Abstract
A fundamental yet poorly understood aspect of gene regulation in eukaryotic organisms is the mechanisms that control allelic exclusion and mutually exclusive gene expression. In the malaria parasite Plasmodium falciparum, this process regulates expression of the var gene family—a large, hypervariable repertoire of genes that are responsible for the ability of the parasite to evade the host immune system and for pathogenesis of the disease. A central problem in understanding this process concerns the mechanisms that limit expression to a single gene at a time. Here, we describe results that provide information on the mechanisms that control silencing and single gene expression and differentiate between several models that have recently been proposed. The results provide the first evidence, to our knowledge, supporting the existence of a postulated var-specific, subnuclear expression site and also reinforce the conclusion that var gene regulation is based on cooperative interactions between the two promoters of each var gene.
Keywords: malaria, antigenic variation, allelic exclusion, silencing, transcription
Introduction
One of the most interesting yet poorly understood aspects of gene regulation in eukaryotic organisms is the process by which cells choose to express only a single member among many within a family of multiple, functionally equivalent genes. This type of gene regulation is responsible for allelic exclusion of immunoglobulin genes in B cells (Corcoran, 2005), single odorant receptor gene expression (Serizawa et al, 2004), genetic imprinting (Arney et al, 2001) and X-chromosome inactivation in female mammals (Okamoto et al, 2004). Although considerable progress has been made in recent years in understanding certain features of these regulatory pathways, many aspects remain ill-defined. In addition, most work has focused on mutually exclusive expression in higher eukaryotes, in particular, in model organisms such as mice or fruitflies.
Plasmodium falciparum, the parasite responsible for the most severe form of human malaria, invades circulating red blood cells, resulting in severe anaemia and circulatory disruption due to vascular obstruction. During its replication in the red blood cell, parasites modify the host cell, placing a cytoadherence protein called PfEMP1 on the infected cell surface (Kyes et al, 2001). Different antigenic forms of PfEMP1 are encoded by different members of the multicopy var gene family. PfEMP1 expression enables P. falciparum-parasitized red blood cells to sequester in the small venules of deep tissue vascular beds and avoid destruction by the spleen, while expression switches between individual var genes allow the parasites to evade antibody responses against PfEMP1 and thereby produce a persistent infection. Crucial to this process of cytoadhesion and antigenic variation is the absolute requirement that each individual parasite expresses only a single var gene at a time; for this, parasites have evolved a mechanism of strict, mutually exclusive, var gene expression analogous to that described for certain genes in higher eukaryotes. However, in contrast to expression patterns that are typically irreversible in the somatic lines of those systems, malaria parasites have the ability to repeatedly switch var gene expression during the course of an infection.
Expression of var genes is regulated at the level of transcription initiation (Scherf et al, 1998), but little is understood of var promoter recognition or of the mechanisms by which mutually exclusive expression is controlled. One model to explain this process postulates that a var-specific, subnuclear expression site exists in the parasite nucleus, and that by limiting access to a single gene at a time, mutually exclusive expression is achieved (Merrick & Duraisingh, 2006). A similar hypothesis has been proposed for monoallelic variant surface glycoprotein (vsg) gene expression in African trypanosomes (Chaves et al, 1998; Navarro & Gull, 2001). Although this is an intuitively attractive model, until now it has not been possible to show whether such an expression site exists, or what role, if any, it might have in controlling var gene expression. Other proposed models have incorporated promoter–promoter interactions or the involvement of noncoding RNAs in coordinating var gene regulation; however, these hypotheses remain controversial.
Here, we explore these hypothesized models using transgenic parasite lines in which var gene expression can be manipulated using drug selection, and in which specific var loci have been tagged for visualization by fluorescent in situ hybridization (FISH). The data show that cooperative interactions between two promoters found in each gene are required for both silencing and mutually exclusive expression. Disruption of these interactions results in simultaneous expression of several var genes in the same parasite, thus definitively showing the necessity of cooperative regulatory elements in var gene regulation. Furthermore, heterologous promoters can act in place of var intron promoter elements for cooperative regulation, providing information on the silencing mechanism. Simultaneously active var genes colocalize within the nucleus, providing strong evidence for a postulated subnuclear expression site, but also suggesting that it is capable of accommodating several transcriptionally active var promoters.
Results And Discussion
A second promoter is required for var gene silencing
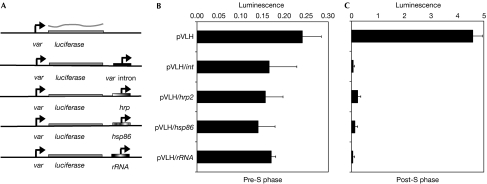
Several previous studies have implicated the intron found in all var genes in the epigenetic silencing of var gene expression (Deitsch et al, 2001a; Calderwood et al, 2003; Gannoun-Zaki et al, 2005; Frank et al, 2006). One possible mechanism for this silencing effect is the classic model of a repressor-binding site existing within the intron that is bound by proteins either preventing transcription complexes from accessing the upstream var promoter, or recruiting chromatin modifiers, which results in the assembly of a silent chromatin state. However, it was also shown that var introns have independent promoter activity and that deleting the part of the intron containing this promoter disrupts the silencing properties of the intron (Calderwood et al, 2003). These observations indicate a possible alternative model for the mechanism that underlies the silencing effect of the intron—namely, that it is the promoter activity itself rather than the DNA sequence that is responsible for the ability of the intron to silence a var gene. To test this hypothesis, we created a series of plasmid constructs in which we placed any one of several, unrelated P. falciparum gene promoters in close proximity to a var promoter that drives expression of a firefly luciferase reporter gene. We found that the P. falciparum heat-shock protein 86 (hsp86) promoter and promoters for genes such as histidine-rich protein 2 (hrp2) and ribosomal RNA somehow interact, either directly or indirectly, with a var upstream promoter, resulting in cooperative silencing similar to that observed with the var intron (Fig 1). Moreover, as previously observed for cooperative interactions with a var intron promoter (Deitsch et al, 2001a), silencing in all cases was S-phase-dependent, strengthening the conclusion that it is the promoter activity of these sequences that contributes to the epigenetic processes responsible for silencing.
Figure 1.
An episomal var promoter is silenced by adjacent promoters. (A) Schematic maps of vectors. Plasmid constructs carry either a ‘free' var promoter (pVLH) or a var promoter paired with a var intron promoter (pVLH/int), the hrp2 promoter (pVLH/hrp), the hsp86 promoter (pVLH/hsp86) or the rRNA promoter (pVLH/rRNA). Luciferase activity was measured in transfected synchronized parasites (B) before S phase, or (C) after proceeding through at least one S phase. S-phase dependence was determined as described previously (Calderwood et al, 2003). Error bars represent calculated standard deviations. hrp2, histidine-rich protein 2; hsp86, heat-shock protein 86; rRNA, ribosomal RNA.
The ability of promoter activity, rather than simple DNA sequence elements, to participate in epigenetic silencing is not without precedent. Replacement of promoters in the X-inactivation centre of mammalian X chromosomes with heterologous promoters from elsewhere in the genome does not disrupt silencing or counting, but can influence choice (Stavropoulos et al, 2001). Our data also provide a possible explanation for var upstream promoter silencing in the absence of a var intron that was recently reported (Voss et al, 2006). The plasmids used in this study also carried an hsp86 promoter (for expression of a drug-resistant cassette) in the neighbourhood of the var upstream promoter. As a consequence, the plasmids were subjected to cooperative silencing from promoter–promoter interactions similar to that observed for the constructs in the experiments shown in Fig 1, thus potentially rendering the intron redundant and unnecessary for silencing.
Cooperative interactions and var gene recognition
An additional question for understanding P. falciparum antigenic variation concerns how var genes are recognized by the mechanism that controls mutually exclusive expression. Previous work has shown that this mechanism normally restricts expression to a single var upstream promoter at any point in time, regardless of whether it is a transgenic promoter on an episome or an endogenous promoter in the chromosome (Dzikowski et al, 2006; Voss et al, 2006). In addition, analysis of concatameric episomes suggested that strict one-to-one pairing between var upstream and intron promoters is required not only for silencing, but also for var gene recognition and mutually exclusive expression (Frank et al, 2006). This is in contrast to a model recently proposed by Voss et al (2006), who reported that parasites are able to express only a single var upstream promoter at a time, regardless of intron pairing. As discussed above, however, the var promoters examined in those experiments might have been interacting with a neighbouring hsp86 promoter, and thus might not in fact have been ‘unpaired'.
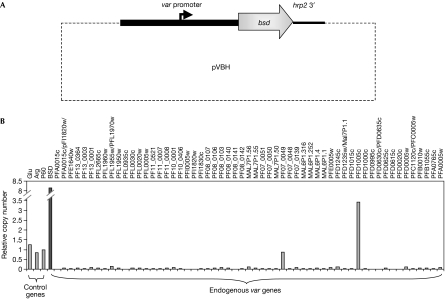
In an attempt to determine definitively whether a ‘free' var promoter is indeed recognized by the P. falciparum allelic exclusion mechanism, we carried out experiments using a plasmid (pVBH; Fig 2A) in which an upstream var promoter was truly isolated from other sequences that could influence its transcriptional activity. Because the blasticidin S deaminase (bsd) cassette in pVBH is driven by this var promoter, blasticidin selection of transfected parasites ensured that all parasites in the drug-selected population contained an active episomal var promoter. Analysis of transcription of the entire var gene family in these transfected parasites detected active transcription of the episomal bsd gene along with two endogenous var genes (PF07-0049 and PFD1005c; Fig 2B), confirming that the ‘unpaired' episomal var promoter was not recognized by the mechanism controlling mutually exclusive expression. Because individual parasites would not survive as drug-sensitive forms in the presence of blasticidin, these results argue strongly for joint expression of bsd under the control of the ‘unpaired' episomal var promoter together with expression of an endogenous var gene in each parasite. Detection of transcripts from two endogenous var genes in the transfectants is consistent with var gene switching and the development of subpopulations carrying two different forms of PfEMP1.
Figure 2.
Simultaneous expression of more than one var promoter in transfected parasites. (A) Schematic map of the plasmid pVBH. (B) Levels of var gene expression detected by quantitative reverse transcription–PCR of complementary DNA obtained from transfected parasites. The columns show messenger RNA levels of each var gene in the parasite genome, the episomal bsd gene and three housekeeping control genes. RNA was extracted from a population in which one var gene (PFD1005c) was predominantly expressed above the others. Note that bsd is transcribed at a relatively high level, reflecting its expression from episomes by all parasites within the population.
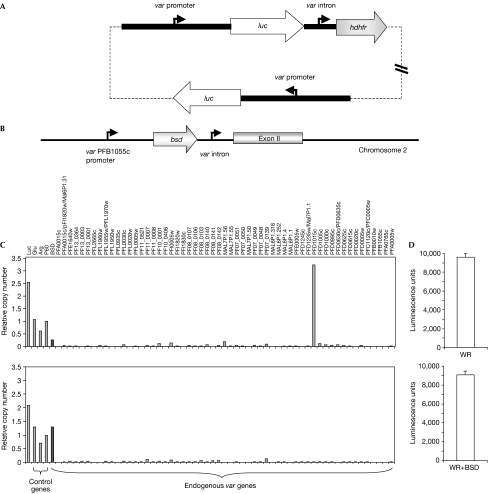
A possible criticism of the above experiments is that the var promoter in pVBH had been selected by drug pressure to be active, so its default state of transcriptional activity thus might not have been accurately observed. To address this possibility, we carried out additional experiments with a plasmid containing two var upstream promoters, one paired with an intron and the other not paired and therefore constitutively active (pVLH/IDH-FP; Fig 3A; Frank et al, 2006). In transfectants carrying this plasmid, it was therefore possible to measure luciferase messenger RNA levels from an unpaired var promoter that had not been under direct selection. This plasmid was transfected into parasites that had been previously engineered by allelic replacement to carry a bsd cassete at var locus PFB1055c (Fig 3B; Dzikowski et al, 2006). The recombinant var promoter driving bsd expression at this locus is paired with the endogenous intron, and previous work has shown that it is recognized and subjected to mutually exclusive expression (Dzikowski et al, 2006).
Figure 3.
Expression of luciferase, bsd and the var gene family in transfected parasite lines. (A) Schematic map of pVLH/IDH-FP. (B) Schematic map of the var gene PFB1055c in the transgenic parasite line B12E3. (C) Results confirming simultaneous transcription of two var promoters in stably transfected parasite lines. Upper panel: B12E3 stably transfected with pVLH/IDH-FP and grown in the absence of blasticidin. Both the endogenous var gene PFD1015c and luciferase are highly expressed, whereas all other var genes are silent. Lower panel: the same parasite line, grown for several weeks under blasticidin pressure. Expression switched to the recombinant var (PFB1055c) containing the bsd-resistant gene, which was then transcribed in a mutually exclusive manner. However, the expression level of the luciferase gene being driven by an ‘unpaired' var promoter remains unchanged. (D) Simultaneous protein expression by two var promoters in stably transfected parasite lines. Luciferase is highly expressed in transfected parasites grown with (lower panel) or without (upper panel) blasticidin pressure. Stable episomes were maintained using WR99210 (WR) pressure to select for expression of the human dihydrofolate reductase gene (hdhfr).
Results from the pVLH/IDH-FP transfectants free of blasticidin pressure showed both active luciferase transcription from the constitutively active plasmid promoter and high levels of transcription from the endogenous var gene PFD1015c (Fig 3C). This suggests that the unpaired promoter was not recognized by the exclusive control mechanism and that more than one var promoter was transcribed in individual parasites. When the pVLH/IDH-FP-transfected parasites were subjected to blasticidin selection, only those parasites that switched to bsd expression under the PFB1055c promoter were able to survive. In these parasites, expression of PFD1015c and all other chromosomal var genes were silenced (Fig 3C), indicating that the endogenous var promoter driving bsd expression that is paired with an intron is recognized and expressed in a mutually exclusive manner. Nevertheless, these parasites continued to express high levels of luciferase (Fig 3D).
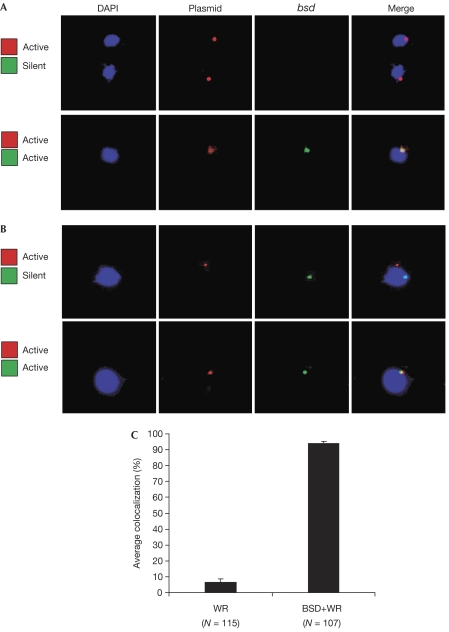
To validate further that both var promoters were, in fact, simultaneously active in the same cell, RNA-FISH was carried out to detect active transcription of both bsd and luciferase in individual nuclei. These experiments detected both var promoter-driven transcripts in 30 out of 30 nuclei examined (Fig 4A, lower panel), confirming that a var promoter that is separated from its intron (or other paired promoter activity) remains active in the presence of an actively transcribed endogenous var locus, and thus is not recognized by the exclusive expression mechanism. Interestingly, in the cells examined, both var promoter-driven transcripts frequently localized within the same region of the nucleus, leading us to investigate the possible existence of a var-specific expression site.
Figure 4.
Nuclear positioning of two var loci in different activation states. Nuclei are stained with DAPI (blue). (A) RNA-FISH. Upper panel: nuclear positioning of messenger RNA actively transcribed from the luciferase gene (red) on the plasmid pVLH/IDH-FP in cells in which the chromosomal bsd cassette is silent. Lower panel: nuclear positioning of mRNA of both luciferase (red) and the endogenous bsd cassette (green) when they are both actively expressed. (B) DNA-FISH. Upper panel: nuclear positioning of actively transcribed pVLH/IDH-FP episomes (red) and of a silent bsd cassette under the control of a chromosomal var promoter (green). Lower panel: nuclear positioning when both luciferase (red) carried on the pVLH/IDH-FP episomes and the endogenous bsd cassette (green) are actively expressed. (C) Quantification of colocalization shown in (B). Error bars show standard deviations of multiple, independent counts. DAPI, 4',6-diamidino-2-phenylindole; FISH, fluorescent in situ hybridization; WR, WR99210 selection.
Detection of a var-specific subnuclear expression site
Investigations into the structure of the P. falciparum nucleus have identified a region of uncondensed euchromatin in the largely heterochromatic nuclear periphery (Ralph et al, 2005). This subnuclear region has been suggested to function as a specific expression site for var promoters, and a model for mutually exclusive expression proposes that this site can accommodate only one var promoter at a time, similar to a model proposed for the mutually exclusive expression of vsg genes in African trypanosomes (Navarro & Gull, 2001). Furthermore, some recent publications have reported that var genes move to a different position in the nucleus on transcriptional activation (Duraisingh et al, 2005; Ralph et al, 2005; Voss et al, 2006). However, it has not yet been determined whether active var genes move to a specific site, or whether they simply move to one of several transcriptionally competent regions of the nucleus. We were therefore interested to localize var transcription in our transgenic parasite lines containing two simultaneously active var promoters.
Using DNA-FISH and probes specific to the pVLH/IDH-FP episome or to the recombinant chromosomal gene expressing bsd, we found that the constitutively active, unpaired episomal var promoter colocalized with the active PFB1055c-driven bsd gene in greater than 90% of the transfectants selected by blasticidin (Fig 4B,C). By contrast, less than 10% of cells showed colocalization in transfected parasite populations without blasticidin pressure and primarily expressing the endogenous var gene PFD1015c. Removal of WR99210 pressure resulted in rapid shedding of the plasmid (confirmed by quantitative PCR) and loss of luciferase signal, indicating that in all cases the pVLH/IDH-FP plasmid had not integrated into the genome.
The level of colocalization between the simultaneously active var promoters detected here (>90%) was higher than previously reported for the colocalization of an active var promoter with the promoter of an unrelated, constitutively active gene (∼60%; Duraisingh et al, 2005). We also found that an active var gene frequently colocalized with the active, unrelated gene PFF1125c; however, the rate of colocalization was lower than that observed for two active var genes (supplementary Fig S1 online). This might indicate that the nucleus of the parasite contains a limited number of sites of transcriptional activity, and thus transcriptionally active genes, regardless of promoter type, tend to colocalize frequently in these subnuclear regions. However, var promoters seem to require more strict localization into a specific subnuclear site, thus resulting in the near-complete colocalization of the two active var promoters detected in this study. This might indicate the presence in the nucleus of a localized, var-specific factor necessary for activation of var gene transcription, although this factor seems to be able to actively transcribe at least two var promoters simultaneously, suggesting that restricted accommodative capacity of the expression site alone does not explain strict mutually exclusive var gene expression. Furthermore, the two active var promoters in the FISH images frequently seemed to be immediately adjacent to one another, rather than completely overlapping, suggesting a relatively large subnuclear region (see supplementary Fig S2 online for images). Interestingly, a silent var promoter colocalized with an active unrelated promoter approximately 25% of the time (supplementary Fig S1 online), whereas active and silent var promoters colocalized in only 10% of cases, indicating that there might be a mechanism that actively separates active and silent var genes. Further work into the structure and function of Plasmodium nuclear subdomains will provide more information on this potentially important aspect of var gene regulation.
Methods
Parasite culture and transfection. All experiments used the P. falciparum NF54 line cultivated by standard methods (Trager & Jensen, 1976). Parasites were transfected by spontaneous uptake of DNA in plasmid-loaded red blood cells as described previously (Deitsch et al, 2001b). Transfected parasites were selected in 4 nM WR99210 and/or 2 μg/ml blasticidin as appropriate.
DNA constructs. The plasmids pVLH, pVLH/int, and pVLH/IDH-FP were described previously (Deitsch et al, 2001a; Dzikowski et al, 2006; Frank et al, 2006). The other plasmid constructs were derived from these as described in detail in the supplementary information online.
Assays of var transcription by real-time reverse transcription–PCR. RNA extraction, production of complementary DNA and analysis of expression of the var gene family were carried out as described previously (Dzikowski et al, 2006; Frank et al, 2006).
Fluorescent in situ hybridization. DNA-FISH was carried out on ring-stage parasites as described previously (Freitas-Junior et al, 2000) with slight modifications (see the supplementary information online). Images of nuclei were collected, randomly coded and scored independently by three individuals. RNA-FISH was carried out as described previously (Thompson, 2002) with slight modifications (see the supplementary information online).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
supplementary Figures and Legends
Acknowledgments
We thank O. Dzikowski and D. Raskolnikov for their help in counting and scoring colocalization in the FISH experiments. This work was supported by National Institutes of Health (NIH) grant AI 52390 and a grant from the Ellison Medical Foundation. The Department of Microbiology and Immunology at Weill Medical College of Cornell University acknowledges the support of the William Randolph Hearst Foundation. T.E.W. is supported by the Intramural Research Program of the NIH, NIAID. K.W.D. is a Stavros S. Niarchos Scholar.
References
- Arney KL, Erhardt S, Drewell RA, Surani MA (2001) Epigenetic reprogramming of the genome—from the germ line to the embryo and back again. Int J Dev Biol 45: 533–540 [PubMed] [Google Scholar]
- Calderwood MS, Gannoun-Zaki L, Wellems TE, Deitsch KW (2003) Plasmodium falciparum var genes are regulated by two regions with separate promoters, one upstream of the coding region and a second within the intron. J Biol Chem 278: 34125–34132 [DOI] [PubMed] [Google Scholar]
- Chaves I, Zomerdijk J, Dirks-Mulder A, Dirks RW, Raap AK, Borst P (1998) Subnuclear localization of the active variant surface glycoprotein gene expression site in Trypanosoma brucei. Proc Natl Acad Sci USA 95: 12328–12333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran AE (2005) Immunoglobulin locus silencing and allelic exclusion. Semin Immunol 17: 141–154 [DOI] [PubMed] [Google Scholar]
- Deitsch KW, Calderwood MS, Wellems TE (2001a) Malaria. Cooperative silencing elements in var genes. Nature 412: 875–876 [DOI] [PubMed] [Google Scholar]
- Deitsch KW, Driskill CL, Wellems TE (2001b) Transformation of malaria parasites by the spontaneous uptake and expression of DNA from human erythrocytes. Nucleic Acids Res 29: 850–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraisingh MT, Voss TS, Marty AJ, Duffy MF, Good RT, Thompson JK, Freitas-Junior LH, Scherf A, Crabb BS, Cowman AF (2005) Heterochromatin silencing and locus repositioning linked to regulation of virulence genes in Plasmodium falciparum. Cell 121: 13–24 [DOI] [PubMed] [Google Scholar]
- Dzikowski R, Frank M, Deitsch K (2006) Mutually exclusive expression of virulence genes by malaria parasites is regulated independently of antigen production. PLoS Pathog 2: e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M, Dzikowski R, Constantini D, Amulic B, Burdougo E, Deitsch K (2006) Strict pairing of var promoters and introns is required for var gene silencing in the malaria parasite Plasmodium falciparum. J Biol Chem 281: 9942–9952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas-Junior LH, Bottius E, Pirrit LA, Deitsch KW, Scheidig C, Guinet F, Nehrbass U, Wellems TE, Scherf A (2000) Frequent ectopic recombination of virulence factor genes in telomeric chromosome clusters of P. falciparum. Nature 407: 1018–1022 [DOI] [PubMed] [Google Scholar]
- Gannoun-Zaki L, Jost A, Mu JB, Deitsch KW, Wellems TE (2005) A silenced Plasmodium falciparum var promoter can be activated in vivo through spontaneous deletion of a silencing element in the intron. Eukaryotic Cell 4: 490–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyes S, Horrocks P, Newbold C (2001) Antigenic variation at the infected red cell surface in malaria. Annu Rev Microbiol 55: 673–707 [DOI] [PubMed] [Google Scholar]
- Merrick CJ, Duraisingh MT (2006) Heterochromatin-mediated control of virulence gene expression. Mol Microbiol 62: 612–620 [DOI] [PubMed] [Google Scholar]
- Navarro M, Gull K (2001) A pol I transcriptional body associated with VSG mono-allelic expression in Trypanosoma brucei. Nature 414: 759–763 [DOI] [PubMed] [Google Scholar]
- Okamoto I, Otte AP, Allis CD, Reinberg D, Heard E (2004) Epigenetic dynamics of imprinted X inactivation during early mouse development. Science 303: 644–649 [DOI] [PubMed] [Google Scholar]
- Ralph SA, Scheidig-Benatar C, Scherf A (2005) Antigenic variation in Plasmodium falciparum is associated with movement of var loci between subnuclear locations. Proc Natl Acad Sci USA 102: 5414–5419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf A, Hernandez-Rivas R, Buffet P, Bottius E, Benatar C, Pouvelle B, Gysin J, Lanzer M (1998) Antigenic variation in malaria: in situ switching, relaxed and mutually exclusive transcription of var genes during intra-erythrocytic development in Plasmodium falciparum. EMBO J 17: 5418–5426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serizawa S, Miyamichi K, Sakano H (2004) One neuron–one receptor rule in the mouse olfactory system. Trends Genet 20: 648–653 [DOI] [PubMed] [Google Scholar]
- Stavropoulos N, Lu N, Lee JT (2001) A functional role for Tsix transcription in blocking Xist RNA accumulation but not in X-chromosome choice. Proc Natl Acad Sci USA 98: 10232–10237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J (2002) In situ detection of RNA in blood- and mosquito-stage malaria parasites. Methods Mol Med 72: 225–233 [DOI] [PubMed] [Google Scholar]
- Trager W, Jensen JB (1976) Human malaria parasites in continuous culture. Science 193: 673–675 [DOI] [PubMed] [Google Scholar]
- Voss TS, Healer J, Marty AJ, Duffy MF, Thompson JK, Beeson JG, Reeder JC, Crabb BS, Cowman AF (2006) A var gene promoter controls allelic exclusion of virulence genes in Plasmodium falciparum malaria. Nature 439: 1004–1008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary Figures and Legends