Abstract
Just as people head to the beaches for a well-deserved rest, accumulating evidence suggests that transcription factors take similar ‘vacations' at the nuclear envelope. Recent studies indicate that the periphery of the nucleus provides a platform for sequestering transcription factors away from chromatin. Several transcriptional regulators, operating in different signal-transduction pathways, have been found to interact physically with components of the inner nuclear membrane. In general, this association seems to restrict access to their target genes and limit their transactivation or transrepression abilities. The mechanisms of inner nuclear membrane association are diverse, and include regulated associations with the nuclear lamina and integral membrane proteins. Together, these findings indicate that the inside of the nuclear envelope functions as a resting place for transcription factors and suggest a more direct role for the nuclear envelope in gene regulation than previously anticipated.
Keywords: gene expression, inner nuclear membrane, lamin, nuclear lamina, signal transduction
Introduction
The nuclear envelope (Franke et al, 1981) is arguably the most important border within the eukaryotic cell: it isolates chromatin from the cytoplasm, which allows additional levels of regulation and, perhaps, defence. Transport across the nuclear envelope occurs through nuclear pore complexes (NPCs), which span both the inner and outer nuclear membranes (INM and ONM, respectively). These membranes contain different sets of proteins and, considering the continuity of the nuclear envelope to the endoplasmic reticulum, most ONM proteins are likely to perform endoplasmic reticulum-related functions. The roles of INM proteins are less intuitive, although several functions have been attributed to them. Here, we briefly summarize the functions before discussing the newly emerging role for these proteins, which is the subject of this review.
Known and putative functions of the INM
In higher eukaryotes, the INM is associated with a network of intermediate filament proteins called lamins (Stuurman et al, 1998), of which there are two types: lamin A/C and lamin B, where lamin A and C are variants of the same gene. Together, these form the nuclear lamina—a stable structure beneath the INM. Many different INM proteins bind to lamins, presumably to tightly anchor the nuclear lamina to the INM (Fig 1A).
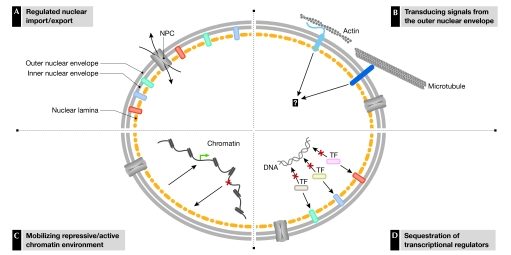
Figure 1.
Proposed parallel functions of the nuclear envelope in regulating gene expression. (A) Regulated access of transcription factors (TFs; curved arrows) through nuclear pore complexes. (B) Signal transduction through nuclear envelope lumen-spanning protein assemblies of nesprin/uncoordinated 84 (UNC84; light blue) and UNC-83–UNC-84 (dark blue). (C) Assembly of a repressive chromatin environment. (D) Sequestering of transcriptional regulators.
Two nuclear envelope protein pairs, vertebrate nesprin–SUN (Sad1 and uncoordinated 84 (UNC84) homology), and Caenorhabditis elegans UNC-83–UNC-84, span the nuclear envelope lumen, and therefore connect the INM and ONM (reviewed in Gruenbaum et al, 2005). In vertebrates, it has been shown that nesprins interact with cytoskeletal elements, whereas SUNs interact with the nuclear lamina. These interactions are thought to regulate the correct positioning of the nucleus within the cell, but might also have a role in signal transduction (Fig 1B).
Another crucial function of the INM, which is not discussed in detail here but was recently reviewed in Akhtar & Gasser (2007), is the organization of chromatin (Fig 1C). The association of genes with the nuclear envelope has been linked to both the repression and activation of transcription. For example, several studies of the regulation of mating-type loci and telomeres in the yeast Saccharomyces cerevisiae have shown that, when silenced, these chromatin regions are preferentially located in close proximity to the nuclear envelope in an environment enriched with repressive factors (Akhtar & Gasser, 2007). In fact, the accumulation of repressive factors on silenced chromatin might even reinforce tethering to the nuclear envelope. By contrast, transcriptional activation has been linked to the translocation of genes to the nuclear periphery, specifically to regions containing NPCs, although such re-localization does not appear to be obligatory.
This review focuses on an additional function of the INM, which has emerged from recent insights. INM proteins engage in direct chromatin-independent interactions with transcription factors (Fig 1D), and the sequestering of transcription factors to the INM limits their transactivation or transrepression abilities. Here, we describe the examples of this function discovered so far, and discuss the roles of both the lamins and the integral INM proteins that participate in these interactions.
Lamins as transcription factor magnets
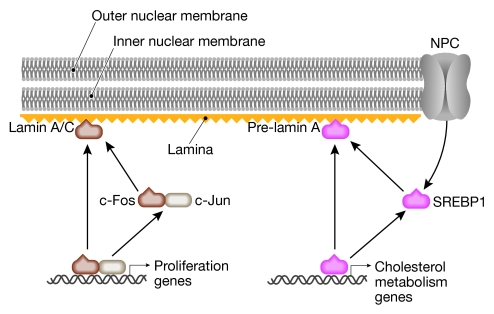
The most well-documented example of the principle of sequestering transcription factors to the nuclear envelope is probably that of c-Fos by lamin A/C (Ivorra et al, 2006). c-Fos, together with c-Jun, forms the transcription factor activating protein 1 (AP1), which participates in several crucial cellular processes including cell proliferation and differentiation. Ivorra and co-workers isolated lamin A/C as a heterodimerization partner of c-Fos. In serum-starved quiescent cells, AP1 activity is low and c-Fos is localized to the nuclear envelope (Fig 2). This perinuclear localization of c-Fos strictly requires lamin A/C, as the addition of serum restores AP1 function and relocates c-Fos from the nuclear periphery to the nucleoplasm. Conversely, lamin A/C remains at the nuclear envelope and is therefore a negative regulator of AP1 through the sequestering of c-Fos to the INM. Interestingly, Lmna−/− cells show enhanced proliferation (Ivorra et al, 2006), indicating that the lamin–c-Fos interaction regulates maintenance of the quiescent state. However, proliferation might also be enhanced by a lack of lamin A/C-dependent anchoring of the retinoblastoma (Rb) protein (Johnson et al, 2004; Pekovic et al, 2007), which is an intranuclear event that also involves the non-membrane lamin A/C-interacting protein, lamina-associated polypeptide 2α (LAP2α; Markiewicz et al, 2002).
Figure 2.
Model of the interactions of transcription factor complexes at the nuclear lamina with lamin A/C (left) or pre-lamin A (right). c-Fos–c-Jun heterodimers activate a proliferative genetic programme. Sequestering of c-Fos by lamin A/C results in the suppression of this programme. Low cholesterol induces the nuclear access of sterol regulatory element-binding protein 1 (SREBP1) so that it can activate cholesterol metabolic genes. This activation is downregulated by the sequestering of SREBP1 by pre-lamin A. NPC, nuclear pore complex.
The mature form of lamin A is generated through a series of post-translational modifications that include farnesylation and proteolytic processing to remove the farnesylated terminus. The unprocessed lamin A—or pre-lamin A—was found to associate with the sterol regulatory element-binding protein 1 (SREBP1; Capanni et al, 2005). SREBP1 is synthesized as a precursor protein embedded in the endoplasmic reticulum membrane. The depletion of cholesterol causes intramembrane proteolysis and the release of the DNA-binding amino-terminal section of SREBP1, which translocates to the nucleus and induces the expression of cholesterol metabolism genes such as peroxisome proliferator-activated receptor-γ (PPARγ; Fajas et al, 1999). Pre-lamin A and SREBP1 co-localize to the nuclear rim, which coincides with the downregulation of PPARγ expression (Capanni et al, 2005). Therefore, it is thought that pre-lamin A limits the access of the transcription factor SREBP1 to the nuclear interior. However, there is evidence that the overexpression of pre-lamin A alone is able to repress the expression of PPARγ, even when localized to the nuclear interior (Capanni et al, 2005), which questions the necessity of SREBP1 sequestration at the nuclear lamina. By contrast, this might reflect a more general inhibitory effect of overexpressed lamins on RNA polymerase II transcription (Kumaran et al, 2002; Spann et al, 2002).
Of notable interest is the disease familial partial lipodystrophy, which is characterized by impaired lamin A/C processing. Pre-lamin A specifically accumulates in lipodystrophy cells (Capanni et al, 2005) and this phenotype seems to be directly correlated with altered PPARγ induction, because the forced accumulation of pre-lamin A in wild-type pre-adipocytes impairs their differentiation and reduces the amount of DNA-bound SREBP1.
Another transcription factor that might be sequestered by a lamin is octamer-binding transcription factor 1 (OCT1; Imai et al, 1997). OCT1 localizes to the nuclear periphery, which correlates with repression of the collagenase gene, a transcriptional target of OCT1. However, at present, it is unclear whether OCT1 localization is the cause or effect of gene repression—that is, whether OCT1 directly associates with the nuclear lamina or only with repressed genes that are located there. Physical interaction studies between OCT1 and lamins, and intranuclear localization of the repressed collagenase gene, might answer this question.
INM transcription factor scavengers
In addition to nuclear lamins, several integral INM proteins have been found to interact with transcription factors. Approximately 15 integral INM proteins have been fully characterized in mammalian cells so far and two recent genomic approaches (Dreger et al, 2001; Schirmer et al, 2003) have substantially increased the number of putative INM proteins. Integral INM proteins seem to have little in common (Schirmer & Gerace, 2005), although three INM proteins share a lamin-associated polypeptide (LAP), emerin and MAN1 (LEM) domain of approximately 40 amino acids (Lin et al, 2000), which has been found to function as a promiscuous protein–protein interaction platform. Most integral INM proteins are not specifically associated with NPCs and localize throughout the INM.
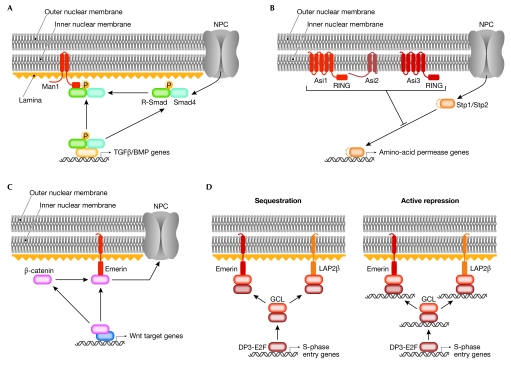
MAN1 is an integral INM protein with an amino-terminal LEM domain, two transmembrane domains and a carboxy-terminal RNA-recognition motif. Several studies have probed the interaction between MAN1 and Smad transcription factors (Bengtsson, 2007; Cohen et al, 2007; Ishimura et al, 2006; Lin et al, 2005; Osada et al, 2003; Pan et al, 2005). Smads are crucial regulators of transforming growth factor-β (TGFβ), bone morphogenic protein (BMP) and activin signalling (reviewed in Massague et al, 2005). Activation of TGFβ and BMP receptors at the plasma membrane leads to phosphorylation of receptor-specific R-Smads, which oligomerize with the common mediator Smad4, and subsequently translocate to the nucleus, where they bind to DNA and regulate transcription (reviewed Bengtsson, 2007). In both Xenopus embryos and human cells, MAN1 has been found to functionally antagonize TGFβ and BMP signalling (Cohen et al, 2007; Ishimura et al, 2006; Lin et al, 2005; Osada et al, 2003; Pan et al, 2005). R-Smads have been shown to physically interact with MAN1 (Lin et al, 2005; Pan et al, 2005), indicating their direct inhibition by MAN1. The mechanism of this interaction is controversial (Bengtsson, 2007; Worman, 2006), although most authors propose that R-Smads are sequestered to the INM, thereby preventing transcription of their target genes (Fig 3A). However, the fact that most R-Smads are cytoplasmic in the absence of TGFβ or BMP signalling, and not clearly at the nuclear envelope, argues against this (Pan et al, 2005), although a detergent-insoluble pool of R-Smads clearly co-localizes with MAN1 (Pan et al, 2005). Therefore, a consensus view could be that MAN1 acts as a nuclear scavenger, sequestering R-Smads that illegitimately enter the nucleus. MAN1 also interacts with several other transcriptional regulators, including the transcription factor germ-cell-less (GCL), the BCL2-associated transcription factor (BTF) and the barrier-to-autointegration factor (BAF; Mansharamani & Wilson, 2005), although the significance of these associations is not yet understood.
Figure 3.
Model of interactions of transcription factors with inner nuclear membrane proteins. (A) In the absence of transforming growth factor-β (TGFβ) or bone morphogenic protein (BMP) signalling, MAN antigen 1 (MAN1) acts as a nuclear scavenger that sequesters R-Smads that illegitimately enter the nucleus, thereby preventing transcription from their target genes. (B) The yeast amino-acid sensor independent (Asi)-complex prevents illegitimate expression of Stp1/Stp2 (species-specific tRNA processing factor)-responsive genes when amino-acid signalling is absent. (C) Overexpression of emerin prevents nuclear accumulation of β-catenin and inhibits its transcriptional activity, presumably by increasing nuclear export of β-catenin. (D) The transcriptional capacity of E2F–DP3 (Early gene 2 factor–Differentiation regulated transcription factor polypeptide 3) is reduced when bound to germ-cell-less (GCL), which itself can associate with emerin and lamina-associated polypeptide 2β (LAP2β). It is unclear whether transcriptional repression works through sequestering (left) the E2F–DP3–GCL trimeric complex, or if the GCL–E2F–DP3 actively represses target genes at the nuclear periphery (right). NPC, nuclear pore complex.
In S. cerevisiae, the cellular import of amino acids is controlled by two homologous transcription factors, Stp1 and Stp2 (species-specific tRNA processing protein), which are synthesized as latent cytoplasmic factors (Andreasson & Ljungdahl, 2002). Amino-acid induction causes cleavage of Stp1 and Stp2, liberating the DNA-binding and transactivating domains (Abdel-Sater et al, 2004; Andreasson et al, 2006). The cleaved forms of Stp1 and Stp2 localize to the nucleus where they bind to specific promoters (de Boer et al, 2000; Nielsen et al, 2001). Mutations in amino-acid sensor independet (ASI) genes constitutively activate target promoters (Forsberg et al, 2001). ASI1, ASI2 and ASI3 each encode integral INM proteins (Boban et al, 2006; Zargari et al, 2007; Fig 3B). Asi1 and Asi3 are homologous proteins with five membrane-spanning domains and a C-terminal RING-finger like region. Asi2 has two transmembrane regions but lacks other functional domains. In asi1Δ, asi2Δ and asi3Δ mutant cells, uncleaved Stp1 and Stp2 can bind to their target genes and activate transcription (Fig 3B). This suggests that Asi proteins might also act as scavengers of transcription factors that have inadvertently entered the nucleus.
Activators of repression or repressors of activation?
The single spanning INM protein emerin is encoded by the EMD gene, which, when mutated, produces the X-linked form of Emery–Dreifuss muscular dystrophy (EDMD; Gruenbaum et al, 2005). The lamin-associated protein LAP2β was originally identified as a single spanning INM protein with a nucleoplasmic binding region for lamin B and chromatin (Foisner & Gerace, 1993). Both emerin and LAP2β associate with several transcriptional regulators, and this association invariably coincides with repression of the transcription factor target genes. In most instances, the mechanism of repression is not clear because it is uncertain whether the transcription factor acts as an activator or repressor of transcription—often transcription factors can do both. If the transcription factor acts as an activator, sequestering the transcription factor away from its target gene is a possible mechanism. If the transcription factor acts as a repressor, a model would be created of a repressive environment for the target gene at the nuclear periphery. An exception to this uncertainty is the β-catenin–emerin interaction (Markiewicz et al, 2006). β-catenin is a co-activator of Wnt-signalling-responsive genes, which are involved in a range of developmental and homeostatic processes. In the presence of Wnt, β-catenin accumulates in the nucleus where it forms active transcriptional complexes with transcription factors of the T-cell factor/lymphocyte enhancing factor (TFC/LEF) family. The level of β-catenin activity is therefore regulated by its rate of accumulation in the nucleus. The overexpression of emerin prevents nuclear accumulation of β-catenin and inhibits its transcriptional activity (Markiewicz et al, 2006). When emerin is mislocalized from the INM to either the cytoplasm or nucleoplasm, it fails to inhibit β-catenin activity, indicating that the correct localization of emerin is required for its repressive effect (Fig 3C). These data indicate that, at least in this case, emerin is not involved in chromatin-mediated repression, but rather in inhibiting β-catenin activity, possibly by stimulating its nuclear export.
By contrast, both mechanisms of repression are still possible explanations for the function of the transcriptional regulator GCL, which was identified as an interaction partner of both emerin (Holaska et al, 2003) and LAP2β (Nili et al, 2001). GCL binds to the DP3 subunit of the heterodimeric transcription factor E2F–DP3, and the transcriptional activity of the E2F–DP3 heterodimer is crucial for cell-cycle progression. Overexpression of either LAP2β or GCL reduces the transcriptional capacity of E2F–DP3 (Nili et al, 2001). It is uncertain whether this repression works through sequestering the E2F–DP3–GCL trimeric complex (Fig 3D, left) or whether the GCL–E2F–DP3 complex actively represses target genes at the nuclear periphery (Fig 3D, right). Both scenarios are possible because it is unclear whether GCL acts as a transcriptional activator or repressor. So far, repressed E2F/DP3 target genes have not been co-localized together with GCL at the nuclear periphery.
Finally, the transcriptional repressor BCL2-associated transcription factor (BTF), which was picked up in a yeast two-hybrid screen, was also identified as a binding partner of emerin (Haraguchi et al, 2004). Under normal growth conditions, BTF is a nuclear protein; however, on induction of apoptosis, the protein accumulates at the nuclear periphery, presumably binding to emerin. Overexpression of BTF causes cell death involving the inhibition of anti-apoptotic BCL2 proteins (Kasof et al, 1999). Importantly, EDMD-associated missense mutations specifically disrupt the binding of BTF to emerin, suggesting that disregulated nuclear access induces apoptosis. However, the precise repressor function of BTF is poorly understood, and it is not clear whether apoptosis coincides with increased or decreased repressor activity.
Concepts and perspectives
On the basis of the examples presented in this review, it seems clear that the nuclear envelope is actively involved in the modulation of gene expression by sequestering transcription factors that have entered the nucleus. Although the groups of nuclear envelope proteins and the transcription factors that they interact with are heterogeneous, interaction with the nuclear envelope proteins clearly restricts the access of some transcription factors to their target genes, and restrains their activating or repressing capacities. The spatial separation from their target promoters therefore appears to be sufficient for inactivation. In several cases it is not possible to discriminate between inactivation of the transcription factor and inactivation of the target gene at the nuclear periphery. Both in situ localization and in vitro analysis of inactive target genes are required to answer these questions.
We speculate that the tethering of transcription factors to the nuclear envelope might aid in fine-tuning pathways that are prone to fluctuations, such as growth factor and amino-acid signalling. Specifically, under non-inducing conditions, some transcription factors are kept latent in the cytoplasm away from their target promoters. However, it is possible that such cytosolic sequestering is not fully effective and that a few DNA-binding-competent transcription factors might illegitimately access the nucleus in the absence of an inducing signal. In these scenarios, the nuclear envelope might bind to and inactivate transcription factors to ensure that no transcription occurs when activating signals are absent. Therefore, in a biological systems context, sequestering might provide a means of enlarging the difference between the on and off states of a gene (Boban et al, 2006).
What happens to the pool of nuclear envelope-bound transcription factors? It is possible that, in some cases, association with the nuclear envelope causes further inactivation of a transcription factor by inducing nuclear export and/or degradation. This strategy might explain why some nuclear envelope proteins are known to regulate transcription factors, even though their co-localization at the nuclear envelope has not been documented. Another possibility is that nuclear envelope localization reflects a stable pool of nuclear envelope bound transcription factors. This pool might be released on activation by the correct signal, which could provide cells with a mechanism to react quickly to changing conditions without the need to import transcription factors from the cytosol.
Another crucial question is to what extent syndromes and disorders associated with mutations in lamins and INM proteins are caused by defects in the sequestering of transcription factors. In light of the many examples of INM-transcription factor interactions documented so far, and the fact that many more INM proteins remain to be characterized, it is likely that the regulation of transcription factor activity by lamins and INM proteins might be a more common phenomenon than previously anticipated. This increases the likelihood that this mechanism is important for understanding and eventually curing nuclear envelope-related diseases.

Stijn Heessen

Maarten Fornerod
Acknowledgments
We thank B. Kalverda for critical reading of the manuscript. SH is supported by a long-term European Molecular Biology Organization (EMBO) fellowship.
References
- Abdel-Sater F, El Bakkoury M, Urrestarazu A, Vissers S, Andre B (2004) Amino acid signaling in yeast: casein kinase I and the Ssy5 endoprotease are key determinants of endoproteolytic activation of the membrane-bound Stp1 transcription factor. Mol Cell Biol 24: 9771–9785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar A, Gasser SM (2007) The nuclear envelope and transcriptional control. Nat Rev Genet 8: 507–517 [DOI] [PubMed] [Google Scholar]
- Andreasson C, Ljungdahl PO (2002) Receptor-mediated endoproteolytic activation of two transcription factors in yeast. Genes Dev 16: 3158–3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasson C, Heessen S, Ljungdahl PO (2006) Regulation of transcription factor latency by receptor-activated proteolysis. Genes Dev 20: 1563–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson L (2007) What MAN1 does to the Smads. TGFβ/BMP signaling and the nuclear envelope. FEBS J 274: 1374–1382 [DOI] [PubMed] [Google Scholar]
- Boban M, Zargari A, Andreasson C, Heessen S, Thyberg J, Ljungdahl PO (2006) Asi1 is an inner nuclear membrane protein that restricts promoter access of two latent transcription factors. J Cell Biol 173: 695–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capanni C et al. (2005) Altered pre-lamin A processing is a common mechanism leading to lipodystrophy. Hum Mol Genet 14: 1489–1502 [DOI] [PubMed] [Google Scholar]
- Cohen TV, Kosti O, Stewart CL (2007) The nuclear envelope protein MAN1 regulates TGFβ signaling and vasculogenesis in the embryonic yolk sac. Development 134: 1385–1395 [DOI] [PubMed] [Google Scholar]
- de Boer M, Nielsen PS, Bebelman JP, Heerikhuizen H, Andersen HA, Planta RJ (2000) Stp1p, Stp2p and Abf1p are involved in regulation of expression of the amino acid transporter gene BAP3 of Saccharomyces cerevisiae. Nucleic Acids Res 28: 974–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreger M, Bengtsson L, Schoneberg T, Otto H, Hucho F (2001) Nuclear envelope proteomics: novel integral membrane proteins of the inner nuclear membrane. Proc Natl Acad Sci USA 98: 11943–11948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajas L, Schoonjans K, Gelman L, Kim JB, Najib J, Martin G, Fruchart JC, Briggs M, Spiegelman BM, Auwerx J (1999) Regulation of peroxisome proliferator-activated receptor γ expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: implications for adipocyte differentiation and metabolism. Mol Cell Biol 19: 5495–5503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foisner R, Gerace L (1993) Integral membrane proteins of the nuclear envelope interact with lamins and chromosomes, and binding is modulated by mitotic phosphorylation. Cell 73: 1267–1279 [DOI] [PubMed] [Google Scholar]
- Forsberg H, Hammar M, Andreasson C, Moliner A, Ljungdahl PO (2001) Suppressors of ssy1 and ptr3 null mutations define novel amino acid sensor-independent genes in Saccharomyces cerevisiae. Genetics 158: 973–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke WW, Scheer U, Krohne G, Jarasch ED (1981) The nuclear envelope and the architecture of the nuclear periphery. J Cell Biol 91: 39s–50s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenbaum Y, Margalit A, Goldman RD, Shumaker DK, Wilson KL (2005) The nuclear lamina comes of age. Nat Rev Mol Cell Biol 6: 21–31 [DOI] [PubMed] [Google Scholar]
- Haraguchi T, Holaska JM, Yamane M, Koujin T, Hashiguchi N, Mori C, Wilson KL, Hiraoka Y (2004) Emerin binding to Btf, a death-promoting transcriptional repressor, is disrupted by a missense mutation that causes Emery–Dreifuss muscular dystrophy. Eur J Biochem 271: 1035–1045 [DOI] [PubMed] [Google Scholar]
- Holaska JM, Lee KK, Kowalski AK, Wilson KL (2003) Transcriptional repressor germ cell-less (GCL) and barrier to autointegration factor (BAF) compete for binding to emerin in vitro. J Biol Chem 278: 6969–6975 [DOI] [PubMed] [Google Scholar]
- Imai S, Nishibayashi S, Takao K, Tomifuji M, Fujino T, Hasegawa M, Takano T (1997) Dissociation of Oct-1 from the nuclear peripheral structure induces the cellular aging-associated collagenase gene expression. Mol Biol Cell 8: 2407–2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimura A, Ng JK, Taira M, Young SG, Osada S (2006) Man1, an inner nuclear membrane protein, regulates vascular remodeling by modulating transforming growth factor β signaling. Development 133: 3919–3928 [DOI] [PubMed] [Google Scholar]
- Ivorra C, Kubicek M, Gonzalez JM, Sanz-Gonzalez SM, Alvarez-Barrientos A, O'Connor JE, Burke B, Andres V (2006) A mechanism of AP-1 suppression through interaction of c-Fos with lamin A/C. Genes Dev 20: 307–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BR, Nitta RT, Frock RL, Mounkes L, Barbie DA, Stewart CL, Harlow E, Kennedy BK (2004) A-type lamins regulate retinoblastoma protein function by promoting subnuclear localization and preventing proteasomal degradation. Proc Natl Acad Sci USA 101: 9677–9682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasof GM, Goyal L, White E (1999) Btf, a novel death-promoting transcriptional repressor that interacts with Bcl-2-related proteins. Mol Cell Biol 19: 4390–4404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran RI, Muralikrishna B, Parnaik VK (2002) Lamin A/C speckles mediate spatial organization of splicing factor compartments and RNA polymerase II transcription. J Cell Biol 159: 783–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Blake DL, Callebaut I, Skerjanc IS, Holmer L, McBurney MW, Paulin-Levasseur M, Worman HJ (2000) MAN1, an inner nuclear membrane protein that shares the LEM domain with lamina-associated polypeptide 2 and emerin. J Biol Chem 275: 4840–4847 [DOI] [PubMed] [Google Scholar]
- Lin F, Morrison JM, Wu W, Worman HJ (2005) MAN1, an integral protein of the inner nuclear membrane, binds Smad2 and Smad3 and antagonizes transforming growth factor-β signaling. Hum Mol Genet 14: 437–445 [DOI] [PubMed] [Google Scholar]
- Mansharamani M, Wilson KL (2005) Direct binding of nuclear membrane protein MAN1 to emerin in vitro and two modes of binding to barrier-to-autointegration factor. J Biol Chem 280: 13863–13870 [DOI] [PubMed] [Google Scholar]
- Markiewicz E, Dechat T, Foisner R, Quinlan RA, Hutchison CJ (2002) Lamin A/C binding protein LAP2α is required for nuclear anchorage of retinoblastoma protein. Mol Biol Cell 13: 4401–4413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markiewicz E et al. (2006) The inner nuclear membrane protein emerin regulates β-catenin activity by restricting its accumulation in the nucleus. EMBO J 25: 3275–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J, Seoane J, Wotton D (2005) Smad transcription factors. Genes Dev 19: 2783–2810 [DOI] [PubMed] [Google Scholar]
- Nielsen PS, van den Hazel B, Didion T, de Boer M, Jorgensen M, Planta RJ, Kielland-Brandt MC, Andersen HA (2001) Transcriptional regulation of the Saccharomyces cerevisiae amino acid permease gene BAP2. Mol Gen Genet 264: 613–622 [DOI] [PubMed] [Google Scholar]
- Nili E et al. (2001) Nuclear membrane protein LAP2β mediates transcriptional repression alone and together with its binding partner GCL (germ-cell-less). J Cell Sci 114: 3297–3307 [DOI] [PubMed] [Google Scholar]
- Osada S, Ohmori SY, Taira M (2003) XMAN1, an inner nuclear membrane protein, antagonizes BMP signaling by interacting with Smad1 in Xenopus embryos. Development 130: 1783–1794 [DOI] [PubMed] [Google Scholar]
- Pan D, Estevez-Salmeron LD, Stroschein SL, Zhu X, He J, Zhou S, Luo K (2005) The integral inner nuclear membrane protein MAN1 physically interacts with the R-Smad proteins to repress signaling by the transforming growth factor-β superfamily of cytokines. J Biol Chem 280: 15992–16001 [DOI] [PubMed] [Google Scholar]
- Pekovic V, Harborth J, Broers JL, Ramaekers FC, van Engelen B, Lammens M, von Zglinicki T, Foisner R, Hutchison C, Markiewicz E (2007) Nucleoplasmic LAP2α–lamin A complexes are required to maintain a proliferative state in human fibroblasts. J Cell Biol 176: 163–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer EC, Gerace L (2005) The nuclear membrane proteome: extending the envelope. Trends Biochem Sci 30: 551–558 [DOI] [PubMed] [Google Scholar]
- Schirmer EC, Florens L, Guan T, Yates JR, Gerace L (2003) Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science 301: 1380–1382 [DOI] [PubMed] [Google Scholar]
- Spann TP, Goldman AE, Wang C, Huang S, Goldman RD (2002) Alteration of nuclear lamin organization inhibits RNA polymerase II-dependent transcription. J Cell Biol 156: 603–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuurman N, Heins S, Aebi U (1998) Nuclear lamins: their structure, assembly, and interactions. J Struct Biol 122: 42–66 [DOI] [PubMed] [Google Scholar]
- Worman HJ (2006) Inner nuclear membrane and regulation of Smad-mediated signaling. Biochim Biophys Acta 1761: 626–631 [DOI] [PubMed] [Google Scholar]
- Zargari A, Boban M, Heessen S, Andreasson C, Thyberg J, Ljungdahl PO (2007) Inner nuclear membrane proteins Asi1, Asi2, and Asi3 function in concert to maintain the latent properties of transcription factors Stp1 and Stp2. J Biol Chem 282: 594–605 [DOI] [PubMed] [Google Scholar]