Abstract
How arterial and venous fates are established is largely unknown. In the past, circulatory dynamics were thought to be the exclusive cause of arteries and veins being structurally and functionally distinct; however, growing evidence indicates that an orderly progression of molecular signals controls arterial–venous specification in the developing vertebrate vascular system.
Keywords: arterial–venous specification, COUP-TFII, Notch signalling, PI3K, Vegf
Introduction
Structural differences between arteries and veins have long been recognized by anatomists. The consensus view was that arteries and veins are distinguished by the direction and pressure of blood flow owing to haemodynamic factors (reviewed in Lawson & Weinstein, 2002). With the discovery of ephrin B2 (Efnb2) and the B4 ephrin receptor (Ephb4) as markers for arteries and veins, respectively, a new concept has arisen in which the specification of arteries and veins is determined by genetic programmes in the developing embryo before the onset of circulation (Wang et al, 1998). Efnb2, which is a transmembrane ligand for the ephrin family, is specifically expressed in endothelial precursors that produce arteries, whereas Ephb4, which is a receptor for Efnb2, is found preferentially in veins. In this review, we summarize recent progress in the characterization of the molecular components involved in arterial–venous fate determination.
Vascular morphogenesis
The vertebrate vasculature is a sophisticated system derived from a complex programme involving sequential genetic and morphological events that drive the formation and specification of blood and lymphatic vasculature.
Angioblasts and pluripotent haematopoietic stem cells are derived from a common mesoderm-derived progenitor cell: the haemangioblast (Vogeli et al, 2006). Angioblasts generate vascular endothelial cells, whereas pluripotent haematopoietic cells produce the various blood cells and lymphocytes. In mice, the blood cells are initially generated in the yolk sac at embryonic day 7–7.5 (Moore & Metcalf, 1970). They initially aggregate to form islands of blood cells, which are then sheathed by the endothelial cells to form vessels. In vertebrates, vascular development proceeds through two main stepwise processes: vasculogenesis and angiogenesis (Poole & Coffin, 1989). During vasculogenesis, endothelial precursor cells, in response to local signals such as growth factors and the extracellular matrix, undergo specification, proliferation, migration, differentiation and finally coalescence to form the lining of nascent vessels. The vascular network is then remodelled and refined into arteries, veins and capillaries through angiogenesis.
In early zebrafish embryos, angioblasts originating from the lateral posterior mesoderm migrate to the midline, where they assemble and coalesce to form the aorta (artery) and cardinal vein (Zhong et al, 2001). Notably, lineage tracing experiments have revealed that the progeny of each angioblast in the lateral posterior mesoderm can be found in the artery or vein but never both, indicating that the fate of each angioblast has been predetermined in the lateral posterior mesoderm (Zhong et al, 2001).
Genes controlling the developmental programmes underlying artery and vein specification have been identified in different vertebrate species. Most are signalling molecules, including ligands and receptors for transmembrane receptor tyrosine kinases, and transcription factors. Among them, the Notch signalling pathway that lies upstream of Efnb2 has been shown to have a crucial role in arterial–venous specification in both zebrafish and mice (reviewed in Gridley, 2007).
Developmental signals that determine arterial identity
Notch guides arterial fate. Notch, which is an evolutionarily conserved transmembrane receptor, has a well-known function in regulating cell-fate decisions during a range of developmental processes in metazoans (reviewed in Artavanis-Tsakonas et al, 1999). Notch signalling occurs through cell-to-cell contact that is mediated by the interactions of Notch receptors and their DSL ligands, which are Delta and Serrate in Drosophila, LAG-2 in Caenorhabditis elegans, Delta in zebrafish, and Delta-like (Dll) and Jagged (Jag) in mice (reviewed in Bray, 2006). Ligand binding activates Notch by inducing two sequential proteolytic cleavages, which results in the translocation of the intracellular domain of the Notch receptor (NICD) into the nucleus. The NICD binds to CSL transcriptional regulators, which are CBF1 in humans, Suppressor of Hairless (Su(H)) in Drosophila and zebrafish, LAG-1 in C. elegans and Rbpj in mice. As a consequence of binding, co-repressors associated with CSL are released, concomitant with transcriptional activation of downstream target genes.
In zebrafish, mindbomb (mib) mutants expressing an inactive Notch receptor and embryos microinjected with a dominant-negative form of Su(H) show arterial–venous shunts with a disorganized dorsal aorta and posterior cardinal vein (Lawson et al, 2001). These embryos also show an absence, or reduced expression, of arterial markers such as efnb2a and notch5, while conversely displaying an elevated level of the venous marker fms-related tyrosine kinase 4 (flt4) within the dorsal aorta. Similarly, ectopic expression of an activated form of notch5 in the posterior cardinal vein results in decreased venous flt4 expression. These results indicate that Notch signalling is necessary for the specification of arterial fate.
One set of downstream target genes regulated by Notch in mammals is the HES (hairy-and-enhancer-of-split) and Hey (hairy-and-enhancer-of-split related) families of transcriptional repressors (reviewed in Iso et al, 2003). Gridlock (grl), which is a zebrafish orthologue of mammalian Hey2, is expressed early in the lateral posterior mesoderm, and its expression becomes restricted to the dorsal aorta during later development (Zhong et al, 2000). The expression of grl can be induced by activation of the notch1 pathway, and can be blocked by inhibition of notch activity (Zhong et al, 2001). Consistent with previous findings, embryos treated with different concentrations of grl antisense morpholino oligonucleotides show variable arterial defects in the posterior trunk, which are dependent on the expression level of grl. A low dose of grl antisense morpholino oligonucleotides, which maintains arterial integrity, causes embryos to show decreased expression of the arterial marker efnb2a with a concomitant increase in venous ephb4 expression. Reciprocally, overexpression of grl reduces the size of the vein and eliminates flt4 expression, without affecting the artery. These observations reveal that the notch–grl pathway regulates the formation of the dorsal aorta and controls arterial cell fate.
Further support for the idea that Notch signalling is essential for arterial fate decisions has come from studies genetically manipulating the activity of Notch in mice. Mammals have four Notch receptors (Notch 1–4) and five Notch ligands (Dll1, Dll3, Dll4, Jag1 and Jag2) (Gridley, 2007). Mice lacking Notch1/4, Dll4, Rbpj, Mib or Hey1/2 display defects in vascular development, including arterial specification (Duarte et al, 2004; Fischer et al, 2004; Kokubo et al, 2005; Koo et al, 2005; Krebs et al, 2000, 2004). Dll4 and Notch4 are particularly important owing to their specific expression patterns in arterial, but not venous, endothelium in the developing mouse embryo (Shutter et al, 2000; Villa et al, 2001). Dll4-null mutants exhibit disrupted arterial endothelial cell differentiation with decreased Efnb2 and Cx37 expression concomitant with increased Ephb4 expression (Duarte et al, 2004). These findings suggest a new role for Notch signalling in suppressing venous cell fate, and therefore promoting arterial differentiation.
Sonic hedgehog and vascular endothelial growth factor control Notch signalling. How is Notch signalling induced? Sonic hedgehog (Shh) is believed to be a crucial inducer of arterial cell fate. Shh encodes a secreted peptide, which is derived from the notochord and floor plate, and is important for neural tube and somite development. Elegant studies in zebrafish have shown that embryos lacking shh activity, including the null-mutant sonic-you (syu) and embryos treated with the inhibitor of shh signalling cyclopamine, fail to establish arterial identity in the dorsal aorta, with the loss of arterial maker expression and the gain of venous marker expression (Lawson et al, 2002). Conversely, microinjection of messenger RNA encoding shh in zebrafish embryos leads to a switch from a venous to an arterial fate in the posterior cardinal vein, indicating that Shh is required for arterial endothelial differentiation (Lawson et al, 2002).
Zebrafish embryos lacking shh activity also fail to express vascular endothelial growth factor (vegf) within the somites. Reduction of vegf expression in zebrafish embryos by antisense morpholino oligonucleotides results in the loss of arterial fate, whereas overexpression of vegf in shh-deficient embryos rescues vascular efnb2a expression, therefore implicating vegf signalling as being important for arterial specification (Lawson et al, 2002). Although vegf is unable to restore artery identity in mutant embryos that are deficient in notch signalling, exogenous induction of notch activity rescues efnb2a and notch5 expression in the absence of vegf, suggesting that vegf lies downstream of shh, but upstream of notch to induce arterial differentiation.
Consistent with these zebrafish studies, recent observations in mice also confirm the prominent roles of Vegf signalling in promoting arterial differentiation (reviewed in Sato, 2003). Murine Vegfa exists—owing to alternative splicing—as three main homodimeric isoforms: Vegf120, Vegf164 and Vegf188 (reviewed in Ng et al, 2006). Besides having different molecular masses, these isoforms can display specific receptor-binding properties. The overexpression of Vegf164 in cardiomyocytes in transgenic mice leads to a proportional increase of Efnb2-positive vessels (Visconti et al, 2002). Similarly, primary embryonic endothelial cells treated with either Vegf120 or Vegf164 show an increased percentage of Efnb2-positive cells without affecting cell proliferation or survival (Mukouyama et al, 2002).
The cell-surface receptors for all Vegf isoforms are Vegf receptor 1 (Vegfr1/Flt1), Vegfr2 (KDR/Flk1) and neuropilin 1 (NP-1) (Ng et al, 2006). During angiogenesis, Vegfr2 seems to be the main signalling receptor, whereas Vegfr1 acts as a decoy receptor that negatively regulates the activity of Vegfr2 (Rahimi et al, 2000). In addition, NP-1 is thought to be a Vegf164 isoform-specific co-receptor that facilitates signalling through Vegfr2. Although NP-1 has arterial-restricted expression, the ubiquitous expression of Vegfr1 and Vegfr2 in all nascent endothelial cells makes it difficult to further decipher the mechanisms underlying the specific arterial effects of Vegf signalling.
Forkhead box c proteins induce Dll4–Notch. Forkhead box c (Foxc) 1 and 2, which are two members of the forkhead family of transcription factors, have been recently shown to control arterial specification by regulating the expression of the Notch ligand Dll4 (Seo et al, 2006). Foxc1 and Foxc2 are localized in both endothelium and smooth-muscle cells of the aorta (Kume et al, 2001; Seo et al, 2006). Mice with targeted inactivation of both Foxc1 and Foxc2 develop arterial–venous malformations, which are abnormal fusions of arteries and veins. The defective vessels of these compound homozygous-null mutants show decreased expression of arterial markers (Dll4, Jag1, Notch1, Notch4, Hey2 and Efnb2), whereas expression of the venous markers chicken ovalbumin upstream promoter transcription factor II (COUP-TFII) and Ephb4 is not altered, suggesting that the vessels have a vein-like nature. Intriguingly, Foxc proteins can directly bind to a forkhead-binding element in the Dll4 promoter and stimulate its activity. Together, these findings suggest that Foxc1 and Foxc2 act upstream from the Notch pathway to induce arterial differentiation.
Factors that control venous identity
COUP-TFII marks the veins. The discovery that Vegf–Notch signalling is required for arterial cell specification in the zebrafish led to the belief that the venous state is derived from a default pathway, whereas arterial identity is conferred by the presence of additional signalling (Thurston & Yancopoulos, 2001). This concept was later reassessed by a breakthrough in understanding of the venous functions of COUP-TFII. COUP-TFII, which is a member of the orphan nuclear receptor superfamily, is specifically expressed in venous, but not arterial, endothelium (You et al, 2005). Conditional ablation of COUP-TFII in the endothelium results in the acquisition of arterial characteristics in veins. This phenotype is characterized by an increase in expression of arterial markers, including efnb2, NP-1 and Notch signalling molecules. In addition, the mutant veins are able to form haematopoietic cell clusters and recruit smooth-muscle cells, which are two functional features of arteries, indicating that the mutant veins not only gain arterial-specific gene expression but also behave like arteries. Intriguingly, ectopic expression of COUP-TFII in the endothelium results in a fusion of arteries and veins, which phenocopies the vascular defects found in mouse embryos lacking NP-1 or Notch1, suggesting that downregulation of Notch signalling could account for such vascular phenotypes (Huppert et al, 2000; Kawasaki et al, 1999). Indeed, the ectopic expression of COUP-TFII results in a loss of arterial markers, including NP-1, and other factors in the Notch signalling pathway. Together, these findings suggest that COUP-TFII is a crucial regulator of venous fate determination.
Phosphatidylinositol-3 kinase also marks the veins. The phosphatidylinositol-3 kinase (PI3K) signalling pathway has also been implicated in the specification of vein identity. Recent studies in zebrafish have identified this pathway as being important for the maintenance of vein identity. PI3K promotes venous cell fate by blocking arterial p42/44 mitogen-activated protein kinase (Mapk; extracellular signal-regulated kinase (Erk)) activation (Hong et al, 2006). Activated Erks are preferentially detected in angioblasts that are fated to become arteries. The identification of phospholipase Cγ-1 (Plc-γ) as a downstream mediator of Vegf signalling in the arterial fate decision links Vegf signalling to Erk activation, thereby forming the molecular cascade Vegf→Plc-γ→Pkc→Raf→Mek→Erk (Pkc for protein kinase c; Mek for mitogen-activated protein kinase kinase) (Lawson et al, 2003). The flavone GS4898 was isolated using a powerful small-molecule screen, and characterized as an inhibitor of the PI3K–Akt pathway. The inactivation of Akt with GS4898 reverses the inhibitory effects of PI3K on Plc-γ–Erk signalling, thereby triggering an arterial fate specification (Hong et al, 2006). Conversely, the mosaic expression of constitutive, active Akt was found to induce venous fate. Together, these studies suggest a new role for the interplay of the Plc-γ–Erk and PI3K–Akt pathways in controlling artery and vein decisions.
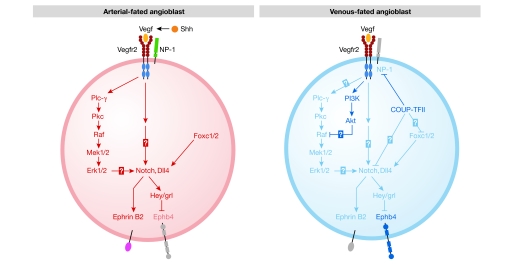
Collectively, the accumulated data from the zebrafish studies delineate a genetic hierarchy of signalling pathways that is responsible for arterial specification (Fig 1). Expression of Shh in the notochord and floor plate induces Vegf expression in the adjacent somites. The interaction of Vegf with its receptor on angioblasts triggers activation signals that are transduced through the Plc-γ–Erk and Notch pathways, which in turn induce efnb2 and other arterial markers to establish an arterial fate. Within an angioblast fated to become a venous cell, COUP-TFII represses the Notch signalling pathway by inhibiting the expression of NP-1 and other participants, thereby suppressing an arterial fate (Fig 1).
Figure 1.
Model of arterial–venous specification in the developing embryo. Sonic hedgehog (Shh) secreted by the notochord and floor plate induces vascular endothelial growth factor (Vegf) levels in the somites, which in turn activate angioblasts arising from the lateral plate mesoderm. Within an arterial-fated angioblast, Vegf interacts with the Vegf receptor 2 (Vegfr2)–neuropilin 1 (NP-1) complex to activate downstream phospholipase Cγ-1 (Plc-γ)–extracellular signal-regulated kinase (Erk) and Notch signalling pathways, thereby inducing arterial marker expression, such as ephrin B2 (efnb2). Forkhead box c 1 (Foxc1)/Foxc2 proteins activate the Notch pathway by inducing the expression of Delta-like 4 (Dll4), thereby leading to an arterial fate. Conversely, chicken ovalbumin upstream promoter transcription factor II (COUP-TFII) and phosphatidylinositol-3 kinase (PI3K)–Akt signalling promote venous fate by suppression of the Notch pathway and Erk signalling, respectively, thereby repressing arterial fate. COUP-TFII not only suppresses the Notch pathway, but also inhibits NP-1 expression, therefore attenuating Vegf and downstream Notch activation. Unconfirmed interactions are indicated by question marks. This figure was adapted from a model presented by Lamont & Childs (2006). Ephb4, B4 ephrin receptor; grl, gridlock; Hey, hairy-and-enhancer-of-split related; Mek, mitogen-activated protein kinase kinase; Pkc, protein kinase c.
Conclusions and perspectives
We have highlighted the recent progress made in understanding artery and vein identity. According to the current model, the concept of artery and vein specification works like the ancient Chinese philosophical concept of yin-yang, which describes a balance of two dynamic, opposing and complementary principles in the universe. The outer circle of the yin-yang symbol reveals everything or, in this case, the whole population of angioblasts, whereas the black and white parts within the circle represent two natural forces or, here, two cell fates. This suggests that arterial and venous fate cannot exist without each other under normal conditions, and that an intrinsic relationship between them coordinates harmony.
Although we have discussed at length the fact that vessel identity is genetically predetermined, there is no doubt that haemodynamic factors, such as blood pressure and flow, also have a crucial role in regulating vascular plasticity (reviewed in Jones et al, 2006). Quail–chicken graft assays show that flow is able to change the identity of nascent endothelial cells, highlighting the importance of the microenvironment (le Noble et al, 2004; Moyon et al, 2001). Understanding the balance between global genetic inputs and local environmental forces in vessel identity and remodelling will provide insight into how a functional vascular architecture forms.
Many of the molecular regulators responsible for the arterial and venous fate specification have now begun to be identified, but their roles are still not fully understood. In the case of COUP-TFII, some questions still remain; for example, does COUP-TFII act in angioblasts? If angioblasts in the lateral posterior mesoderm are restricted to an arterial or venous lineage, does COUP-TFII regulate the venous differentiation programme within a subset of angioblasts while the remaining cells take on an arterial fate? Alternatively, if all angioblasts are COUP-TFII-positive, could inactivation of COUP-TFII by an unknown signal during angioblast migration confer arterial identity? It is equally possible that COUP-TFII acts even higher in the hierarchy, for example in the haemangioblast, to regulate cell fate. Other interesting questions that remain include whether PI3K activation is linked to COUP-TFII-mediated signalling, whether Foxc proteins regulate the Plc-γ–Erk cascade downstream of Vegfr2 and, finally, what is the signal/factor regulating the expression of COUP-TFII specifically in the endothelium of the vein? We believe that COUP-TFII might directly suppress the expression of many participants, such as NP-1, Hey, Foxc and Notch, which lie upstream and downstream of the Notch signalling pathway or within the Notch pathway, to ensure that Notch signalling is inactivated in this process. The precise mechanism by which these complicated hierarchies of signalling interactions are coordinated and the interplays between them remain to be elucidated.

Fu-Jung Lin
Ming-Jer Tsai

Sophia Y. Tsai
Acknowledgments
We thank C.E. Foulds for critical reading of the manuscript. This work was supported by National Institutes of Health grants HL076448 and DK59820 to S.Y.T., and HD17379 and DK45641 to M.-J.T.
References
- Artavanis-Tsakonas S, Rand MD, Lake RJ (1999) Notch signaling: cell fate control and signal integration in development. Science 284: 770–776 [DOI] [PubMed] [Google Scholar]
- Bray SJ (2006) Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7: 678–689 [DOI] [PubMed] [Google Scholar]
- Duarte A, Hirashima M, Benedito R, Trindade A, Diniz P, Bekman E, Costa L, Henrique D, Rossant J (2004) Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev 18: 2474–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M (2004) The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev 18: 901–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gridley T (2007) Notch signaling in vascular development and physiology. Development 134: 2709–2718 [DOI] [PubMed] [Google Scholar]
- Hong CC, Peterson QP, Hong JY, Peterson RT (2006) Artery/vein specification is governed by opposing phosphatidylinositol-3 kinase and MAP kinase/ERK signaling. Curr Biol 16: 1366–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert SS, Le A, Schroeter EH, Mumm JS, Saxena MT, Milner LA, Kopan R (2000) Embryonic lethality in mice homozygous for a processing-deficient allele of Notch1. Nature 405: 966–970 [DOI] [PubMed] [Google Scholar]
- Iso T, Kedes L, Hamamori Y (2003) HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol 194: 237–255 [DOI] [PubMed] [Google Scholar]
- Jones EA, le Noble F, Eichmann A (2006) What determines blood vessel structure? Genetic prespecification vs. hemodynamics. Physiology 21: 388–395 [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H (1999) A requirement for neuropilin-1 in embryonic vessel formation. Development 126: 4895–4902 [DOI] [PubMed] [Google Scholar]
- Kokubo H, Miyagawa-Tomita S, Nakazawa M, Saga Y, Johnson RL (2005) Mouse hesr1 and hesr2 genes are redundantly required to mediate Notch signaling in the developing cardiovascular system. Dev Biol 278: 301–309 [DOI] [PubMed] [Google Scholar]
- Koo BK et al. (2005) Mind bomb 1 is essential for generating functional Notch ligands to activate Notch. Development 132: 3459–3470 [DOI] [PubMed] [Google Scholar]
- Krebs LT et al. (2000) Notch signaling is essential for vascular morphogenesis in mice. Genes Dev 14: 1343–1352 [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T (2004) Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev 18: 2469–2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume T, Jiang H, Topczewska JM, Hogan BL (2001) The murine winged helix transcription factors, Foxc1 and Foxc2, are both required for cardiovascular development and somitogenesis. Genes Dev 15: 2470–2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont RE, Childs S (2006) MAPping out arteries and veins. Sci STKE 355: pe39. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM (2002) Arteries and veins: making a difference with zebrafish. Nat Rev Genet 3: 674–682 [DOI] [PubMed] [Google Scholar]
- Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM (2001) Notch signaling is required for arterial–venous differentiation during embryonic vascular development. Development 128: 3675–3683 [DOI] [PubMed] [Google Scholar]
- Lawson ND, Vogel AM, Weinstein BM (2002) Sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell 3: 127–136 [DOI] [PubMed] [Google Scholar]
- Lawson ND, Mugford JW, Diamond BA, Weinstein BM (2003) Phospholipase C gamma-1 is required downstream of vascular endothelial growth factor during arterial development. Genes Dev 17: 1346–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Noble F, Moyon D, Pardanaud L, Yuan L, Djonov V, Matthijsen R, Breant C, Fleury V, Eichmann A (2004) Flow regulates arterial–venous differentiation in the chick embryo yolk sac. Development 131: 361–375 [DOI] [PubMed] [Google Scholar]
- Moore MA, Metcalf D (1970) Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol 18: 279–296 [DOI] [PubMed] [Google Scholar]
- Moyon D, Pardanaud L, Yuan L, Breant C, Eichmann A (2001) Plasticity of endothelial cells during arterial–venous differentiation in the avian embryo. Development 128: 3359–3370 [DOI] [PubMed] [Google Scholar]
- Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ (2002) Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell 109: 693–705 [DOI] [PubMed] [Google Scholar]
- Ng YS, Krilleke D, Shima DT (2006) VEGF function in vascular pathogenesis. Exp Cell Res 312: 527–537 [DOI] [PubMed] [Google Scholar]
- Poole TJ, Coffin JD (1989) Vasculogenesis and angiogenesis: two distinct morphogenetic mechanisms establish embryonic vascular pattern. J Exp Zool 251: 224–231 [DOI] [PubMed] [Google Scholar]
- Rahimi N, Dayanir V, Lashkari K (2000) Receptor chimeras indicate that the vascular endothelial growth factor receptor-1 (VEGFR-1) modulates mitogenic activity of VEGFR-2 in endothelial cells. J Biol Chem 275: 16986–16992 [DOI] [PubMed] [Google Scholar]
- Sato TN (2003) Vascular development: molecular logic for defining arteries and veins. Curr Opin Hematol 10: 131–135 [DOI] [PubMed] [Google Scholar]
- Seo S, Fujita H, Nakano A, Kang M, Duarte A, Kume T (2006) The forkhead transcription factors, Foxc1 and Foxc2, are required for arterial specification and lymphatic sprouting during vascular development. Dev Biol 294: 458–470 [DOI] [PubMed] [Google Scholar]
- Shutter JR, Scully S, Fan W, Richards WG, Kitajewski J, Deblandre GA, Kintner CR, Stark KL (2000) Dll4, a novel Notch ligand expressed in arterial endothelium. Genes Dev 14: 1313–1318 [PMC free article] [PubMed] [Google Scholar]
- Thurston G, Yancopoulos GD (2001) Gridlock in the blood. Nature 414: 163–164 [DOI] [PubMed] [Google Scholar]
- Villa N, Walker L, Lindsell CE, Gasson J, Iruela-Arispe ML, Weinmaster G (2001) Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech Dev 108: 161–164 [DOI] [PubMed] [Google Scholar]
- Visconti RP, Richardson CD, Sato TN (2002) Orchestration of angiogenesis and arteriovenous contribution by angiopoietins and vascular endothelial growth factor (VEGF). Proc Natl Acad Sci USA 99: 8219–8224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeli KM, Jin SW, Martin GR, Stainier DY (2006) A common progenitor for haematopoietic and endothelial lineages in the zebrafish gastrula. Nature 443: 337–339 [DOI] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ (1998) Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93: 741–753 [DOI] [PubMed] [Google Scholar]
- You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY (2005) Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature 435: 98–104 [DOI] [PubMed] [Google Scholar]
- Zhong TP, Rosenberg M, Mohideen MA, Weinstein B, Fishman MC (2000) Gridlock, an HLH gene required for assembly of the aorta in zebrafish. Science 287: 1820–1824 [DOI] [PubMed] [Google Scholar]
- Zhong TP, Childs S, Leu JP, Fishman MC (2001) Gridlock signalling pathway fashions the first embryonic artery. Nature 414: 216–220 [DOI] [PubMed] [Google Scholar]