Abstract
Objective:
ErbB receptors and their ligands play crucial roles in development. During late gestation, they might also be involved in the pathogenesis of prematurity-associated disorders. ErbB receptor dimerization leads to a diversity of biologic signals. We studied the expression and localization patterns of erbB receptors in the developing human umbilical endothelial cell system. It is still unclear, whether expression patterns might be developmentally regulated and depend on the cell type studied.
Methods:
Primary human umbilical venous endothelial cells (HUVEC) and arterial endothelial cells (HUAEC) were isolated between 24 and 42 weeks of gestation and used for immunoprecipitation, western blotting, and confocal microscopy.
Results:
All four erbB receptors were present in HUVEC and HUAEC. Expression patterns were similar for cell types at gestational ages examined. ErbB4 always co-precipitated with erbB1 in both cell types independent of the gestational age. Confocal microscopy revealed that all erbB receptors were localized in the nucleus, erbB1 and erbB3 in the nucleoli, while erbB2 and erbB4 spared the nucleolar region. All receptors showed a tendency to co-localize. Growth factor stimulation altered localization patterns. Cellular subfractionation experiments for erbB4 largely confirmed microscopy results. Pretreatment with lipopolysaccharide enhanced this nuclear localization of erbB4, particularly of its intracellular domain.
Conclusions:
All erbB receptors are present in both HUVEC and HUAEC at all gestational ages tested. ErbB receptor expression patterns were independent of the developmental stage of the endothelial cell, at least in the third trimester. We speculate that endothelial erbB receptors might play a role in normal development in mid and late gestation. We also speculate that these findings, together with the known involvement of erbB receptors in development, inflammation, and angiogenesis, will open new avenues for erbB receptor-related research in the pathogenesis of fetal and neonatal inflammation-associated disorders.
Keywords: erbB receptors, umbilical endothelial cells, newborns
1. Introduction
Multiple ligands of the transmembrane tyrosinekinase erbB receptor family are involved in developmental and maturation processes [1-4], as well as in the pathogenesis of neonatal disorders. In the fetal lung, neuregulin (NRG) 1, the ligand of erbB3 and erbB4, plays a role in signaling the onset of surfactant synthesis [5]. In the postnatal lung, epidermal growth factor (EGF), transforming growth factor (TGF)- α, and their receptor erbB1 might be involved in the pathogenesis of bronchopulmonary dysplasia (BPD) [6-8]. In the gut, inactivation of erbB1 leads to hemorrhagic enteritis that is similar to necrotizing enterocolitis (NEC) [9] and EGF seems to be protective in experimental NEC [10]. In preterm infants, EGF might might be involved in the etiology of cerebral palsy (CP) [11]. One unifying theme involved in the pathogenesis of all of the above (and other) neonatal disorders of prematurity is exposure to perinatal infection/inflammation [12-15].
In light of these intriguing functions of erbB receptors and their ligands in development and various neonatal disorders, we wanted to explore the expression patterns of erbB receptors in developing human endothelial cells. The endothelial system is present in all organs affected by the above neonatal disorders and is involved in inflammatory and angiogenic processes. We used human umbilical venous (HUVEC) and arterial endothelial cells (HUAEC) for our experiments.
First, we wanted to confirm the presence of all erbB receptors in fetal endothelium. Second, we hypothesized that erbB receptor expression and localization patterns are different in arterial versus venous cells isolated from the same umbilical cord. Third, we hypothesized that these patterns might differ between cells from different gestational ages. Fourth, we wanted to further explore the most recent observations that erbB receptors not only reside in the cell membrane, but also in the nucleus [16,17]. Since nuclear erbB4 shuttling appears to play an important role in cellular function [18,19] as well as in human disease [20-22], we wanted to study the effects of growth factor stimulation and exposure to lipopolysaccharide (LPS) on nuclear shuttling of erbB4.
2. Methods
2.1 Approval
This study was approved by the Ethics Committee of Hannover Medical School. Informed written parental consent was obtained.
2.2 Reagents
Type I collagenase was purchased from GIBCO Invitrogen; endothelial cell growth medium MV was from Promocell; Dulbecco’s Modified Eagle’s medium (DMEM), LPS (Lipopolysaccharide from E.coli – L 2880) and Gelvatol/DABCO were from Sigma; Protein-A-Sepharose CL-4B was from Amersham Biosciences; polyclonal rabbit anti-phosphotyrosine antibody 610010 was from Transduction Laboratories; for western blotting: erbB1 (1005) and erbB4 (C-18) antibodies were from Santa Cruz, erbB2 (Ab-1) and erbB3 (Ab-2, Ab-6) antibodies were from Neomarkers; for subcellular fractionation: LAP2 antibody was from BD Transduction Laboratories and Anti-ß-Tubulin was from Sigma; for immunoprecipitation (IP): erbB1 (1005), erbB4 (C-7) antibodies, and normal rabbit IgG (sc-2027) were from Santa Cruz; erbB2 (Ab-6, Ab-15) and erbB3 (Ab-2, Ab-6) were from Neomarkers; for confocal microscopy: erbB1 (1005), erbB3 (C-17) and erbB4 (C-7) antibodies were from Santa Cruz; erbB2 (Ab-1, Ab-15) and erbB3 (Ab-5) were from Neomarkers; anti-mouse and anti-rabbit antibodies conjugated with Alexa488 and Alexa568 for confocal microscopy were from Molecular Probes. EGF was from Upstate Biotechnology; HRP-fused goat anti-mouse IgG and goat anti-rabbit IgG were from Zymed. The recombinant EGF-like domain of NRG1β, a generous gift from Dr. K. L. Carraway 3rd (U.C. Davis, CA), was expressed and purified as previously described [23,24].
2.3 Umbilical endothelial cell preparation
Endothelial cells were harvested from human umbilical cord vessels within 1 − 5 days of delivery. We excluded umbilical cords with histologic evidence of inflammation (funisitis) to reduce the likelihood of obtaining biased results from activated endothelial cells. Umbilical cords were from babies born between 24 weeks and 42 weeks gestation. To study erbB receptor expression patterns over gestation, we grouped our samples from gestational age 24 to 42 into four groups, namely weeks 24 − 27 (group 1, N=13), weeks 28 −31 (group 2, N=4), weeks 32 − 37 (group 3, N=5), and weeks 38 − 42 (group 4, N=6). The umbilical veins and arteries were cannulated with blunt needles and rinsed with sterile PBS buffer. Type I collagenase (0,04%) was installed, the segments were clamped at both ends, and placed in an incubator at 37°C for 25 minutes. Collagenase was collected and segments were rinsed with 6ml PBS. Cell suspension was centrifuged at 250 × g for 5 minutes at 4°C, cell pellet was resuspended and plated in a 25 ml flask in endothelial cell growth medium containing 10% fetal calf serum (FCS). Cells were washed after 2−3 hours of adherence, new medium was added, and cells were grown to confluence. Passage 3 was used for all expression experiments. This was necessary to gather enough protein in the first two splits for the IP protocols. The purity of the cell cultures was determined with a fluorescence-activated cell sorter (FACSCalibur; Becton Dickinson, Heidelberg) using the endothelial cell markers lektin UEA-1 and CD31, and the fibroblast marker CD90. Human fibroblasts were used as a negative control (Table 1).
Table 1.
Cell purity determination by FACS analysis of HUVEC and HUAEC cell cultures at 24 and 40 weeks of gestation. Purity of the cells studied using the endothelial cell marker UEA-1 and CD31 and fibroblast marker CD90.
| 24 weeks of gestation HUAEC | 24 weeks of gestation HUVEC | 40 weeks of gestation HUAEC | 40 weeks of gestation HUVEC | fibroblasts | |
|---|---|---|---|---|---|
| UEA-1 | 99,98% | 99,2% | 100% | 99,85% | 3,84% |
| CD 31 | 99,95% | 98,5% | 97% | 97,4% | 2,24% |
| CD 90 | 0,9% | 0,8% | 0,1% | 0,1% | 93,6% |
2.4 ErbB receptor expression by immunoprecipitation (IP)
Cells were grown in 100 mm culture dishes in endothelial cell growth medium containing 10% FCS until they reached 80−90% confluence. After serum starvation in Dulbecco’s Modified Eagle’s medium (DMEM) for 2 hours, cells were washed with ice cold PBS, and lysed in lysis buffer (20 mM HEPES, pH 7.4, 150 mM NaCl, 1 mM MgCl2, 1% Igepal CA-630, 10% glycerol, 1 mM Na3VO4, 1 mM NaF, 1 mM ZnCl2, 10mM β-glycerolphosphate, 5 mM tetrasodium pyrophosphate, 1 mM phenylmethylsulfonyl fluoride, and 4 μg/ml each aprotinin, leupeptin, and pepstatin) as described before [5,24]. Lysates were cleared by microcentrifugation for 15 minutes at 4°C and supernatants were incubated with the precipitating antibody for 1.5 hours at 4°C with gentle rocking. Protein-A-Sepharose was added and incubation continued overnight at 4°C. Beads were collected by microcentrifugation, washed three times in washing buffer (20 mM HEPES, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 1 mM Na3VO4, 1 mM ZnCl2, 10 mM β-glycerolphosphate, 5 mM tetrasodium pyrophosphate, 0.2 mM phenylmethylsulfonyl fluoride, and 4 μg/ml each aprotinin, leupeptin, and pepstatin), and boiled in Laemmli sample buffer for 5 minutes. Proteins were separated by 7% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Blots were blocked in 2% ECL Advance Blocking Agent for 1 hour at room temperature, incubated with polyclonal anti-phosphotyrosine antibody overnight at 4°C, washed three times, followed by a 1 hour incubation with an anti-rabbit secondary antibody coupled to horseradish peroxidase. Proteins were visualized by enhanced chemiluminescence using x-ray film. Blots were stripped in stripping buffer (62.5mM Tris, pH 6.8, 2%SDS, 0,8% beta-mercaptoethanol) for 30 minutes at 50°C, blocked with either 2% ECL Advance Blocking Agent or 1% BSA, and reprobed with erbB receptor-specific antibodies as indicated.
2.5 Subcellular fractionation and LPS treatment
At 80−90% confluence, cells were serum starved in Dulbecco’s Modified Eagle’s medium (DMEM) for 2 hours, and incubated with 500 ng LPS/ml DMEM or 10 μg LPS/ml DMEM for 1 hour or 8 hours. After the incubation cells were stimulated with EGF (100ng/ml) or NRG (33nmol) for 2 minutes at 37°C or kept untreated as controls. For subcellular fractionation, cells were pelleted and suspended in Nuclei Buffer (pH 7.9, 10mM Hepes, 1.5 mM MgCl2, 10mM KCl, 1% Triton X-100, 0.5 mM DTT), kept on ice for 10 minutes and centrifuged at 10000g at 4°C for 10 minutes. The supernatants (cytoplasmic fractions) were stored at −80°C and the pellets were suspended in High Salt Buffer (pH 7.9, 10 mM Hepes, 400 mM NaCl, 0.1 mM EDTA, 5% glycerol, 0.5 mM DTT), kept on ice for 20 minutes and centrifuged at 10000g at 4°C for 10 minutes. The supernatants (nuclear fractions) were stored at − 80°C until further processed. Proteins were separated, transferred, and probed as described in the IP section. Purity of the cytoplasmic and nuclear fraction was confirmed by using nuclear specific and cytoplasmic specific protein antibodies.
2.6 ErbB receptor localization by confocal microscopy
HUVEC and HUAEC were grown on glass slides for 24 hours and starved in serum-free DMEM for 2 hours. We isolated cells from 10 umbilical cords at different gestational ages. Cells were stimulated with EGF (100ng/ml) or NRG (33nmol) for 2 minutes at 37°C. Immunofluorescence was performed as previously described [25]. Cells were rinsed three times with DMEM, fixed either with 3% paraformaldehyde for 20 minutes followed by permeabilisation with 0.2% Triton X-100 for 2 minutes, or with 3% paraformaldehyde containing 0.5% Triton X-100, followed by 3% paraformaldehyde for 30 minutes at room temperature. After 1 hour blocking in 10% normal goat serum, fixed cells were incubated with the specific primary erbB-antibody at room temperature for 1−2 hours. Cells were washed with PBS and incubated with the appropriate secondary antibody conjugated with Alexa488 or Alexa568. Cells were mounted in Gelvatol/DABCO and analysed using a Leica TCS–SP2 confocal laser scanning microscope.
3. Results
3.1 ErbB receptor expression
All four receptors were present in unstimulated human umbilical venous and arterial endothelial cells (Fig.1A, Fig.1B). We grouped our samples from gestational age 24 to 42 into four groups, namely weeks 24 − 27 (group 1, N=13) (Fig.1 A1-A4), weeks 28 −31 (group 2, N=4) (data not shown), weeks 32 − 37 (group 3, N=5) (data not shown), and weeks 38 − 42 (group 4, N=6) (Fig.1 B1-B4). Expression patterns were similar for all gestational age groups tested, except for absent erbB1 phosphorylation in HUAECs at late gestation (Fig.2 B1). Otherwise, there was no difference in expression pattern between arterial and venous cells. Immunoprecipitation of erbB1 always co-precipitated erbB4 independent of the gestational age or cell type, suggesting a strong tendency of erbB4 to dimerize with erbB1 (Fig.1 A1, Fig.1 B1).
Figure 1.
Immunoprecipitation of erbB1 (1005) (A1), erbB2 (Ab-15) (A2), erbB3 (Ab-2, Ab-6) (A3) and erbB4 (C-18) (A4) in unstimulated HUVEC (left panel) and HUAEC (right panel) at 27 weeks of gestation. Immunoprecipitation of erbB1 (1005) (B1), erbB2 (Ab-15) (B2), erbB3 (Ab-2, Ab-6) (B3) and erbB4 (C-18) (B4) in unstimulated HUVEC (left panel) and HUAEC (right panel) at 38 weeks of gestation. The upper blots show the phosphorylated receptors, the lower blots show the specific receptor protein. Representative blot of four independent experiments. Reprobing of the same blot up to five times. Immunoprecipitation with control IgG (left panels) of erbB1 (C1), erbB2 (C2), erbB3 (C3). and erbB4 (C4). The right panels show the lysates of the cells probed with the specific receptor protein.
Figure 2.

Subcellular fractionation of HUVECs control cells (Figure 2 A) and in cells after LPS treatment (Figure 2 B). Cells were treated with 500 ng LPS / ml for 1 hour. Some cells (lane 3, 4, 7, 8) were stimulated for 2 minutes with EGF (100ng/ml).
The upper blots show the phosphorylated receptors, the lower blots show the whole length erbB receptor or the intracellular domain of erbB4 (80kD) protein. The left lane of a pair of lanes shows the cytoplasmic (cyto) and the right lane shows the nuclear (nucl) fraction.
Figure 2 C shows fractionation purity controls, using anti-ß-Tubulin as a cytoplasmic marker and LAP2 as a nuclear marker.
In order to exclude any antibody-based non-specific binding, we performed immunoprecipitations with control IgG antibody (Fig.1C). There was no erbB receptor protein band detectable on the IgG IP’s.
3.2 ErbB4 localization after growth factor stimulation and LPS exposure
Subcellular fractionation revealed cytoplasmic staining of erbB4 in unstimulated cells and an increase in nuclear localization after stimulation with growth factors NRG (data not shown) and EGF (Fig.2A). Growth factor stimulation led to an even more pronounced nuclear staining of the 80 kD intracellular domain of erbB4 (Fig.2A).
LPS treatment led to different expression and localization patterns of erbB4 receptor with an increase in phosphorylation and protein content in the nuclear fraction of erbB4 (Fig.2B). This effect was even more pronounced for the intracellular 80kD domain of the receptor which is cleaved by a proteolytic mechanism leading to a nuclear translocation of the intracellular domain. Optimal results were obtained with 500 ng LPS / ml DMEM treatment for 1 hour. In order to confirm the purity of the subcellular fractionation, we used anti-ß-Tubulin as cytoplasmic marker and LAP2 as nuclear marker (Fig 2C).
3.3 ErbB receptor localization and co-localization
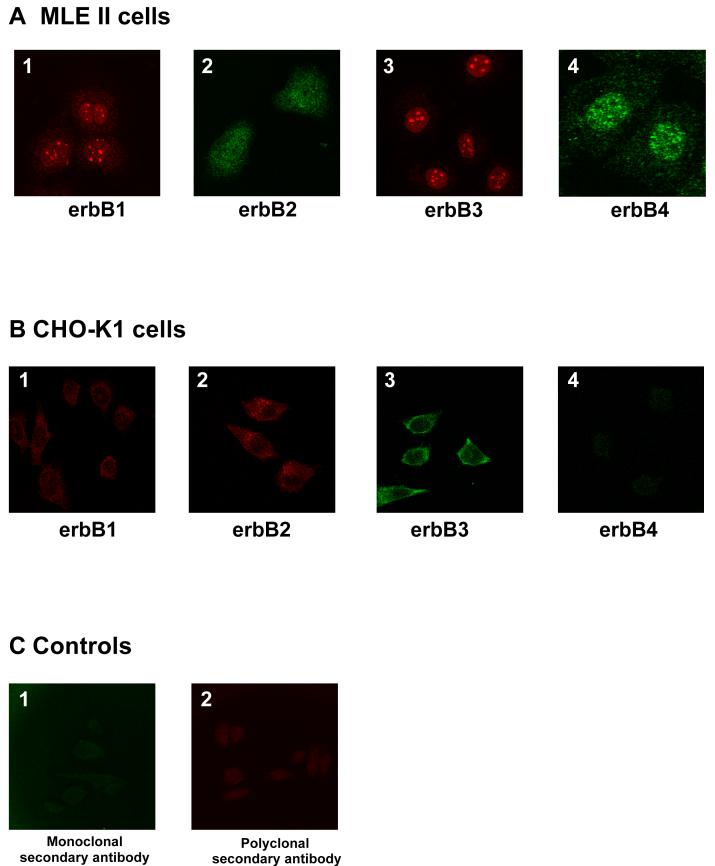
Although the erbB receptors are transmembrane receptors and, thus, usually located on the cell surface, we found that all four erbB receptors strongly localized to the nucleus in both HUVEC (Fig. 3) and HUAEC (data not shown). ErbB1 staining was intense in the nucleus and nucleoli, while being weak and diffuse in the cytoplasm (Fig. 3A, 3B, 3C). ErbB2 was localized in the nuclear compartment but spared the nucleolar region with a diffuse cytoplasmic pattern (Fig. 3A, 3D, 3E). ErbB3 localized in the nucleus with a pronounced nucleolar staining when labeled with the polyclonal antibody (Fig. 3D, 3F). In unstimulated cells, erbB4 showed a more diffuse localization pattern in all cell compartments with a tendency to spare the nucleolar region (Fig. 3C, 3E, 3F). The localization of the specific erbB receptors was independent of the cell fixation procedure and the antibody (mono- or polyclonal) used, except for the erbB3 receptor. We found different patterns for two different erbB3 receptor antibodies used in our cells. While the monoclonal antibody showed a more diffuse pattern (Fig. 3B), the polyclonal antibody revealed a strong nucleolar pattern (Fig. 3D, 3F). Since primary antibodies sometimes yield non-specific background we performed a confocal microscopy study with both a positive cell line (MLE12 cells), highly expressing all four erbB receptors (Fig.4A) and a negative cell line (CHO cells) (Fig.4B), showing no obvious erbB receptor expression. Although we did see some non-specific staining in the CHO cells (especially for erbB2 and erbB3; Fig.4 B2 and B3), these staining patterns differed appreciably from the specific staining patterns present in HUVECs and MLE12 cells (Fig. 3 and Fig. 4A).
Figure 3.


Subcellular localization and co-localization of (A) erbB1(red dye; 1005) and erbB2 (green dye; Ab-15), (B) erbB1 (red dye; 1005) and erbB3 (green dye; Ab-5), (C) erbB1 (red dye; 1005) and erbB4 (green dye; C-7), (D) erbB2 (green dye; Ab-15) and erbB3 (red dye; C-17), (E) erbB2 (red dye; Ab-1) and erbB4 (green dye; C-7), and (F) erbB3 (red dye; C-17) and erbB4 (green dye; C-7) in non-stimulated HUVEC and HUAEC detected by confocal microscopy. The right panel shows the effect of EGF on subcellular localization.
Figure 4.
Controls for non-specific immunofluorescent staining: Localization of erbB1 (A1), erbB2 (A2), erbB3 (A3) and erbB4 (A4) in non-stimulated mouse lung type II epithelial cells (MLE-12 cells). These cells are well known for high expression of all four erbB receptors and were therefore used as a positive control. Localization of erbB1 (B1), erbB2 (B2), erbB3 (B3) and erbB4 (B4) in non-stimulated chinese hamster ovary cells (CHO-K1). Since CHO-K1 cells do not express erbB receptors, these cells were used as a negative control. Negative control for immunostaining in HUVEC cells, stained with monoclonal secondary antibody (C1) and polyclonal secondary antibody (C2) only.
Unstimulated cells exhibited co-localization tendency for erbB1 with erbB3 (Fig. 3B) and erbB4 (Fig. 3C) as well as for erbB2 with erbB4 (Fig. 3E). Again, there was no obvious difference in the co-localization at the different gestational ages or cell types studied. Stimulation with EGF and NRG changed localization and co-localization patterns of the erbB receptors in these endothelial cells. Because there was no prominent difference between the stimulation with these two growth factors, we present the effects of EGF only. EGF induced a more pronounced co-localization of erbB1 with all other erbB receptors (Fig. 3A, 3B, 3C) and to a lesser extent of erbB3 with erbB2 (Fig. 3D) and erbB4 (Fig. 3F). Interestingly, growth factor stimulation led to an intense nucleolar immunolabeling of erbB3 with both the monoclonal and the polyclonal erbB3 antibody (Fig. 3B). EGF and NRG led to a complex shifting pattern towards the nucleus for erbB4, and to the nucleoli for erbB1 and erbB2, while erbB3 staining became visible in the cytoplasm (Table 2).
Table 2.
ErbB receptor localization and co-localization patterns, and effects of EGF/NRG stimulation in HUVEC.
| Predominant location (unstimulated) | ||||
|---|---|---|---|---|
| erbB | Plasma | Nucleus | Nucleoli | Stimulation with EGF / NRG |
| 1 | (+) | ++ | +++ | = |
| 2 | (+) | ++ | - | Nucleoli ↑ |
| 3 | - | + | +++ | Cytoplasma ↑ |
| 4 | (+) | + | - | Nucleoli ↑ |
4. Discussion
The goal of our studies was to explore the expression patterns of erbB receptors in HUVEC and HUAEC from preterm and term newborns. The first major result is that we found all four erbB receptors (including erbB1) expressed in human umbilical endothelial cells. ErbB1 was previously difficult to detect in human umbilical endothelial cells [26], which is somewhat surprising given the well-documented effects that EGF exerts on these cells [27]. We have identified erbB1 protein by immunoprecipitation and by confocal microscopy. Additional support for the presence of erbB1 comes from findings of others that betacellulin, a ligand of erbB1, stimulates endothelial cell migration and tube formation in HUVEC [28], and that EGF inhibits endothelin-1 synthesis by HUVEC [29].
Second, we found all four erbB receptors in both HUVEC and HUAEC. This suggests that the erbB receptors and their ligands might have a role in both, venous and arterial, systems.
Third, constitutive erbB receptor expression in HUVEC/HUAEC does not appear to be developmentally regulated, at least not in the last trimester, because expression patterns for all four erbB receptors did not change from 24 to 42 weeks. Thus, endothelial erbB receptors might play a role in both mid- and late gestation, as well as in disorders of preterm (<37 weeks gestation) and term infants. Since we assume that expression patterns in preterm infants might be confounded by a common characteristic of preterm delivery (i.e., perinatal infection), we excluded cords with umbilical vasculitis. However, the biologic functions of erbB receptors at different gestational ages await more detailed elucidation.
Fourth, the cellular localization pattern is different from what one might expect for transmembrane receptors. All four erbB receptors are prominently localized in the nucleus of HUVEC and HUAEC. Our results are in agreement with the finding in Schwann cells, where erbB2 and erbB3 are localized in the cytoplasm and the nucleus [17]. However, we found a more diffuse distribution pattern for erbB4 in human umbilical endothelial cells than others did in fetal renal cells, where erbB4 mainly localized to the nucleus [16]. Receptor tyrosine kinases are found in the nucleus in two forms, i.e., either as the intact molecule or as its cytoplasmic domain fragment. While the means by which intact receptors are translocated from the plasma membrane to the nucleus are still poorly understood, it has been reported that stimulation of erbB4 results in proteolytic cleavage and translocation of the cytoplasmic fragment to the nucleus in renal cells [16].
We found that nuclear translocation of the intracellular component of erbB4 was enhanced by erbB4 ligands EGF and NRG. This effect was even more pronounced after pre-treatment with LPS. This suggests that erbB4 nuclear shuttling might be enhanced in the context of systemic infection. Since EGF is not a ligand of erbB4, but of erbB1, we further speculate that erbB1/erbB4 interactions might play a role in this scenario.
ErbB1 receptor stimulation at the cell surface leads to migration of the full length transmembrane erbB1 receptor to the nucleus [30], where it binds an AT-rich consensus sequence via an undefined domain and enhances transcription via a proline-rich region near its carboxy-terminal domain [30]. A second possible translocation mechanism is that the receptors carry other molecules into the nucleus. These receptor-associated molecules are functional and it might be possible that the operative molecule in the nucleus is the ligand rather than its receptor. For example, erbB1 may transport STAT-1, a tyrosine-phosphorylated transcription factor, from the cytosol to the nucleus [31]. Again, it is unclear how the receptors translocate from the membrane to the nucleus and whether the nuclear localization of the receptor is a necessary part of the cellular response to growth factor stimulation.
All four erbB receptors co-localize within the nucleus (Fig. 2A-F). This is well compatible with the observation that erbB receptors dimerize with different erbB partners leading to markedly different cellular responses via different intracellular signaling pathways depending on the heterodimers formed [32]. For example, dimerization of erbB2 and erbB3 appears to be strongly involved in VEGF-associated angiogenesis in breast cancer cells [33].
Finally, we found that stimulation with EGF and NRG leads to a shifting towards the nucleus for erbB4, and to the nucleoli for erbB1, erbB2, and erbB3. This is in keeping with the finding that shuttling of erbB1 and erbB3 between nuclear and non-nuclear compartments is stimulated by EGF and NRG, respectively [30]. ErbB1 is especially enriched in the nuclei of highly proliferating cells [30]. It has been speculated that nuclear erbB receptors are involved in activating gene expression [30]. However, their exact function in the nucleus is still unclear. Our finding that LPS treatment promotes nuclear shuttling of erbB4 in fetal endothelium prompts us to speculate that nuclear functions of erbB4 in fetal endothelium might be involved in endothelial responses to perinatal infection/inflammation.
Using isolated human umbilical endothelial cells after the third passage has potential drawbacks. In order to be able to use immunoprecipitation protocols, we had to passage the cells, which led to the cells being more remote from the in vivo situation compared to fresh material. Further in vivo studies would be helpful to further elucidate the regulation of these receptors.
ErbB receptors are crucial in organ development and neonatal disorders. One possible link between these disorders and the erbB-ligand family of growth factors might be inflammation, which plays an important role in the etiology of BPD [34,35], brain white matter damage leading to CP [36], and NEC [37]. EGF and TGF-α induce production of the pro-inflammatory chemokine CXCL8 (interleukin-8, IL-8) [38,39]. There is also a signaling interaction between erbB and interleukin-6 (IL-6)-receptor components [40,41]. Another potential link how erbB receptors might contribute to disorders such as BPD [8,42] or retinopathy of prematurity (ROP) [43] is aberrant angiogenesis/neo-vascularization, either with [44] or without involving vascular endothelial growth factor (VEGF) [26].
If NRG was involved in neonatal disease as a signaling molecule acting via the systemic circulation, its biologic effects were dependent upon the expression of its receptors in the endothelial cell system. Therefore, disturbance of the endothelial erbB signaling network might be the link between the systemic fetal inflammatory response (e.g., in the setting of intrauterine infection) [45] and the fetal response to this inflammatory response.
We conclude that all four erbB receptors are present in fetal endothelium in mid- and late gestation, and exhibit stimulus-specific localization and co-localization patterns. Our most interesting result of LPS-associated shuttling of erbB4 into the endothelial nucleus should lead to experiments that include all erbB receptors to further elucidate the functional effects of this phenomenon. We believe that our findings, taken together with the known involvement of erbB receptors in development, inflammation, and angiogenesis, will open new avenues for erbB receptor-related research in the pathogenesis of neonatal inflammation-associated disorders.
Acknowledgements
This work was supported by National Institutes of Health HL 04436, Charles H. Hood Foundation, Boston, MA, Wilhelm Hirte Stiftung, Hannover and an intramural grant (HiLF) from Hannover Medical School, Germany. We thank Dr. K. Kamino for the histologic examination of the umbilical cords, Mrs. B. Haarmeijer for technical assistance in the endothelial cell isolation, Dr. M. Winkler for determining the purity of endothelial cell cultures, Mrs. M. Haidukiewicz for helping to collect umbilical cords, and Mrs. C. Acevedo for helpful assistance in setting up systems.
Abbreviations
- BPD
bronchopulmonary dysplasia
- CP
cerebral palsy
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- FIR
fetal inflammatory response
- HUAEC
human umbilical arterial endothelial cell
- HUVEC
human umbilical venous endothelial cell
- ICAM
intercellular adhesion molecule
- IP
immunoprecipitation
- LPS
lipopolysaccharide
- NEC
necrotizing enterocolitis
- NRG
neuregulin
- ROP
retinopathy of prematurity
- VCAM
vascular cell adhesion molecule
- VEGF
vascular endothelial growth factor
References
- 1.Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378(6555):390–4. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- 2.Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–8. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- 3.Sibilia M, Wagner EF. Strain-dependent epithelial defects in mice lacking the EGF receptor. Science. 1995;269:234–8. doi: 10.1126/science.7618085. [DOI] [PubMed] [Google Scholar]
- 4.Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC, et al. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269:230–4. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 5.Dammann CE, Nielsen HC, Carraway KL., 3rd Role of neuregulin-1 beta in the developing lung. Am J Respir Crit Care Med. 2003;167(12):1711–6. doi: 10.1164/rccm.200205-468OC. [DOI] [PubMed] [Google Scholar]
- 6.Strandjord TP, Clark JG, Guralnick DE, Madtes DK. Immunolocalization of transforming growth factor-alpha, epidermal growth factor (EGF), and EGF-receptor in normal and injured developing human lung. Pediatr Res. 1995;38(6):851–6. doi: 10.1203/00006450-199512000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Currie AE, Vyas JR, MacDonald J, Field D, Kotecha S. Epidermal growth factor in the lungs of infants developing chronic lung disease. Eur Respir J. 2001;18(5):796–800. doi: 10.1183/09031936.01.00088201. [DOI] [PubMed] [Google Scholar]
- 8.Le Cras TD, Hardie WD, Deutsch GH, Albertine KH, Ikegami M, Whitsett JA, Korfhagen TR. Transient induction of TGF-{alpha} disrupts lung morphogenesis, causing pulmonary disease in adulthood. Am J Physiol Lung Cell Mol Physiol. 2004;287(4):L718–L29. doi: 10.1152/ajplung.00084.2004. [DOI] [PubMed] [Google Scholar]
- 9.Miettinen PJ, Berger JE, Meneses J, Phung Y, Pedersen RA, Werb Z, Derynck R. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995;376:337–41. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- 10.Dvorak B, Halpern MD, Holubec H, Williams CS, McWilliam DL, Dominguez JA, Stepankova R, Payne CM, McCuskey RS. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J Physiol Gastrointest Liver Physiol. 2002;282(1):G156–64. doi: 10.1152/ajpgi.00196.2001. [DOI] [PubMed] [Google Scholar]
- 11.Kaukola T, Satyaraj E, Patel DD, Tchernev VT, Grimwade BG, Kingsmore SF, Koskela P, Tammela O, Vainionpaa L, Pihko H, Aarimaa T, Hallman M. Cerebral palsy is characterized by protein mediators in cord serum. Ann Neurol. 2004;55(2):186–94. doi: 10.1002/ana.10809. [DOI] [PubMed] [Google Scholar]
- 12.Dammann O, Leviton A. Inflammation, brain damage and visual dysfunction in preterm infants. Semin Fetal Neonatal Med. 2006;11(5):363–8. doi: 10.1016/j.siny.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Martin CR, Walker WA. Intestinal immune defences and the inflammatory response in necrotising enterocolitis. Semin Fetal Neonatal Med. 2006;11(5):369–77. doi: 10.1016/j.siny.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Speer CP. Inflammation and bronchopulmonary dysplasia: a continuing story. Semin Fetal Neonatal Med. 2006;11(5):354–62. doi: 10.1016/j.siny.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Rousset CI, Hagberg H, Mallard C. Lipopolysaccharide-induced inflammation and perinatal brain injury. Semin Fetal Neonatal Med. 2006;11(5):343–53. doi: 10.1016/j.siny.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Komuro A, Nagai M, Navin NE, Sudol M. WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxyl-terminal fragment of ErbB-4 that translocates to the nucleus. J Biol Chem. 2003;278(35):33334–41. doi: 10.1074/jbc.M305597200. [DOI] [PubMed] [Google Scholar]
- 17.Raabe TD, Deadwyler G, Varga JW, Devries GH. Localization of neuregulin isoforms and erbB receptors in myelinating glial cells. Glia. 2004;45(2):197–207. doi: 10.1002/glia.10311. [DOI] [PubMed] [Google Scholar]
- 18.Williams CC, Allison JG, Vidal GA, Burow ME, Beckman BS, Marrero L, Jones FE. The ERBB4/HER4 receptor tyrosine kinase regulates gene expression by functioning as a STAT5A nuclear chaperone. J Cell Biol. 2004;167(3):469–78. doi: 10.1083/jcb.200403155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sardi SP, Murtie J, Koirala S, Patten BA, Corfas G. Presenilin-Dependent ErbB4 Nuclear Signaling Regulates the Timing of Astrogenesis in the Developing Brain. Cell. 2006;127(1):185–97. doi: 10.1016/j.cell.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 20.Chaudhury AR, Gerecke KM, Wyss JM, Morgan DG, Gordon MN, Carroll SL. Neuregulin-1 and erbB4 immunoreactivity is associated with neuritic plaques in Alzheimer disease brain and in a transgenic model of Alzheimer disease. J Neuropathol Exp Neurol. 2003;62(1):42–54. doi: 10.1093/jnen/62.1.42. [DOI] [PubMed] [Google Scholar]
- 21.Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, Gallop RJ, Arnold SE. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 2006;12(7):824–8. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- 22.Earp HS, 3rd, Calvo BF, Sartor CI. The EGF receptor family--multiple roles in proliferation, differentiation, and neoplasia with an emphasis on HER4. Trans Am Clin Climatol Assoc. 2003;114:315–33. discussion 33-4. [PMC free article] [PubMed] [Google Scholar]
- 23.Carraway KL, 3rd, Weber JL, Unger MJ, Ledesma J, Yu N, Gassmann M, Lai C. Neuregulin-2, a new ligand of ErbB3/ErbB4-receptor tyrosine kinases. Nature. 1997;387(6632):512–6. doi: 10.1038/387512a0. [DOI] [PubMed] [Google Scholar]
- 24.Crovello CS, Lai C, Cantley LC, Carraway KL., 3rd Differential signaling by the epidermal growth factor-like growth factors neuregulin-1 and neuregulin-2. J Biol Chem. 1998;273(41):26954–61. doi: 10.1074/jbc.273.41.26954. [DOI] [PubMed] [Google Scholar]
- 25.Padmakumar VC, Abraham S, Braune S, Noegel AA, Tunggal B, Karakesisoglou I, Korenbaum E. Enaptin, a giant actin-binding protein, is an element of the nuclear membrane and the actin cytoskeleton. Exp Cell Res. 2004;295(2):330–9. doi: 10.1016/j.yexcr.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Russell KS, Stern DF, Polverini PJ, Bender JR. Neuregulin activation of ErbB receptors in vascular endothelium leads to angiogenesis. Am J Physiol. 1999;277(6 Pt 2):H2205–11. doi: 10.1152/ajpheart.1999.277.6.H2205. [DOI] [PubMed] [Google Scholar]
- 27.Gospodarowicz D, Brown KD, Birdwell CR, Zetter BR. Control of proliferation of human vascular endothelial cells. Characterization of the response of human umbilical vein endothelial cells to fibroblast growth factor, epidermal growth factor, and thrombin. J Cell Biol. 1978;77(3):774–88. doi: 10.1083/jcb.77.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim HS, Shin HS, Kwak HJ, Cho CH, Lee CO, Koh GY. Betacellulin induces angiogenesis through activation of mitogen-activated protein kinase and phosphatidylinositol 3'-kinase in endothelial cell. Faseb J. 2003;17(2):318–20. doi: 10.1096/fj.02-0570fje. [DOI] [PubMed] [Google Scholar]
- 29.Haug C, Schmid-Kotsas A, Linder T, Bachem MG, Gruenert A, Rozdzinski E. Influence of hepatocyte growth factor, epidermal growth factor, and mycophenolic acid on endothelin-1 synthesis in human endothelial cells. Nephrol Dial Transplant. 2001;16(12):2310–6. doi: 10.1093/ndt/16.12.2310. [DOI] [PubMed] [Google Scholar]
- 30.Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3(9):802–8. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- 31.Bild AH, Turkson J, Jove R. Cytoplasmic transport of Stat3 by receptor-mediated endocytosis. Embo J. 2002;21(13):3255–63. doi: 10.1093/emboj/cdf351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemmon MA, Schlessinger J. Transmembrane signaling by receptor oligomerization. Methods Mol Biol. 1998;84:49–71. doi: 10.1385/0-89603-488-7:49. [DOI] [PubMed] [Google Scholar]
- 33.Yen L, Benlimame N, Nie ZR, Xiao D, Wang T, Al Moustafa AE, Esumi H, Milanini J, Hynes NE, Pages G, Alaoui-Jamali MA. Differential regulation of tumor angiogenesis by distinct ErbB homo- and heterodimers. Mol Biol Cell. 2002;13(11):4029–44. doi: 10.1091/mbc.E02-02-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jobe AH, Ikegami M. Antenatal infection/inflammation and postnatal lung maturation and injury. Respir Res. 2001;2(1):27–32. doi: 10.1186/rr35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Speer CP. Inflammation and bronchopulmonary dysplasia. Semin Neonatol. 2003;8:29–38. doi: 10.1016/s1084-2756(02)00190-2. [DOI] [PubMed] [Google Scholar]
- 36.Dammann O, Leviton A. Inflammatory brain damage in preterm newborns - dry numbers, wet lab, and causal inference. Early Hum Dev. 2004;79(1):1–15. doi: 10.1016/j.earlhumdev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Caplan MS, Jilling T. New concepts in necrotizing enterocolitis. Curr Opin Pediatr. 2001;13:111–5. doi: 10.1097/00008480-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Subauste MC, Proud D. Effects of tumor necrosis factor-alpha, epidermal growth factor and transforming growth factor-alpha on interleukin-8 production by, and human rhinovirus replication in, bronchial epithelial cells. Int Immunopharmacol. 2001;1(7):1229–34. doi: 10.1016/s1567-5769(01)00063-7. [DOI] [PubMed] [Google Scholar]
- 39.Hamilton LM, Torres-Lozano C, Puddicombe SM, Richter A, Kimber I, Dearman RJ, Vrugt B, Aalbers R, Holgate ST, Djukanovic R, Wilson SJ, Davies DE. The role of the epidermal growth factor receptor in sustaining neutrophil inflammation in severe asthma. Clin Exp Allergy. 2003;33(2):233–40. doi: 10.1046/j.1365-2222.2003.01593.x. [DOI] [PubMed] [Google Scholar]
- 40.Qiu Y, Ravi L, Kung HJ. Requirement of ErbB2 for signalling by interleukin-6 in prostate carcinoma cells. Nature. 1998;393(6680):83–5. doi: 10.1038/30012. [DOI] [PubMed] [Google Scholar]
- 41.Grant SL, Hammacher A, Douglas AM, Goss GA, Mansfield RK, Heath JK, Begley CG. An unexpected biochemical and functional interaction between gp130 and the EGF receptor family in breast cancer cells. Oncogene. 2002;21(3):460–74. doi: 10.1038/sj.onc.1205100. [DOI] [PubMed] [Google Scholar]
- 42.Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol. 1998;29(7):710–7. doi: 10.1016/s0046-8177(98)90280-5. [DOI] [PubMed] [Google Scholar]
- 43.Smith LE. Pathogenesis of retinopathy of prematurity. Growth Horm IGF Res. 2004;14(Suppl A):S140–4. doi: 10.1016/j.ghir.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 44.Finkenzeller G, Weindel K, Zimmermann W, Westin G, Marme D. Activated Neu/ErbB-2 Induces Expression of the Vascular Endothelial Growth Factor Gene by Functional Activation of the Transcription Factor Sp 1. Angiogenesis. 2004;7(1):59–68. doi: 10.1023/B:AGEN.0000037332.66411.f0. [DOI] [PubMed] [Google Scholar]
- 45.Dammann O, Leviton A. Role of the fetus in perinatal infection and neonatal brain damage. Curr Opin Pediatr. 2000;12(2):99–104. doi: 10.1097/00008480-200004000-00002. [DOI] [PubMed] [Google Scholar]