric assembly or GTP binding and hydrolysis among the Ser 656 variants. It is possible that Drp1 phosphorylation affects the subcellular distribution of the enzyme, its ubiquitination or sumoylation and/or its association with as yet unidentified effector or regulatory proteins.
A CDK phosphorylation site in Drp1 located just 20 residues to the N-terminal of Ser 656 was recently identified (Taguchi et al, 2007). Intriguingly, the corresponding alanine mutant Drp1 was shown to promote mitochondrial elongation, suggesting that phosphorylation at that site activates the enzyme. In the absence of structural information for Drp1, it is difficult to predict how close the CDK and PKA phosphorylation sites are within three-dimensional space. However, opposite regulation of enzymes by neighbouring phosphorylation sites is well documented (Hudmon & Schulman, 2002). How could CDK and PKA phosphorylation have opposite effects on Drp1 activity? Perhaps phosphorylation at one site prevents access of the kinase or recruits the phosphatase to the other site. Indeed, our results implicate different phosphatases in the regulation of Drp1 by multisite phosphorylation, as PP1/PP2A inhibitors increased total 32P incorporation into Drp1 but not Ser 656 phosphorylation. Regardless of the precise mechanisms involved, reversible phosphorylation of Drp1 by multiple kinases and phosphatases ties the enzyme into a complex regulatory network that allows for rapid mitochondrial remodelling in response to intrinsic and extrinsic signals.
Methods
Metabolic labelling and in vitro phosphorylation. COS cells or PC12 cells were metabolically labelled with 0.5 mCi/ml 32PO42− in phosphate-free medium containing 1% dialysed fetal bovine serum for 4–5 h, with inhibitors and agonists added during the last 1–2 h. Immunoprecipitation of ectopically expressed or endogenous Drp1 was carried out in lysis buffer (1% Triton X-100, 150 mM NaCl, 20 mM Tris (pH 7.5), 1 mM EDTA, 1 mM EGTA, 1 mM β-glycerolphosphate, 1 mM Na3VO4, 1 mM Na4P2O7, 1 μM microcystin-LR, 1 mM phenylmethylsulphonyl fluoride, 1 μg/ml leupeptin, 1 mM benzamidine). Immunoprecipitates were separated by SDS–polyacrylamide gel electrophoresis, immunoblotted for Drp1 and analysed for 32P incorporation by PhosphorImager. 32P signals were divided by Drp1 chemiluminescence signals to obtain relative levels of phosphorylation. Several different volumes of each sample were loaded to ensure that signals were in the linear range of detection. Additional methods can be found in the Supplementary information online.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary information
Supplementary Figurs
Acknowledgments
We are grateful to C. Allamargot of the Central Microscopy Research Facility for electron microscopy, J. Kim for the GTP-agarose pull-down data, N. Martinez for some of the mitochondrial shape analysis, A. Halt and J. Hell for donating mice, C. Blackstone (National Institutes of Health (NIH)) for bacterial expression vectors for Drp1, and Y. Yoon (University of Rochester) for GFP–Drp1 expression vectors. This work was supported by NIH grants NS043254 and NS056244 and United Mitochondrial Disease Foundation grant 04-65 to S.S.
References
- Affaitati A, Cardone L, de Cristofaro T, Carlucci A, Ginsberg MD, Varrone S, Gottesman ME, Avvedimento EV, Feliciello A (2003) Essential role of A-kinase anchor protein 121 for cAMP signaling to mitochondria. J Biol Chem 278: 4286–4294 [DOI] [PubMed] [Google Scholar]
- Alexander C et al. (2000) OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet 26: 211–215 [DOI] [PubMed] [Google Scholar]
- Alto NM, Soderling J, Scott JD (2002) Rab32 is an A-kinase anchoring protein and participates in mitochondrial dynamics. J Cell Biol 158: 659–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramburu J, Heitman J, Crabtree GR (2004) Calcineurin: a central controller of signalling in eukaryotes. EMBO Rep 5: 343–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsoum MJ et al. (2006) Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J 25: 3900–3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard G, Bellance N, James D, Parrone P, Fernandez H, Letellier T, Rossignol R (2007) Mitochondrial bioenergetics and structural network organization. J Cell Sci 120: 838–848 [DOI] [PubMed] [Google Scholar]
- Delettre C et al. (2000) Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet 26: 207–210 [DOI] [PubMed] [Google Scholar]
- Gewirtz DA (1999) A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol 57: 727–741 [DOI] [PubMed] [Google Scholar]
- Harada H, Becknell B, Wilm M, Mann M, Huang LJ, Taylor SS, Scott JD, Korsmeyer SJ (1999) Phosphorylation and inactivation of BAD by mitochondria-anchored protein kinase A. Mol Cell 3: 413–422 [DOI] [PubMed] [Google Scholar]
- Harder Z, Zunino R, McBride H (2004) Sumo1 conjugates mitochondrial substrates and participates in mitochondrial fission. Curr Biol 14: 340–345 [DOI] [PubMed] [Google Scholar]
- Hoppins S, Lackner L, Nunnari J (2007) The machines that divide and fuse mitochondria. Annu Rev Biochem 76: 751–780 [DOI] [PubMed] [Google Scholar]
- Hudmon A, Schulman H (2002) Neuronal Ca2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem 71: 473–510 [DOI] [PubMed] [Google Scholar]
- Koopman WJ, Visch HJ, Verkaart S, van den Heuvel LW, Smeitink JA, Willems PH (2005) Mitochondrial network complexity and pathological decrease in complex I activity are tightly correlated in isolated human complex I deficiency. Am J Physiol Cell Physiol 289: C881–C890 [DOI] [PubMed] [Google Scholar]
- Lee Y-j, Jeong S-Y, Karbowski M, Smith CL, Youle RJ (2004) Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell 15: 5001–5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinou JC, Youle RJ (2006) Which came first, the cytochrome c release or the mitochondrial fission? Cell Death Differ 13: 1291–1295 [DOI] [PubMed] [Google Scholar]
- Muller M, Mironov SL, Ivannikov MV, Schmidt J, Richter DW (2005) Mitochondrial organization and motility probed by two-photon microscopy in cultured mouse brainstem neurons. Exp Cell Res 303: 114–127 [DOI] [PubMed] [Google Scholar]
- Nakamura N, Kimura Y, Tokuda M, Honda S, Hirose S (2006) MARCH-V is a novel mitofusin 2- and Drp1-binding protein able to change mitochondrial morphology. EMBO Rep 7: 1019–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintoul GL, Filiano AJ, Brocard JB, Kress GJ, Reynolds IJ (2003) Glutamate decreases mitochondrial size and movement in primary forebrain neurons. J Neurosci 23: 7881–7888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K (2007) Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem 282: 11521–11529 [DOI] [PubMed] [Google Scholar]
- Wasiak S, Zunino R, McBride HM (2007) Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol 177: 439–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterham HR, Koster J, van Roermund CW, Mooyer PA, Wanders RJ, Leonard JV (2007) A lethal defect of mitochondrial and peroxisomal fission. N Engl J Med 356: 1736–1741 [DOI] [PubMed] [Google Scholar]
- Zanelli SA, Trimmer PA, Solenski NJ (2006) Nitric oxide impairs mitochondrial movement in cortical neurons during hypoxia. J Neurochem 97: 724–736 [DOI] [PubMed] [Google Scholar]
- Zuchner S et al. (2004) Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet 36: 449–451 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Supplementary Figurs