Summary
Adult neurogenesis occurs throughout life in discrete regions of the adult mammalian brain. Little is known about the mechanism governing the sequential developmental process that leads to integration of new neurons from adult neural stem cells into the existing circuitry. Here, we investigated roles of Disrupted-In-Schizophrenia 1 (DISC1), a schizophrenia susceptibility gene, in adult hippocampal neurogenesis. Unexpectedly, down regulation of DISC1 leads to accelerated neuronal integration, resulting in aberrant morphological development and mis-positioning of new dentate granule cells in a cell-autonomous fashion. Functionally, newborn neurons with DISC1 knockdown exhibit enhanced excitability and accelerated dendritic development and synapse formation. Furthermore, DISC1 cooperates with its binding partner Ndel1 in regulating adult neurogenesis. Taken together, our study identifies DISC1 as a key regulator that orchestrates the tempo of functional neuronal integration in the adult brain and demonstrates essential roles of a susceptibility gene for major mental illness in neuronal development, including adult neurogenesis.
Introduction
Adult neurogenesis, a process of generating functionally integrated new neurons from adult neural progenitors, represents a striking form of structural plasticity in the adult mammalian brain (Kempermann and Gage, 1999). In the hippocampus, immature neurons, originating from adult progenitors at the subgranular zone, migrate into the inner granule cell layer to become new dentate granule cells (Ming and Song, 2005). These new neurons extend axonal and dendritic projections and establish new synaptic connections to integrate into the existing circuitry (van Praag et al., 2002). Recent studies have characterized the basic process of adult neurogenesis and defined many physiological and pathological stimuli important for its regulation. Mechanistic studies have been largely concentrated on early events of adult neurogenesis and identified several key players that control the proliferation and fate specification of adult neural progenitors, including Shh, BMPs and Wnts (Lledo et al., 2006). Little is known about the molecular mechanism that regulates the integration of adult-born neurons, an orchestrated process including neuronal morphogenesis, migration, acquisition of intrinsic excitability and synapse formation.
One distinct feature of adult neurogenesis is its tempo of neuronal integration. While adult and fetal neurogenesis of dentate granule cells show remarkable similarities in the developmental process, a major difference is the prolonged course for adult-born neurons (Esposito et al., 2005; Overstreet-Wadiche et al., 2006a; Zhao et al., 2006). Interestingly, neuronal activation, such as seizures, accelerates integration of new neurons in the adult hippocampus (Overstreet-Wadiche et al., 2006b). Together, the difference in the timing of integration between fetal and adult-born granule cells and stimulation of integration pace by neuronal activities in adult indicate that proper tempo regulation of neuronal integration may be critical for the physiological consequence of adult neurogenesis. The molecular mechanism underlying this important aspect of adult neurogenesis remains to be defined.
In an effort to address the molecular mechanism regulating neuronal integration during adult neurogenesis, we investigated the role of Disrupted-In-Schizophrenia 1 (disc1), a susceptibility gene for schizophrenia (Blackwood et al., 2001; Millar et al., 2000) and possibly some mood disorders (Hamshere et al., 2005; Hashimoto et al., 2006). DISC1 expression is broad in many brain regions during embryonic development and fairly restricted in the adult brain with particularly high expression in dentate granule cells of the hippocampus and interneurons of the olfactory bulb (Austin et al., 2004), two neuronal types that are continuously generated through adult neurogenesis. A role of DISC1 in neuronal development was first suggested by biochemical identification of interacting proteins (Millar et al., 2003; Morris et al., 2003; Ozeki et al., 2003). For example, DISC1 binds Ndel1 (NUDEL), a molecule involved in embryonic neuronal development including migration (Sasaki et al., 2005; Shu et al., 2004). In vitro studies with PC12 cells and primary neurons showed that blocking DISC1 function impairs neurite outgrowth (Kamiya et al., 2005; Miyoshi et al., 2003; Shinoda et al., 2007; Taya et al., 2007). Furthermore, in utero electroporation-mediated expression of short-hairpin RNAs (shRNAs) against disc1, or a truncation mutant of disc1, in E14.5 embryos leads to retarded migration and mis-oriented dendrites of cortical neurons (Kamiya et al., 2005). The finding that DISC1 promotes migration in the embryonic cortex and neurite outgrowth in vitro, as well as its restricted expression in neurons produced during adult neurogenesis, raise a tantalizing possibility that DISC1 may play an important role in regulating the process of adult neurogenesis.
To ascertain the in vivo function of DISC1 in adult neurogenesis, we employed an oncoretrovirus-mediated RNA interference approach to genetically manipulate DISC1 expression within individual cells in specific brain regions (Ge et al., 2006). Such in vivo “single-cell genetic” approach allows characterization of cell-autonomous roles of DISC1 specifically in adult neurogenesis, without the complication of potential developmental defects and/or compensations in traditional germ-line knockout animals. Here we demonstrated that DISC1 regulates almost all essential steps of neuronal integration in adult neurogenesis. In contrast to what has been found in embryonic cortical development and cultured neuronal cells, DISC1 knockdown in newborn dentate granule cells of the adult hippocampus leads to soma hypertrophy, accelerated dendritic outgrowth with appearance of ectopic dendrites, mis-positioning from over-extended migration, enhanced intrinsic excitability and accelerated synapse formation of new neurons. These findings indicate that DISC1, a schizophrenia susceptibility gene, serves as a key regulator that controls the tempo of neuronal development and therefore keeps the progress of new neuron integration in the adult brain in check.
Results
Role of DISC1 in morphogenesis of new neurons in the adult brain
We used an oncoretrovirus-mediated approach for birth-dating and genetic manipulation of individual new neurons in the adult mouse dentate gyrus (Ge et al., 2006). Retroviral constructs were engineered to co-express enhanced green fluorescent protein (GFP) and an shRNA to knock down expression of endogenous mouse DISC1 (mDISC1, Figure 1A). Specific shRNAs were designed against conserved regions of all known mouse disc1 isoforms (Figure S1 in the Supplementary Data; See Experimental Procedures). Two different shRNAs (shRNA-D1 and D2) effectively knocked down the expression of a full-length mDISC1 in vitro (Figure 1B). Another two shRNAs against mDISC1 (shRNA-D3 and D4) exhibited partial knockdown, while a control shRNA against DsRed (shRNA-C1) was ineffective (Figure 1B). High titers of engineered retroviruses were stereotaxically injected into the hilar region of the adult C57BL/6 mouse hippocampus to infect proliferating neural progenitors in vivo. Immunocytochemistry confirmed the knockdown of mDISC1 in shRNA-D1/GFP+ cells in vivo (Figures S1).
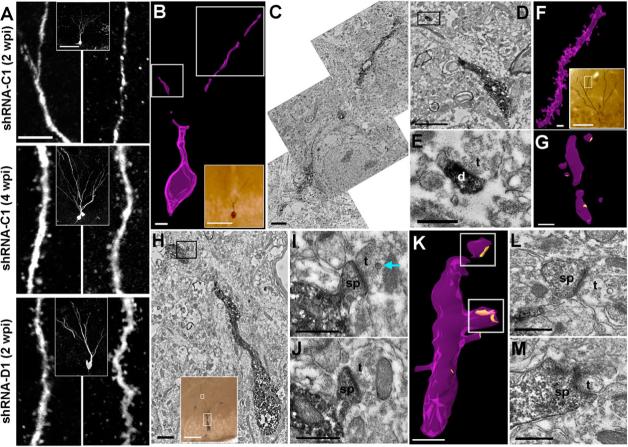
Figure 1. DISC1 regulates morphogenesis of adult-born neurons.
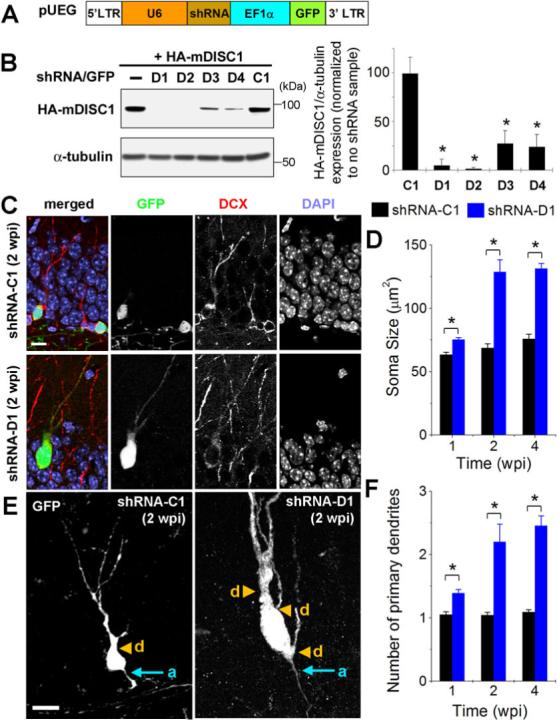
(A) A schematic diagram of the retroviral vector (pUEG) used for in vivo birth-dating and genetic manipulation. (B) Validation of the efficacy of mDISC1-shRNAs in vitro. Retroviral constructs expressing different shRNAs were co-transfected with an expression construct for HA-tagged mDISC1 into 293 cells and equal amount of cell lysate samples were subjected to Western Blot analysis for HA and α-tubulin. A sample blot is shown on the left. Densitometry quantification is shown on the right. For each experiment, the densitometry measurement of DISC1 band was first normalized to that of α-tubulin and then normalized to no shRNA expression sample. Values represent mean ± SD (n = 3; *: p < 0.01, ANOVA). (C, D) Soma size of adult-born neurons. Shown in (C) are sample confocal images of GFP, DAPI and immunostaining for DCX. Scale bar: 10 μm. Shown in (D) is the summary of soma area of GFP+ neurons at 1, 2 and 4 wpi. Values represent mean ± SEM (n = 4 animals; *: p < 0.05, ANOVA). (E, F) Primary dendrites of adult-born neurons. Shown in (E) are sample projections of Z-series confocal images of GFP+ neurons at 2 wpi. Arrows and arrowheads point to initiation sites of axons (a) and primary dendrites (d), respectively. Scale bar: 10 μm. Shown in (F) is summary of primary dendrite numbers of GFP+ neurons at 1, 2 and 4 wpi. Values represent mean ± SEM (same group of cells as in D; *: p < 0.05, ANOVA).
We first examined whether DISC1 regulates neuronal fate specification of adult neural progenitors. Immunostaining of doublecortin (DCX; Figure 1C), an immature neuronal marker (Brown et al., 2003), revealed that 84.5 ± 9.5% of shRNA-C1/GFP+ cells and 89.3 ± 6.4% of shRNA-D1/GFP+ cells (n = 4 animals) became neurons at one week post injection (wpi). Thus, DISC1 knockdown under this condition does not appear to affect neuronal fate specification during adult hippocampal neurogenesis.
We next examined the morphology of adult-born neurons. Surprisingly, cell bodies of shRNA-D1/GFP+ neurons were significantly larger than those of shRNA-C1/GFP+ neurons at all developmental stages examined (Figures 1C and 1D). Several other effective mDISC1-shRNAs also showed different degrees of soma hypertrophy at 2 wpi (Figure S2), suggesting that the observed phenotype is specifically due to DISC1 knockdown.
Dentate granule cells in rodents do not maintain basal dendrites and normally extend only one primary apical dendrite that branches to form elaborated arborization (Seress and Pokorny, 1981; Shapiro and Ribak, 2006). In contrast, neurons with DISC1 knockdown exhibited multiple primary dendrites (Figures 1E and 1F). Strikingly, 21% of shRNA-D1/GFP+ neurons maintained basal dendrites that initiated near the site of axon initiation and extended toward the molecular layer (Figure 1E). Variable degrees of the ectopic dendrite phenotype were observed with other mDISC1-shRNAs (Figure S2). Taken together, these results showed that DISC1 controls morphogenesis of adult-born neurons, including soma size and dendritic initiation.
Role of DISC1 in controlling positioning of new neurons in the adult brain
We next examined the effect of DISC1 knockdown on migration of adult-born neurons. It has been established that adult-born neurons in the hippocampus contribute almost exclusively to only the inner two thirds of the granule cell layer (Figure 2A)(Esposito et al., 2005; Kempermann et al., 2003). At 1 wpi, GFP+ neurons were distributed largely within the inner granule cell layer (Figure 2B). By 2 wpi, while some shRNA-C1/GFP+ neurons had migrated into the middle third layer, majority of shRNA-D1/GFP+ neurons had already migrated into the outer third of the granule cell layer with some even into the molecular layer. By 4 wpi, about 50% of shRNA-D1/GFP+ neurons, but none of shRNA-C1/GFP+ neurons, were in the molecular layer (Figure 2C). Additional studies with BrdU labeled new neurons exhibited similar pattern of positioning as those of shRNA-C1/GFP+ neurons (Figure 2C), suggesting that retroviral manipulation itself does not have any “procedure effects”. Different degrees of over-extended migration phenotype were also observed with two other mDISC1-shRNAs (Figure S3). These results indicate that, instead of directly mediating neuronal migration, DISC1 is required for relaying positional signals during migration.
Figure 2. DISC1 regulates positioning of adult-born neurons but not neuronal subtype differentiation.
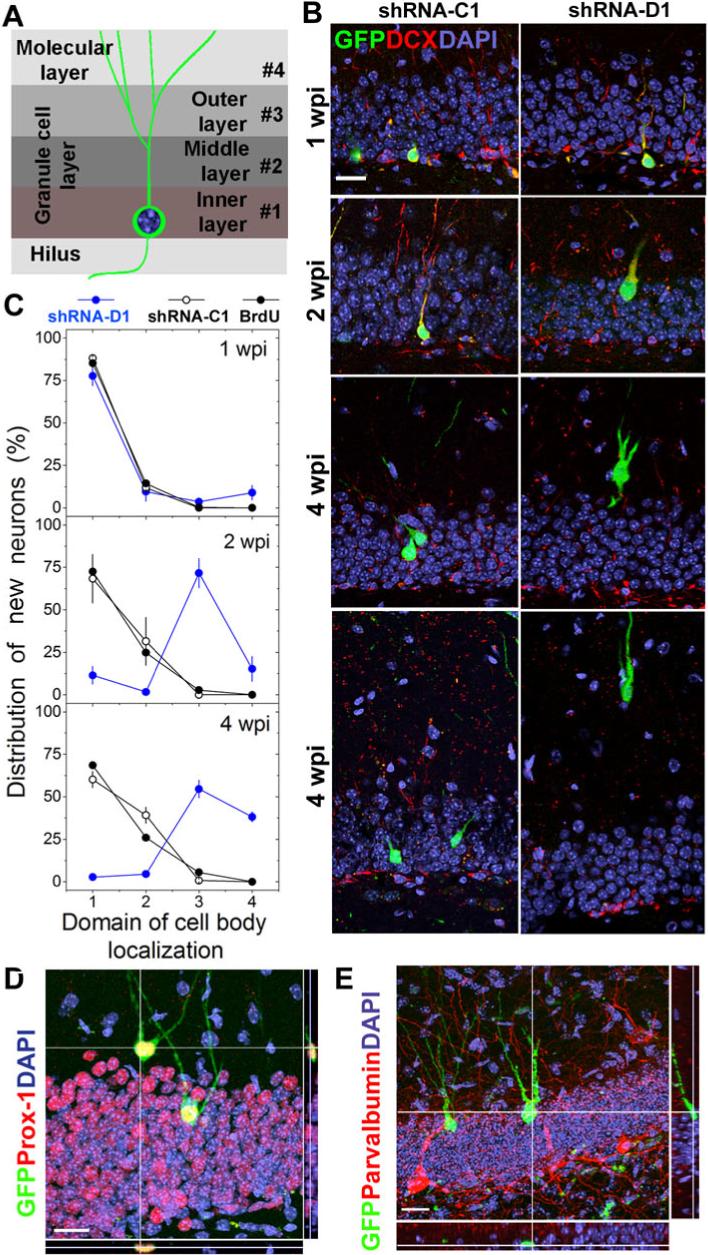
(A) A schematic diagram of adult mouse dentate gyrus region divided into four domains. (B, C) Positioning of adult-born neurons. Shown in (B) are sample confocal images of GFP, DAPI and immunostaining for DCX. Scale bar: 20 μm. Shown in (C) are distribution plots of GFP+ neurons expressing shRNA-D1 or shRNA-C1, or BrdU labeled neurons at 1, 2 or 4 wpi. Values represent mean ± SEM (n = 4 animals). (D, E) Neuronal subtype differentiation of adult neural progenitors. Shown are sample confocal images of GFP, DAPI and immunostaining for Prox-1 (D) or Parvalbumin (E) for shRNA-D1/GFP+ neurons at 2 wpi. Scale bar: 50 μm.
To rule out the possibility that mis-positioning was due to a change in neuronal subtype differentiation of adult neural progenitors with DISC1 knockdown, we examined expression of Prox-1, a dentate granule cell marker (Pleasure et al., 2000), and Parvalbumin, an interneuron marker (Freund and Buzsaki, 1996). Immunocytochemistry showed that all GFP+ neurons at 2 wpi were Prox-1+Parvalbumin−, including those located within the molecular layer (Figures 2D and 2E). Thus, DISC1 knockdown under this condition does not affect neuronal subtype differentiation during adult hippocampal neurogenesis.
Rescue of DISC1-knockdown phenotype by expression of shRNA-resistant mDISC1
Our results with multiple shRNAs targeting different regions of mDISC1 suggest that the observed effects are specific to DISC1 down-regulation. To further confirm the specificity of shRNA manipulations, we carried out in vivo rescue experiments using lentiviruses, which allow sustained transgene expression (Lois et al., 2002). We generated lentiviruses expressing a fusion protein of GFP and an shRNA-resistant form of mDISC1 (GFP-mDISC1R) that harbors 8 silent mutations within the sequence targeted by shRNA-D1 (Figures 3A and 3B). We also generated oncoretroviruses that co-express a red fluorescent protein mCherry and shRNA-D1 or C1 (Figure 3A). Western blot analysis confirmed the resistance of GFP-mDISC1R to shRNA-D1 in vitro (Figure 3C). After co-injection of two types of viruses into the dentate gyrus of adult mice, there were many cells expressing GFP or mCherry alone and a limited number of cells co-expressing both at 2 wpi (Figure 3D). Expression of shRNA-D1 alone in new neurons (mCherry+GFP−) resulted in soma hypertrophy, ectopic dendrities and mis-positioning (Figures 3D to 3G), similar to what we described earlier (Figures 1 and 2). In contrast, double-labeled cells expressing both shRNA-D1/mCherry and GFP-mDISC1R in the same animal exhibited normal soma size, a single primary dendrite and proper positioning (Figures 3D to 3G), suggesting that defects from knockdown of endogenous DISC1 were rescued by expression of exogenous DISC1. Control experiments with co-expression of shRNA-C1 and GFP-mDISC1R showed no obvious phenotypes in neuronal morphology and positioning (Figures 3D to 3G). Taken together, these rescue experiments further confirmed the specificity of shRNA-mediated DISC1 knockdown in vivo.
Figure 3. Rescue of defects from DISC1-knockdown by mDISC1R expression.
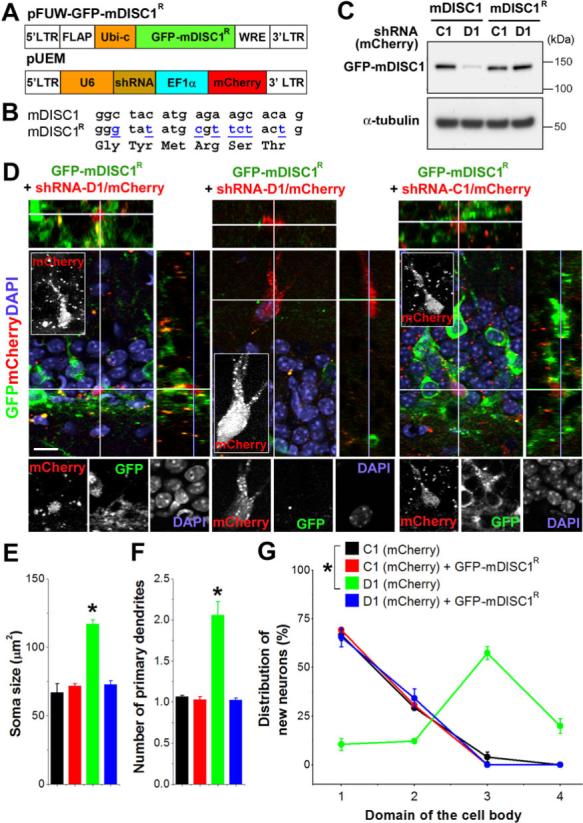
(A) Schematic diagrams of lentiviral (pFUW) and retroviral (pUEM) vectors for rescue experiments. (B) Alignment of shRNA-D1 targeting sequence in mDISC1 and silent mutations made in mDISC1R (underlined). (C) Validation of the resistance of mDISC1R to shRNA-D1 in vitro. Expression constructs for GFP-mDISC1 or GFP-mDISC1R and retroviral constructs were co-transfected into 293 cells and cell lysate samples were subjected to Western Blot analysis for GFP and α-tubulin. (D) Sample confocal images of new neurons at 2 wpi. Lentiviruses expressing GFP-mDISC1R and oncoretroviruses co-expressing mCherry and shRNA-D1, or C1 were co-injected in adult mice. Orthogonal views are shown to reveal co-localization of GFP and mCherry and individual channels are shown at the bottom panel. Insets are projection images (mCherry) to show neuronal morphology. Scale bar: 10 μm. (E-G) Summaries of morphological and positioning phenotypes of new neurons at 2 wpi. Shown are summaries for soma size (E), number of primary dendrites (F), and neuronal positioning (G, domains as defined in Figure 2A). Values represent mean ± SEM (n = 4 animals; *: p < 0.05, ANOVA).
Role of DISC1 in dendritic development of new neurons in the adult brain
To examine whether DISC1 regulates dendritic development in vivo, we reconstructed the dendritic arborization of individual GFP+ neurons with confocal microscopy. Surprisingly, shRNA-D1/GFP+ neurons exhibited much more elaborated dendrites than shRNA-C1/GFP+ neurons at 2 wpi (Figures 4A and 4B), as reflected by significant increases in both total dendritic length and branch numbers (Figure 4C). Sholl analysis further demonstrated an increase in the dendritic complexity of shRNA-D1/GFP+ neurons (Figure 4D). Thus, DISC1 knockdown accelerates dendritic development of adult-born neurons.
Figure 4. DISC1 regulates dendritic development of adult-born neurons.
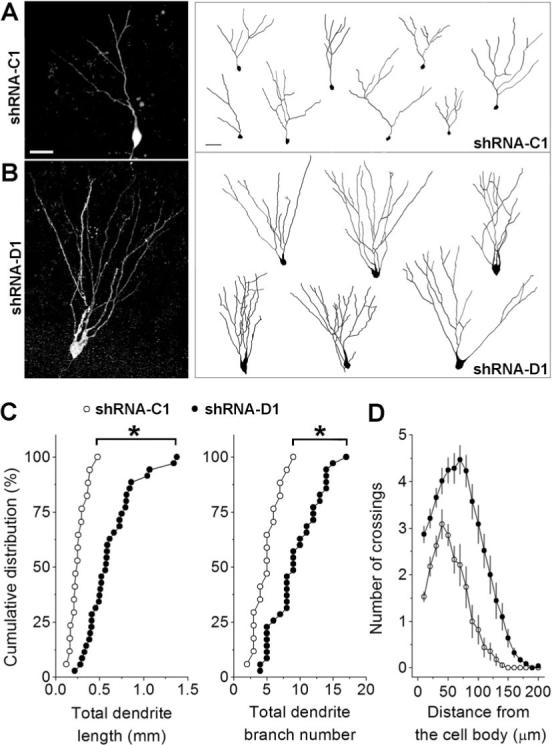
(A, B) Three-dimensional confocal reconstruction of dendrites of GFP+ dentate granule cells at 2 wpi. Shown on the left are sample projections of Z-series confocal images of an shRNA-C1/GFP+ neuron (A) and an shRNA-D1/GFP+ neuron (B). Shown on the right are samples of 2D projection trajectories of 3D confocal reconstruction of cell bodies and dendrites of GFP+ neurons at 2 wpi. Scale bars: 20 μm. (C) Summaries of dendrite properties of new neurons at 2 wpi. Shown are cumulative distribution plots of total dendrite length and branch numbers. Each symbol represents a single GFP+ neuron expressing shRNA-C1 or shRNA-D1 (*: p < 0.01, Kolmogorov-Smirnov test). (D) Sholl analysis of dendritic complexity of GFP+ neurons at 2 wpi. Values represent mean ± SEM (same groups of cells as in C).
Is increased dendritic arborization in shRNA-D1/GFP+ neurons simply an outcome of presence of ectopic dendrites? Re-analysis of the data without contributions from ectopic dendrites showed that single major dendrites of shRNA-D1/GFP+ neurons were still more elaborated than those of shRNA-C1/GFP+ neurons (Figure S3). To examine more closely whether accelerated dendritic development was due to a secondary effect of neuronal positioning, we divided shRNA-D1/GFP+ neurons into two groups, those with normal positioning (within the inner two thirds of the granule cell layer) and those with mis-positioning (within the outer one third of the granule cell layer and the molecular layer). Both groups exhibited more complex dendrites than shRNA-C1/GFP+ neurons at 2 wpi (Figure S3). Thus, DISC1 may regulate neuronal positioning and dendritic development independently.
Role of DISC1 in development of intrinsic excitability of new neurons in the adult brain
To examine whether morphological changes resulted from DISC1 knockdown leads to alterations of physiological properties, we performed electrophysiological analysis of newborn granule cells in acute slices from virus-injected animals. Previous studies have established that the membrane resistance of new neurons decreases along their maturation (Esposito et al., 2005; Schmidt-Hieber et al., 2004). Electrophysiology recording at 2 wpi showed that shRNA-D1/GFP+ neurons exhibited significantly lower membrane resistances (373 ± 26 MΩ, n = 7) than those of shRNA-C1/GFP+ neurons (786 ± 50 MΩ, n = 7), suggesting an accelerated functional maturation of new neurons with DISC1 knockdown.
We then examined the ability of new neurons to fire repetitive action potentials, a hallmark of neuronal maturation. Under the whole-cell current-clamp, shRNA-C1/GFP+ neurons at 2 wpi generally fired one to three tetrodotoxin (TTX)-sensitive action potentials in response to a depolarizing current injection (300 ms and 100 pA; Figure 5A), consistent with their immature neuronal excitability. Strikingly, shRNA-D1/GFP+ neurons fired repetitive action potentials up to 46 Hz in response to the same stimulation (Figure 5B). Thus, DISC1 regulates the development of intrinsic excitability of adult-born neurons.
Figure 5. DISC1 regulates development of intrinsic excitability and formation of functional synapses of adult-born neurons.
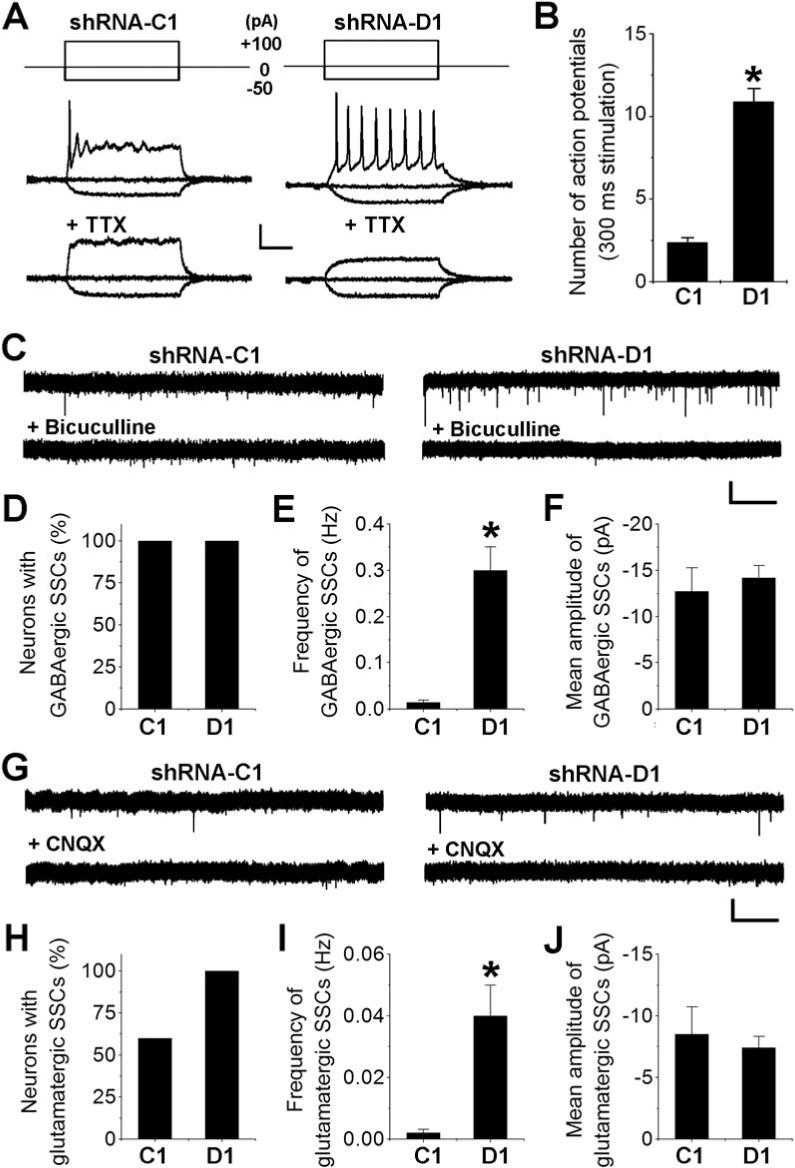
(A, B) Firing of repetitive action potentials by GFP+ neurons at 2 wpi. Shown in (A) are sample traces recorded from a GFP+ neuron expressing shRNA-C1 or shRNA-D1 in response to 300 ms current injections (+100, 0, or −50 pA) under the whole-cell current-clamp before and after the addition of TTX (1 μM). Scale bars: 30 mV and 100 ms. Shown in (B) is the summary of the number of action potentials fired in response to current injections (100 pA, 300 ms). Values represent mean ± SEM (n = 9; *: p < 0.05, ANOVA). (C-F) GABAergic synaptic transmission recorded in GFP+ neurons at 2 wpi. Shown in (C) are sample traces of SSCs recorded in a GFP+ neuron expressing shRNA-C1 or shRNA-D1 under the whole-cell voltage-clamp (Vm = −65 mV) in the presence of TTX (1 μM) and kynurenic acid (5 mM). Shown are continuous recordings before and after the addition of bicuculline (10 μM). Scale bars: 20 pA and 100 ms. Also shown are the percentage of GFP+ neurons recorded that exhibited active GABAergic SSCs (D), mean frequency (E) and peak amplitude (F). Values represent mean ± SEM (*: p < 0.01; ANOVA). (G-J) Glutamatergic synaptic transmission recorded in GFP+ neurons at 2 wpi. Similar as in (C-F), except that the recordings were carried out in the presence of TTX (1 μM) and bicuculline (10 μM). Sample traces shown in (G) are continuous recordings before and after the addition of CNQX (50 μM).
Role of DISC1 in synaptic integration of new neurons in the adult brain
We next examined synapse formation of newborn neurons. Confocal imaging revealed significant numbers of dendritic spines in shRNA-C1/GFP+ neurons at 4 wpi, but not at 2 wpi (Figure 6A). In contrast, numerous dendritic spines were already present in shRNA-D1/GFP+ neurons at 2 wpi, suggesting that DISC1 knockdown accelerates synapse formation of new neurons. To examine synaptic structures in detail, we carried out electron microscopic reconstruction of GFP+ new neurons. While dendritic spines were largely absent in shRNA-C1/GFP+ neurons at 2 wpi (Figures 6B and 6C), in rare cases there were synaptic structures associated with dendritic shafts of GFP+ neurons (Figures 6D and 6E). Increased number of dendritic spines and synaptic structures were observed in shRNA-C1/GFP+ neurons at 4 wpi (Figures 6F and 6G). On the other hand, synaptic structures associated with shRNA-D1/GFP+ neurons were readily observed at 2 wpi, with postsynaptic densities facing presynaptic boutons that contain synaptic vesicles (Figures 6H to 6M). Surprisingly, dense core vesicles were present in some of these new synapses (Figures 6I and 6J), suggesting that presynaptic differentiation might have occurred.
Figure 6. Formation of dendritic spines and synapses in adult-born neurons.
(A) Sample confocal images of shRNA-C1/GFP+ neurons at 2, 4 wpi and an shRNA-D1/GFP+ neuron at 2 wpi. Shown are projections of Z-series confocal images at high magnification to reveal dendritic spine structures. Scale bar: 5 μm. Insets show projection views of GFP+ neurons. Scale bar: 20 μm. (B-G) Electron microscopy analysis of formation of dendritic spines and synapses in normal new granule cells. Shown in (B) is a 3D reconstruction of serial sections through dendrites and soma of an shRNA-C1/GFP+ neuron at 2 wpi (a light micrograph view in the inset, scale bar: 50 μm). Scale bar: 5 μm. The regions within the right and left white boxes in (B) are shown at a higher magnification in (C) and (D), respectively. Scale bar: 2 μm. The boxed region in (D) is shown at a higher magnification in (E) and is an example of a synapse formed onto the dendritic shaft of this neuron. Scale bar: 0.5 μm. “d”: dendrite; “t”: terminal. Shown in (F) is a 3D reconstruction of serial sections through dendrites of an shRNA-C1/GFP+ neuron at 4 wpi (a light micrograph view in the inset). Scale bar: 50 μm. Yellow regions shown in (G) indicate postsynaptic densities identified by EM. Scale bar: 1 μm. (H-M) Presence of dendritic spines and synapses in new neurons with DISC1 knockdown at 2 wpi. Shown in (H) is an electron micrograph of a dendrite of an shRNA-D1/GFP+ neuron at 2wpi (large boxed region in the inset, scale bar: 50 μm). Scale bar: 1 μm. The boxed region in (H) is shown at higher magnification in (I) and (J), which are serial sections through a dendritic spine with an asymmetric synapse containing a dense core vesicle in the axonal terminal (arrows). Scale bars: 0.5 μm. Shown in (K) is a 3D reconstruction of serial sections through a more distal segment of dendrites from the same neuron (the small boxed region within the inset of H). Scale bar: 1 μm. Shown in (L) and (M) are higher magnification images of the synapses found on the dendritic segment within the top and bottom white boxes in (K), respectively. Scale bar: 0.5 μm. “sp”: spine.
To directly examine whether increased synaptic inputs to new neurons were functional, we carried out whole-cell voltage-clamp recording of GFP+ neurons at 2 wpi in acute slices prepared from injected animals. GABAergic spontaneous synaptic currents (SSCs) were recorded from GFP+ neurons (Vm = −65 mV) in the presence of kynurenic acid (5 mM) to block ionotropic glutamatergic currents (Figure 5C). While active GABAergic SSCs were detected in all neurons recorded (Figure 5D), expression of shRNA-D1 resulted in a 20-fold increase in the mean frequency of GABAergic SSCs (Figure 5E) without affecting the mean peak amplitude (Figure 5F). Next, we recorded glutamatergic SSCs in the presence of bicuculline (10 μM) to block GABAAR-mediated currents (Figure 5G). Glutamatergic SSCs were detected from all shRNA-D1/GFP+ neurons recorded at 2 wpi, but only from 60% of shRNA-C1/GFP+ neurons (Figure 5H). Furthermore, the mean frequency of glutamatergic SSCs in shRNA-D1/GFP+ neurons was about 18-fold higher than that of shRNA-C1/GFP+ neurons (Figure 5I), whereas the mean peak amplitudes were similar (Figure 5J). Taken together, these results demonstrate that knockdown of DISC1 leads to accelerated formation of functional GABAergic and glutamatergic synaptic inputs to new neurons in the adult brain.
Cooperation of Ndel1 and DISC1 in regulating development of new neurons
To elucidate the mechanism underlying regulation of neuronal integration by DISC1, we examined effects of down regulation of Ndel1, a direct binding partner of DISC1 (Brandon et al., 2004; Millar et al., 2003; Morris et al., 2003; Ozeki et al., 2003). We engineered a retrovirus expressing a previously characterized shRNA against Ndel1 (shRNA-N1)(Nguyen et al., 2004; Shu et al., 2004) and confirmed its effectiveness both in vitro and in vivo (Figure S4). At 2 wpi, shRNA-N1/GFP+ neurons exhibited ectopic primary dendrites and mis-positioning in the adult dentate gyrus (Figures 7B and 7C), resembling some features of DISC1 knockdown. There was also a modest increase in the total dendritic length (Figure 7D). We further examined the potential epistatic interaction between DISC1 and Ndel1 in regulating neuronal development. Together with retrovirus expressing shRNA-N1/GFP, we co-injected retrovirus expressing shRNA-D3/mCherry, which by themselves led to only partial knockdown of DISC1 (Figure 1B) and had little effects on positioning and dendritic development of new dentate granule cells at 2 wpi (Figures 7C to 7E). Interestingly, double-labeled neurons (GFP+mCherry+) exhibited much more dramatic defects in positioning and dendritic development than either of the single knockdown (Figures 7C to 7E). Together with biochemical evidence for direct interaction between DISC1 and Ndel1 and their co-localization in primary neurons (Figure S6), such synergistic effects in vivo strongly suggest that Ndel1 is a major partner of DISC1 in regulating integration of adult-born neurons.
Figure 7. DISC1 cooperates with Ndel1 to regulate development of adult-born neurons.
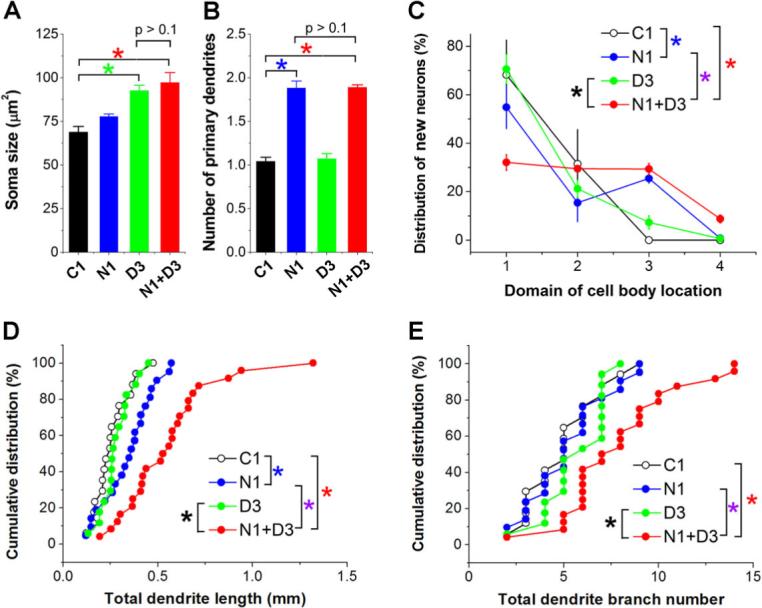
(A to C) Summaries of morphological and positioning phenotypes of new neurons at 2 wpi with different genetic manipulations. Shown are summaries of soma size (A), number of primary dendrites (B), and neuronal positioning (C, domains as defined in Figure 2A). Values represent mean ± SEM (n = 4 animals; *: p < 0.05, ANOVA). (D, E) Summary of dendritic development of new neurons at 2 wpi. Shown are cumulative distribution plots of total dendritic length and dendritic branch numbers. Each symbol represents a newborn neuron under each condition. Same group of cells as in (A-C). *: p < 0.01 (Kolmogorov-Smirnov test).
Discussion
The disc1 gene has been linked to schizophrenia and other serious mental illness in multiple pedigrees (Blackwood et al., 2001; Hamshere et al., 2005; Hashimoto et al., 2006; Millar et al., 2000). The neurobiology of DISC1 in the normal developing and adult brain and its roles in these mental illness are not well understood (Ishizuka et al., 2006; Porteous and Millar, 2006; Ross et al., 2006). Previous cellular studies have had limited success largely due to limitations of in vitro systems and difficulties in generating knockout mice because of complex alternative splicing, resulting in multiple isoforms of DISC1 proteins that remain to be fully characterized (Ishizuka et al., 2006). Using an in vivo “single-cell genetic” approach, we have identified a number of novel functions of DISC1 in integration of new neurons in the adult brain (Figure S5). First, down-regulation of DISC1 accelerates morphological development of adult-born neurons, resulting in soma hypertrophy and enhancement of dendritic outgrowth. This finding is unexpected, given the stimulating effect of DISC1 on axonal outgrowth of embryonic neurons in culture. Second, inhibition of DISC1 functions leads to mis-positioning of new neurons in the molecular layer from over-extended migration, suggesting that DISC1 serves as an interpreter that relays positional signals to the intracellular migratory machinery rather than a direct mediator of neuronal migration. Third, new neurons with DISC1 knockdown exhibit more mature neuronal firing patterns. Thus, regulation of DISC1 expression may alter sustained and synchronized firing, a notion with profound implications in cognitive brain function and schizophrenia. Finally, electron microscopy and electrophysiology studies demonstrated that down-regulation of DISC1 accelerates synapse formation of newborn neurons. Taken together, these results identify DISC1 as a master regulator controlling the tempo of the entire process of neuronal integration in the adult brain.
Our finding on aberrant integration of adult-born neurons with DISC1 knockdown reinforces the view that tempo regulation is critical for functional neurogenesis within an active neuronal network. Previous studies have implicated an important role of neuronal activity in integration during adult neurogenesis. For example, seizures accelerate new neuron integration (Overstreet-Wadiche et al., 2006b) and prolonged seizures lead to inappropriate integration of new neurons into the adult hippocampal circuitry (Scharfman and Hen, 2007). Recent studies have identified neurotransmitter signaling, such as GABA and glutamate, as part of extrinsic mechanisms in stimulating certain steps of neuronal integration (Ge et al., 2007). Different from these extrinsic positive regulators, DISC1 negatively regulates all essential steps of integration of adult-born neurons. Thus, DISC1 may serve as a key intrinsic determinant that balances stimulating effects of extrinsic mechanisms and keep the process in check. Whether DISC1 functions are in turn regulated by extrinsic factors remains to be determined.
Functions of DISC1 in neural development
The expression pattern of DISC1 together with biochemical characterization of interacting proteins (Camargo et al., 2007) implicates a role of DISC1 in neuronal development. Several studies have suggested that DISC1 may function to stimulate neurite outgrowth of PC12 cells and axonal growth of embryonic neurons in culture (Kamiya et al., 2005; Kamiya et al., 2006; Ozeki et al., 2003; Taya et al., 2007). Our preliminary study also supports a similar role of DISC1 in axonal and dendritic outgrowth of early postnatal dentate granule cells in vitro (Figure S6). Surprisingly, DISC1 knockdown in adult-born dentate granule cells in vivo accelerates dendritic development, resulting in a complex and elaborated pattern of dendritic growth, ectopic apical dendrites and unusual appearance of basal dendrites. Thus, instead of a direct mediator of neurite outgrowth, DISC1 may function as a regulator that promotes dendritic development of embryonic neurons in vitro, while suppressing that of adult-born dentate granule cells in vivo. Whether such differential regulation by DISC1 reflects differences in intrinsic properties of neurons at different stages or extrinsic local environment remains to be determined. Interestingly, dentate granule cells exhibit significantly more basal dendrites in some schizophrenic patients (Lauer et al., 2003; Senitz and Beckmann, 2003).
DISC1 has been implicated in neuronal migration during embryonic cortical development (Kamiya et al., 2005). Our preliminary studies with co-injection of two different retroviruses into mouse forebrain ventricles at E13.5 in utero showed that shRNA-D1/GFP+ cells exhibited less extended migration in comparison to shRNA-C1/mCherry+ cells in the cortex of the same individual animals at P1 (Figure S7). In contrast, DISC1 knockdown in adult-born dentate granule cells leads to over-extended migration (Figure 2). It is well-established that different cortical layers are developed in an “inside-out” fashion during embryonic stages (Ayala et al., 2007), while dentate granule cells in rodents are arranged in an orderly “outside-in” sequence within a single cell layer (Altman and Das, 1965; Angevine, 1965)(Figure S8). Thus, both phenotypes are consistent with a model in which DISC1 functions to relay positional signals to the intracellular migratory machinery, rather than a direct mediator of cell migration. Interestingly, MAP2ab+ neurons have been found to be mis-positioned within the white matter of temporal and parahippocampal regions in some schizophrenic patients (Arnold et al., 2005).
The role of DISC1 in maturation of neuronal excitability was not expected. Recent studies have provided indirect evidence for a potential role of DISC1 at synapses. Light and electron microscopic studies showed the localization of DISC1 immunoreactivity at postsynaptic site of both symmetric and asymmetric synapses in humans (Kirkpatrick et al., 2006). Bioinformatics and yeast two-hybrid screens have revealed a “DISC1 interactome”, in which DISC1 interacts with many proteins involved in synaptic functions (Camargo et al., 2007; Carter, 2006). Our study provides direct evidence for a functional role of DISC1 in regulating the tempo of synapse formation of adult-born neurons in vivo. Whether DISC1 plays similar roles in neuronal maturation and synaptogenesis during embryonic development remains to be determined.
Molecular basis of DISC1 functions in neural development
Our findings of functional roles of DISC1 in multiple steps of neuronal development is consistent with the large number of DISC1 interacting protein involved in centrosome assembly, cytoskeleton reorganization and intracellular transport (Camargo et al., 2007). Previous in vitro experiments have implicated a role of DISC1-Ndel1 interaction in regulating neurite outgrowth (Kamiya et al., 2005; Ozeki et al., 2003). We provide in vivo evidence that Ndel1 is a major partner of DISC1 in neuronal functions. Ndel1 knockdown in new neurons mimics several major defects of down regulation of DISC1, including ectopic dendrites and aberrant positioning (Figure 7). More importantly, simultaneous knocking down of Ndel1 and DISC1 results in synergistic effects on neuronal positioning and dendritic development. Interestingly, a recent study has found a link between risk alleles of DISC1 and Ndel1 in schizophrenia patients (Hennah et al., 2007). Consistent with our findings, Ndel1 homozygous knockout mice (Sasaki et al., 2005), as well as Ndel1 knockdown in developing neocortex, exhibited impaired neuronal positioning (Shu et al., 2004). In the heterozygous germ-line knockout mice of Lis1, which binds to Ndel1, some dentate granule cells migrated to the molecular layer and exhibited hypertrophy and basal dendrites (Fleck et al., 2000), resembling morphological and positioning defects of new neurons with DISC1 knockdown. Taken together, these results support a model in which Ndel1 and DISC1 cooperate to control neuronal morphogenesis and positioning during neuronal integration.
It is likely that other DISC1 interacting proteins also play important roles. Furthermore, the existence of multiple isoforms of DISC1 protein raises the possibility that different isoforms may exhibit differential localization and interaction with different partners, thus performing varied functions. Future goals are to define the spatio-temporal nature of DISC1 interactions and functions at different subcellular locations and during different developmental stages in regulating neural development under normal and pathological conditions.
Implications for mental disorders
disc1 was initially identified as a gene disrupted by a balanced translocation on chromosome 1q42 that segregates with schizophrenia, bipolar disorder, and recurrent major depression in a large Scottish family (Blackwood et al., 2001; Millar et al., 2000). Genetic linkage and association studies have suggested that disc1 may be a general risk factor for schizophrenia and some other mental disorders, such as bipolar disorder and depression (Harrison and Weinberger, 2005; Ishizuka et al., 2006; Mackie et al., 2007). The relevance of disc1 to schizophrenia was further demonstrated by a functional correlation between genetic variations of disc1 in humans and specific defects in hippocampal structures and functions (Callicott et al., 2005). In rodent models, one study with an endogenous mutant disc1 identified in the 129S6/SvEv mice supports a role of DISC1 in modulating working memory (Koike et al., 2006). A recent study showed that mice with a missense mutation (Q31L) exhibit deficits in the force swim test and depression-like behaviors, while L100P mutant mice exhibit schizophrenia-like phenotypes, such as defects in pre-pulse inhibition and latent inhibition (Clapcote et al., 2007). Whether defects in adult neurogenesis contribute to schizophrenia, bipolar and major depression, psychological disorders with adult onsets, remains unknown and is an interesting topic for future investigation. Interestingly, a recent study suggests that cell proliferation in the adult human dentate gyrus is decreased in schizophrenia, but not in depression (Reif et al., 2006). Emerging evidence also suggests that many mental disorders are developmental in nature and may result from defects in neuronal integration (Arnold et al., 2005; Lewis and Levitt, 2002; Zoghbi, 2003). Our identification of DISC1 as a critical regulator controlling sequential steps of neuronal integration raises the possibility that DISC1 may be a key molecular player in the etiology of major mental illness.
Experimental Procedures
Materials and other experimental procedures are described in the Supplementary Data.
Genetic marking and manipulation with engineered oncoretroviruses and lentiviruses
Engineered self-inactivating murine oncoretroviruses were used to express shRNAs and GFP (pUEG) or mCherry (pUEM) specifically in proliferating cells and their progeny (van Praag et al., 2002). Several shRNAs against different regions of mDISC1 (Figure S1), mouse Ndel1, and DsRed were used (See supplementary data). For rescue experiments, self-inactivating lentiviruses (Lois et al., 2002) were engineered to express a fusion protein of GFP and mDISC1 harboring 8 silent mutations (GFP-mDISC1R; Figure 3A). To validate the specificity and efficiency of shRNAs, retroviral shRNA vectors and expression constructs for HA-tagged full-length mDISC1, GFP-mDISC1 or GFP-mDISC1R, were co-transfected into 293 cells and cell lysates were prepared for Western blot analysis of DISC1 expression using anti-HA or anti-GFP antibodies, respectively.
High titers of engineered retroviruses were produced (Ge et al., 2006; Lois et al., 2002) and were stereotaxically injected into adult female C57BL/6 mice (7−8 weeks old, Charles River) housed under standard conditions as described (Ge et al., 2006). All animal procedures were in accordance with institutional guidelines.
Immunostaining, confocal imaging and electron microcopy
Coronal brain sections (40-μm thick) were prepared from injected mice and processed for immunostaining as described (Ge et al., 2006). The following primary antibodies were used: BrdU (rat, 1:400, Accurate), DCX (goat, 1:250, Santa Cruz), DISC1 (goat, 1:100, Santa Cruz, N-16), GFP (rabbit, 1:1000, Abcam), Ndel1 (rat monoclonal, 1:50, gift of A. Sawa), Prox-1 (rabbit, 1:1000, Abcam), Parvalbumin (mouse, 1:2000, Sigma). Images were acquired on a Zeiss510 multiphoton confocal system using a multi-track configuration.
For analysis of cell morphology, Z-series stacks of confocal images were taken and a single confocal image slice with the largest soma area for individual GFP+ neurons was used for quantification using NIH ImageJ program. For analysis of neuronal positioning, single section confocal images of GFP+ neurons with 4′,6-diaminodino-2-phenylindole (DAPI, 1:5000) staining were used to determine the cell localization within 4 domains defined in Figure 2A. For one set of control experiments, BrdU (50 mg/kg body weight) was injected. A minimum of 10 neurons of randomly picked sections from each animal and at least 4 animals were analyzed for neuronal morphology and positioning under each experimental condition. Statistic significance was determined with ANOVA.
For analysis of dendritic development, 3D reconstruction of entire dendritic processes of each neuron was made from Z-series stacks of confocal images. The 2D projection images were traced with NIH ImageJ. All GFP+ dentate granule cells with largely intact dendritic trees were analyzed for total dendritic length and branch number as described (Ge et al., 2006). Data shown was from a minimal of 17 individual GFP+ neurons from at least 4 animals for each condition. Statistic significance was determined with Kolmogorov-Smirnov test.
For immunoelectron microscopy analysis, shRNA-D1 and C1 expressing samples from a minimum of two animals each, was simultaneously processed as described (Liu and Jones, 2003). Serial EM images were processed with Reconstruct 2.4 for 3D reconstruction and rendered using 3D Studio Max (Discreet).
Electrophysiology
Mice housed under standard conditions were processed at 2 wpi for slice preparation and electrophysiology recording at 32°C - 34°C as described (Ge et al., 2006). To examine intrinsic excitability, GFP+ dentate granule cells were recorded under the whole-cell current-clamp with current pulses (300 ms) injected. SSCs were examined in the presence of 1 μM TTX and ten-minute continuous sweeps were recorded under the voltage-clamp (Vm = −65 mV) in the presence of 10 μM bicuculline or 5 mM kynurenic acid for glutamatergic or GABAergic SSCs, respectively.
Acknowledgements:
We thank D. Weinberger, A. Sawa, A. Kolodkin, D. Ginty, W. Chen, K.H. Wang, A. Kamiya and members of Song, Lu and Ming Laboratories for help and critical comments, L. Liu, K. Sailor and Y. Cai for technical support, A. Sawa for Ndel1 antibody, T. Dawson, C. Lois and R. Tsien for constructs. This work was supported by NIH (NS047344 and AG024984) and McKnight Scholar Award to H.S., by NIH (NS048271), Whitehall Foundation and a Klingenstein Fellowship Award in the Neurosciences to G-l.M., and by NIH (HD045757) to H-j.C.
Author contributions: X.D. contributed to all aspects of experiments and data analysis; J.H.C. performed shRNA design and in vitro characterization; S-y. G., C-h.Y. performed electrophysiology; R.L.F., X-b.L, H-j.C. performed EM; J.Y.K. performed in vitro experiments; Y.K. performed in utero experiment; J.D.J., D.K.M., C.Y.L. helped with molecular biology; S.G. performed FLIM; G-l.M., B.L., H.S. were co-senior authors and contributed to experiment planning, data analysis and writing. All authors discussed results and commented on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Supplementary Data
Supplementary Data includes 8 figures and Supplementary Experimental Procedures.
References
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Angevine JB., Jr. Time of neuron origin in the hippocampal region. An autoradiographic study in the mouse. Exp Neurol Suppl. 1965;(Suppl 2):1–70. [PubMed] [Google Scholar]
- Arnold SE, Talbot K, Hahn CG. Neurodevelopment, neuroplasticity, and new genes for schizophrenia. Prog Brain Res. 2005;147:319–345. doi: 10.1016/S0079-6123(04)47023-X. [DOI] [PubMed] [Google Scholar]
- Austin CP, Ky B, Ma L, Morris JA, Shughrue PJ. Expression of Disrupted-In-Schizophrenia-1, a schizophrenia-associated gene, is prominent in the mouse hippocampus throughout brain development. Neuroscience. 2004;124:3–10. doi: 10.1016/j.neuroscience.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Ayala R, Shu T, Tsai LH. Trekking across the Brain: The Journey of Neuronal Migration. Cell. 2007;128:29–43. doi: 10.1016/j.cell.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Blackwood DH, Fordyce A, Walker MT, St Clair DM, Porteous DJ, Muir WJ. Schizophrenia and affective disorders--cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am J Hum Genet. 2001;69:428–433. doi: 10.1086/321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon NJ, Handford EJ, Schurov I, Rain JC, Pelling M, Duran-Jimeniz B, Camargo LM, Oliver KR, Beher D, Shearman MS, Whiting PJ. Disrupted in Schizophrenia 1 and Nudel form a neurodevelopmentally regulated protein complex: implications for schizophrenia and other major neurological disorders. Mol Cell Neurosci. 2004;25:42–55. doi: 10.1016/j.mcn.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Straub RE, Pezawas L, Egan MF, Mattay VS, Hariri AR, Verchinski BA, Meyer-Lindenberg A, Balkissoon R, Kolachana B, et al. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc Natl Acad Sci U S A. 2005;102:8627–8632. doi: 10.1073/pnas.0500515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo LM, Collura V, Rain JC, Mizuguchi K, Hermjakob H, Kerrien S, Bonnert TP, Whiting PJ, Brandon NJ. Disrupted in Schizophrenia 1 Interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol Psychiatry. 2007;12:74–86. doi: 10.1038/sj.mp.4001880. [DOI] [PubMed] [Google Scholar]
- Carter CJ. Schizophrenia susceptibility genes converge on interlinked pathways related to glutamatergic transmission and long-term potentiation, oxidative stress and oligodendrocyte viability. Schizophr Res. 2006;86:1–14. doi: 10.1016/j.schres.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Clapcote SJ, Lipina TV, Millar JK, Mackie S, Christie S, Ogawa F, Lerch JP, Trimble K, Uchiyama M, Sakuraba Y, et al. Behavior phenotypes of Disc1 missense mutation in mice. Neuron. 2007;54:387–402. doi: 10.1016/j.neuron.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Esposito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck MW, Hirotsune S, Gambello MJ, Phillips-Tansey E, Suares G, Mervis RF, Wynshaw-Boris A, McBain CJ. Hippocampal abnormalities and enhanced excitability in a murine model of human lissencephaly. J Neurosci. 2000;20:2439–2450. doi: 10.1523/JNEUROSCI.20-07-02439.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Pradhan DA, Ming GL, Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Hamshere ML, Bennett P, Williams N, Segurado R, Cardno A, Norton N, Lambert D, Williams H, Kirov G, Corvin A, et al. Genomewide linkage scan in schizoaffective disorder: significant evidence for linkage at 1q42 close to DISC1, and suggestive evidence at 22q11 and 19p13. Arch Gen Psychiatry. 2005;62:1081–1088. doi: 10.1001/archpsyc.62.10.1081. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Numakawa T, Ohnishi T, Kumamaru E, Yagasaki Y, Ishimoto T, Mori T, Nemoto K, Adachi N, Izumi A, et al. Impact of the DISC1 Ser704Cys polymorphism on risk for major depression, brain morphology and ERK signaling. Hum Mol Genet. 2006;15:3024–3033. doi: 10.1093/hmg/ddl244. [DOI] [PubMed] [Google Scholar]
- Hennah W, Tomppo L, Hiekkalinna T, Palo OM, Kilpinen H, Ekelund J, Tuulio-Henriksson A, Silander K, Partonen T, Paunio T, et al. Families with the risk allele of DISC1 reveal a link between schizophrenia and another component of the same molecular pathway, NDE1. Hum Mol Genet. 2007;16:453–462. doi: 10.1093/hmg/ddl462. [DOI] [PubMed] [Google Scholar]
- Ishizuka K, Paek M, Kamiya A, Sawa A. A review of Disrupted-In-Schizophrenia-1 (DISC1): neurodevelopment, cognition, and mental conditions. Biol Psychiatry. 2006;59:1189–1197. doi: 10.1016/j.biopsych.2006.03.065. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Kubo K, Tomoda T, Takaki M, Youn R, Ozeki Y, Sawamura N, Park U, Kudo C, Okawa M, et al. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat Cell Biol. 2005;7:1167–1178. doi: 10.1038/ncb1328. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Tomoda T, Chang J, Takaki M, Zhan C, Morita M, Cascio MB, Elashvili S, Koizumi H, Takanezawa Y, et al. DISC1-NDEL1/NUDEL protein interaction, an essential component for neurite outgrowth, is modulated by genetic variations of DISC1. Hum Mol Genet. 2006;15:3313–3323. doi: 10.1093/hmg/ddl407. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. New nerve cells for the adult brain. Sci Am. 1999;280:48–53. doi: 10.1038/scientificamerican0599-48. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Xu L, Cascella N, Ozeki Y, Sawa A, Roberts RC. DISC1 immunoreactivity at the light and ultrastructural level in the human neocortex. J Comp Neurol. 2006;497:436–450. doi: 10.1002/cne.21007. [DOI] [PubMed] [Google Scholar]
- Koike H, Arguello PA, Kvajo M, Karayiorgou M, Gogos JA. Disc1 is mutated in the 129S6/SvEv strain and modulates working memory in mice. Proc Natl Acad Sci U S A. 2006;103:3693–3697. doi: 10.1073/pnas.0511189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer M, Beckmann H, Senitz D. Increased frequency of dentate granule cells with basal dendrites in the hippocampal formation of schizophrenics. Psychiatry Res. 2003;122:89–97. doi: 10.1016/s0925-4927(02)00122-1. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Liu XB, Jones EG. Fine structural localization of connexin-36 immunoreactivity in mouse cerebral cortex and thalamus. J Comp Neurol. 2003;466:457–467. doi: 10.1002/cne.10901. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- Mackie S, Millar JK, Porteous DJ. Role of DISC1 in neural development and schizophrenia. Curr Opin Neurobiol. 2007;17:95–102. doi: 10.1016/j.conb.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Millar JK, Christie S, Porteous DJ. Yeast two-hybrid screens implicate DISC1 in brain development and function. Biochem Biophys Res Commun. 2003;311:1019–1025. doi: 10.1016/j.bbrc.2003.10.101. [DOI] [PubMed] [Google Scholar]
- Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, Devon RS, Clair DM, Muir WJ, Blackwood DH, Porteous DJ. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9:1415–1423. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Honda A, Baba K, Taniguchi M, Oono K, Fujita T, Kuroda S, Katayama T, Tohyama M. Disrupted-In-Schizophrenia 1, a candidate gene for schizophrenia, participates in neurite outgrowth. Mol Psychiatry. 2003;8:685–694. doi: 10.1038/sj.mp.4001352. [DOI] [PubMed] [Google Scholar]
- Morris JA, Kandpal G, Ma L, Austin CP. DISC1 (Disrupted-In-Schizophrenia 1) is a centrosome-associated protein that interacts with MAP1A, MIPT3, ATF4/5 and NUDEL: regulation and loss of interaction with mutation. Hum Mol Genet. 2003;12:1591–1608. doi: 10.1093/hmg/ddg162. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Shu T, Sanada K, Lariviere RC, Tseng HC, Park SK, Julien JP, Tsai LH. A NUDEL-dependent mechanism of neurofilament assembly regulates the integrity of CNS neurons. Nat Cell Biol. 2004;6:595–608. doi: 10.1038/ncb1139. [DOI] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Bensen AL, Westbrook GL. Delayed development of adult-generated granule cells in dentate gyrus. J Neurosci. 2006a;26:2326–2334. doi: 10.1523/JNEUROSCI.4111-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Bromberg DA, Bensen AL, Westbrook GL. Seizures accelerate functional integration of adult-generated granule cells. J Neurosci. 2006b;26:4095–4103. doi: 10.1523/JNEUROSCI.5508-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozeki Y, Tomoda T, Kleiderlein J, Kamiya A, Bord L, Fujii K, Okawa M, Yamada N, Hatten ME, Snyder SH, et al. Disrupted-in-Schizophrenia-1 (DISC-1): mutant truncation prevents binding to NudE-like (NUDEL) and inhibits neurite outgrowth. Proc Natl Acad Sci U S A. 2003;100:289–294. doi: 10.1073/pnas.0136913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasure SJ, Collins AE, Lowenstein DH. Unique expression patterns of cell fate molecules delineate sequential stages of dentate gyrus development. J Neurosci. 2000;20:6095–6105. doi: 10.1523/JNEUROSCI.20-16-06095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porteous DJ, Millar JK. Disrupted in schizophrenia 1: building brains and memories. Trends Mol Med. 2006;12:255–261. doi: 10.1016/j.molmed.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch KP. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Mori D, Toyo-oka K, Chen A, Garrett-Beal L, Muramatsu M, Miyagawa S, Hiraiwa N, Yoshiki A, Wynshaw-Boris A, Hirotsune S. Complete loss of Ndel1 results in neuronal migration defects and early embryonic lethality. Mol Cell Biol. 2005;25:7812–7827. doi: 10.1128/MCB.25.17.7812-7827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Hen R. Neuroscience. Is more neurogenesis always better? Science. 2007;315:336–338. doi: 10.1126/science.1138711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Senitz D, Beckmann H. Granule cells of the dentate gyrus with basal and recurrent dendrites in schizophrenic patients and controls. A comparative Golgi study. J Neural Transm. 2003;110:317–326. doi: 10.1007/s00702-002-0776-6. [DOI] [PubMed] [Google Scholar]
- Seress L, Pokorny J. Structure of the granular layer of the rat dentate gyrus. A light microscopic and Golgi study. J Anat. 1981;133:181–195. [PMC free article] [PubMed] [Google Scholar]
- Shapiro LA, Ribak CE. Newly born dentate granule neurons after pilocarpine-induced epilepsy have hilar basal dendrites with immature synapses. Epilepsy Res. 2006;69:53–66. doi: 10.1016/j.eplepsyres.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Shinoda T, Taya S, Tsuboi D, Hikita T, Matsuzawa R, Kuroda S, Iwamatsu A, Kaibuchi K. DISC1 regulates neurotrophin-induced axon elongation via interaction with Grb2. J Neurosci. 2007;27:4–14. doi: 10.1523/JNEUROSCI.3825-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu T, Ayala R, Nguyen MD, Xie Z, Gleeson JG, Tsai LH. Ndel1 operates in a common pathway with LIS1 and cytoplasmic dynein to regulate cortical neuronal positioning. Neuron. 2004;44:263–277. doi: 10.1016/j.neuron.2004.09.030. [DOI] [PubMed] [Google Scholar]
- Taya S, Shinoda T, Tsuboi D, Asaki J, Nagai K, Hikita T, Kuroda S, Kuroda K, Shimizu M, Hirotsune S, et al. DISC1 regulates the transport of the NUDEL/LIS1/14−3−3epsilon complex through kinesin-1. J Neurosci. 2007;27:15–26. doi: 10.1523/JNEUROSCI.3826-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr., Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghbi HY. Postnatal neurodevelopmental disorders: meeting at the synapse? Science. 2003;302:826–830. doi: 10.1126/science.1089071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data
Supplementary Data includes 8 figures and Supplementary Experimental Procedures.