Abstract
Cats were instrumentally conditioned to generate grouped fast (20- to 50-Hz) oscillations in motor cortex (area 4). Over seven experimental sessions, there was a spatially selective increased generation of grouped fast oscillations in that electroencephalogram lead. This locally increased generation of fast oscillations in cortex was associated with a widespread increase in synchrony of fast oscillations in thalamocortical networks, as demonstrated by cross-correlations between intracortical, corticothalamic, and intrathalamic field potentials. A three-session extinction period abolished the local increase in generation of grouped fast oscillations and reset the thalamocortical synchrony of fast oscillations to control values. A subsequent series of seven sessions with instrumental conditioning of fast oscillations in visual cortex (area 17) reproduced the results from area 4, with a spatially selective increased generation of grouped fast oscillations in the criterion lead, associated with a widespread increase in thalamocortical synchrony of fast oscillations. In addition to their presence during the conditioning sessions, the changes in synchrony of fast oscillations were expressed during periods of quiet waking, rapid-eye-movement sleep, and nonrapid-eye-movement sleep recorded during the first hour after the end of the conditioning.
Fast spontaneous oscillations (mainly 20–50 Hz) are present in neocortical and thalamic neurons during wake and sleep states (1, 2). Fast oscillations have been shown to be evoked by optimal sensory stimuli (3–5), but they are also part of the background electrical activity, due to the depolarizing actions exerted by generalized modulatory systems on thalamic and cortical neurons (1, 2, 6). Synchronization of fast oscillations has been shown by magnetoencephalography (7, 8) and electroencephalography combined with multisite, extracellular, and intracellular recordings from reciprocally connected cortical and thalamic regions (1, 2). In the visual system, synchronized fast oscillations have been hypothesized to underlie the perceptual unity of spatially distributed visual activity (5, 9). It has been proposed that the 40-Hz waves in the human brain are organized in a coherent rostrocaudal wave, having a phase shift that appears to scan large portions of the brain, and that this mechanism may be the basis for global binding (8).
In this paper we report the results from experiments designed to study the generation and synchronization of fast oscillations in thalamocortical networks of cats performing a behavioral task. We also report on the vigilance specific expression of synchronization.
METHODS
Cats to be conditioned (n = 2) were chronically implanted under ketamine (15 mg/kg, i.m.) followed by barbiturate anesthesia (Somnotol, 35 mg/kg i.p.). Bipolar coaxial electrodes were inserted into neocortical areas 4 (motor), 17 (primary visual), and 5 and 7 (association), and in thalamic intralaminar centrolateral (CL) and lateral geniculate nuclei. A hole in the calvarium above the left suprasylvian gyrus (areas 5 and 7), which was sealed between recording sessions, allowed the placement of tungsten microelectrodes (impedance of 1–5 MΩ) for unit recordings. The electromyogram from neck muscles and electrooculogram also were recorded to assess the behavioral state of the animal. After surgery the animals were allowed to recover for 2 weeks. During training and recording sessions, the heads of the cats were kept rigid without pain or pressure as previously described (10). The signals were bandpass-filtered (0–9 kHz), digitized at 20 kHz, and stored on tape for off-line computer analysis.
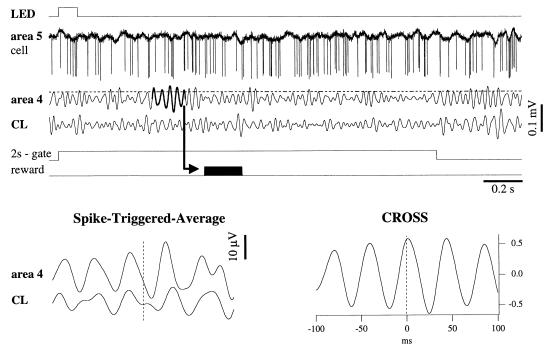
The experimental paradigm is described in Fig. 1. We trained the animals to generate groups of fast oscillations (hereafter termed bursts) by instrumental conditioning. An electronic device produced the time sequence of signals and automatically detected the bursts of fast oscillations. At the input of this device we connected the electroencephalogram (EEG) lead that served as criterion lead (area 4 in Fig. 1). The device filtered the EEG signal between 20 and 50 Hz (5th-order Chebyshev filters, 100 decibels per decade attenuation) and presented a visual stimulus every 10 s through a light-emitting diode (LED). After the LED stimulus, there was a 2-s window during which a qualifying burst would be rewarded. The qualifying burst would consist of at least five consecutive cycles with an amplitude higher than a threshold set before the first conditioning session, at the average peak amplitude of the filtered criterion EEG lead. When a qualifying burst was detected, the reward, a jet of water, was delivered into the oral cavity of the animal 100 ms later. The reward delivery was contingent upon the absence of an increase in electromyogram activity. The burst-detection procedure was blocked during the reward delivery (200 ms). To increase the incentive to learn, the cat had no access to water for the 12 h preceding the training session. The training of each animal consisted of seven sessions of motor cortex (MC) conditioning, three sessions of extinction, and seven sessions of visual cortex (VC) conditioning. During each experimental session the cat received at least 200 visual stimuli. There were no pauses during the experimental session.
Figure 1.
The procedure used for instrumental conditioning. A light flash (LED) of 100-ms duration was delivered in the visual field of the cat. The onset of the conditioned stimulus also triggered a 2-s gate during which a qualifying response was expected in the EEG lead designated for conditioning (in this case, motor cortical area 4, filtered between 20 and 50 Hz). This conditioned response had to be a burst of fast oscillations made of at least five cycles with an amplitude crossing a threshold (horizontal broken line). The appearance of such a burst would command the reward to be delivered (arrow and black impulse). Simultaneously with the criterion EEG lead, fast activities from other cortical and thalamic sites were recorded (in this case, a cell in association cortical area 5 and field potentials from the centrolateral CL nucleus of the thalamus). Spike-triggered averages (STA, Lower Left) were computed to disclose relations between spike discharges in area 5 (vertical broken line) and field potentials in motor cortex and thalamic CL nucleus. STA were calculated by extracting, from each filtered EEG lead, sweeps of ± 100 ms around the time of a spike, and averaging them. We applied the STA to spikes occurring in a window of 2 s. Note the similar shapes and oscillating frequencies (about 25 Hz) of the two leads. The synchrony between field potentials was assessed by cross-correlating 500-ms windows (CROSS, here between area 4 and CL).
The extinction sessions were identical to the conditioning sessions, except a reward was not delivered. At the beginning, animals displayed signs of impatience, but this behavioral feature diminished with time. After three sessions of extinction, seven sessions of conditioning for the visual cortical lead (area 17) took place.
Whenever a cortical cell was recorded during the conditioning procedure (n = 115), we looked for the relation between its firing and field potential oscillations recorded from the cortex and thalamus. To assess the synchrony between cell and field potentials we computed spike-triggered averages (STA; Fig. 1, Lower Left) that provide evidence of relations between the firing of a neuron (at time zero) and the shape of a field potential.
Spectral analysis was used to quantify the fast oscillations. Two-second windows of EEG were analyzed with a fast Fourier transform procedure, and total power density in the 20- to 50-Hz range was calculated. The influence of movements during recording sessions was determined by performing cross-correlations between active EEG leads and electromyogram. In none of the circumstances presented in this article did these correlations indicate a contribution to the EEG activity greater than 5%.
RESULTS
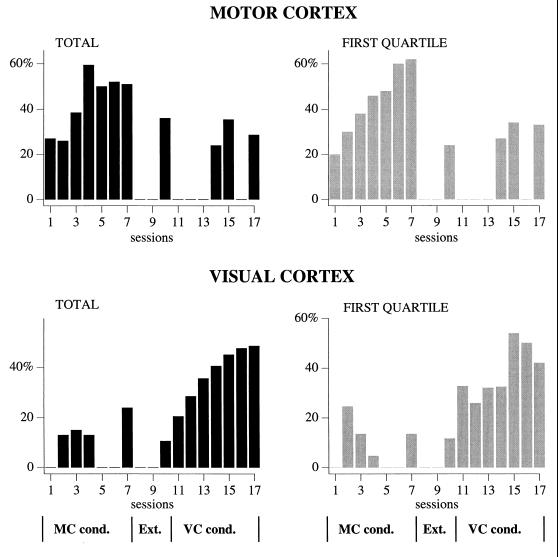
During the course of conditioning, there was an increase in the number of LED flashes followed by qualifying bursts (successful bursts) in the criterion lead, as shown in Fig. 2. Note that the increased numbers of qualifying bursts were spatially selective for the chosen criterion lead. Indeed, there was no such increase in area 17 during sessions 1–7, or in area 4 during sessions 11–17. The extinction sessions reduced the number of bursts in area 4 back to control levels. As can be seen in Fig. 2 Right, in the first quartile of stimulations there was a virtually linear increase in the percentage of qualifying bursts from session 1 to 4. We presume that this was due to the animals being more thirsty and attentive in the first part of the sessions. In the VC conditioning sessions, there was less variation in the course of each session. Statistical testing of these data substantiated that the observed differences were not due to chance, but reflected learning. The experimental sessions differed significantly (Friedman’s nonparametric ANOVA, sessions 1–7: P < 0.0001, sessions 11–17: P < 0.0001). When all sessions within each conditioning series were compared, session 1 and 2 differed from session 4–7 (P < 0.05), and sessions 11 and 12 differed from sessions 14–17 (P < 0.05, all significance values corrected for multiple comparisons).
Figure 2.
Learning performance of one of the cats as disclosed by the percentages of conditioning stimuli followed by qualifying bursts of fast (20- to 50-Hz) activities out of a total of 200 stimulus presentations (Left), and out of the first 50 stimuli presented in a training session (Right). (Upper) Results from the motor (area 4) cortical lead. (Lower) Results from the same 17 sessions, but recorded in the visual (area 17) cortex. The evolution of the training was the following: During the first seven sessions, the animal was conditioned to elicit fast oscillatory bursts in the motor cortex (MC cond.), during the next three sessions (8–10) extinction occurred (no reward in spite of the presence of qualifying bursts; Ext.), and finally, during sessions 11–17 the visual cortex was the criterion lead (VC cond.).
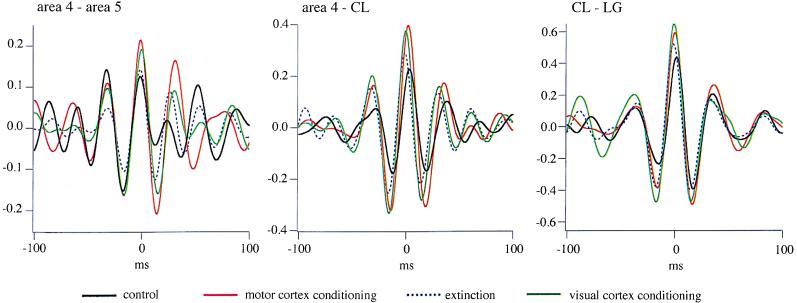
As animals learned to produce bursts of fast rhythms to be rewarded, the synchrony of fast oscillations increased. To demonstrate this, we computed cross-correlations between field potentials from various recorded leads. The cross-correlations depicted in Fig. 3 are averages of 20 cross-correlations derived from individual 2-s windows after the LED stimulus. In all presented cases, intracortical, corticothalamic, and intrathalamic synchrony increased from the control situation (black trace) to the last session of MC conditioning (red trace), returned to control values after extinction (dotted blue trace), and increased again after VC conditioning (green trace). This phenomenon occurred also between sites that apparently are not related to the conditioned one (e.g., area 4–CL during VC conditioning). The increased synchrony from control condition was continuous from one session to the other and the amount of increase ranged from 35% to 70% in the last of the seven conditioning sessions.
Figure 3.
Evolution of intracortical (Left), corticothalamic (Center), and intrathalamic (Right) synchrony before conditioning (black trace), during motor cortex conditioning (red trace), at the end of extinction (dotted blue trace), and after visual cortex conditioning (green trace), as disclosed by averaged (n = 20) cross-correlations between filtered (20- to 50-Hz) field potentials taken from the 2-s period after the LED stimulus. Note increased synchrony induced by the conditioning procedures (red and green traces) as compared with control (black trace), and recovery of control values after extinction.
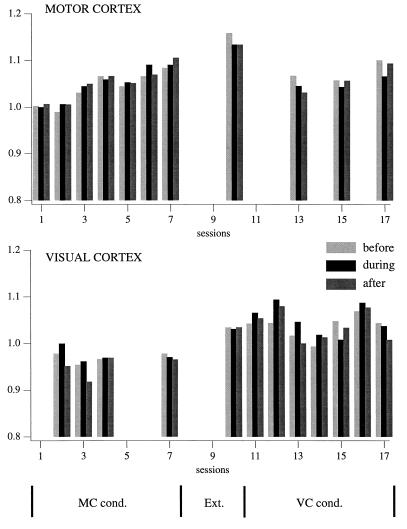
The increased generation of burst of fast oscillation and the increased synchrony could be due to a diffuse activation of the network. To assess the level of activation, we calculated the power spectral density of fast oscillations in the EEG leads used for conditioning (Fig. 4). For each session we calculated the average EEG power density in the fast oscillation range during three time periods, the 2-s period before LED stimulation (before, light gray bar), the 2-s period during which a qualifying burst would be rewarded (during, black bar), and the 2-s period after that (after, dark gray bar). For each session, the results from all 200 presentations of the conditioning stimulus were averaged, and the average of power density was expressed as a percentage of average power density in the during period of the first session of that EEG lead.
Figure 4.
Evolution of the power spectrum of motor and visual cortex EEGs, used alternatively as criterion leads. Each bar represents the averaged fast Fourier transform area between 20 and 50 Hz from a 2-s window extracted from each of the 200 trials of a conditioning session. The 2-s windows were chosen as follows: light gray (before), before the onset of the LED flash; black (during), immediately after LED onset; and dark gray (after), from the next 2 s. The calculated values were normalized by dividing them with the during-value of the first day. Note local increase (<10%) of the activation in motor and visual cortex during their respective conditioning procedures. The motor cortex reaches its maximum activation during the extinction period (session 10).
In area 4, there was an increase in power density of the fast oscillation parallel to the increase in generation of qualifying bursts. The magnitude of the increase was different, however, with a maximal change of 10% in power density, compared with a 140% increase in number of qualifying bursts. Another difference was that the activation reached the peak value in the last extinction session (session 10), when both the generation of qualifying bursts and the synchrony of fast oscillations had returned to control values. In area 17 there was no clear parallel relationship.
The data suggest that there was a spatially selective increase in activation during the course of the conditioning session, as assessed by the power density in the fast oscillations range. However, there was no simple causal relationship between the level of activation and the generation of qualifying bursts or the synchrony of fast oscillations.
The spike-triggered averages did not reveal changes in relationship between cellular firing and field potentials as a function of conditioning (data not shown). We did not observe phase-locked fast oscillatory responses to the LED stimulus in any of the cortical or thalamic regions recorded.
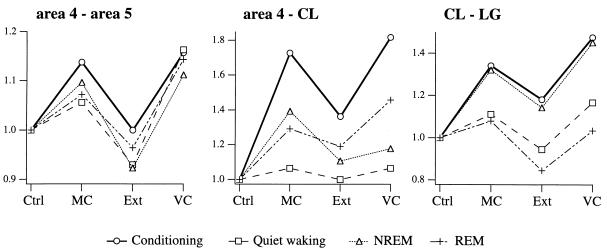
The cats also were recorded for 1 h after the end of the conditioning session. Analyzing the quiet waking from this period, as well as the NREM and REM sleep epochs, allowed us to study the expression of the conditioning-induced changes in corticothalamic synchrony during these wake-sleep states (Fig. 5). The data suggest that the changes in thalamocortical synchrony of fast oscillations during the behavioral task also are expressed during the behavioral states of vigilance, including NREM and REM sleep.
Figure 5.
Evolution of the cross-correlation peak for intracortical (Left), thalamocortical (Center), and intrathalamic (Right) synchrony as a function of behavioral state (conditioning task, thick line and ○; quiet waking, dashed line and □; NREM sleep, dotted line and ▵; and REM sleep: dash-dot-dot line and +) and learning (Ctrl, session 1; MC, from the end of MC conditioning; Ext, from the end of extinction; VC, from the end of VC conditioning). The field potentials for the conditioning task were taken from the 2-s period before the LED stimulus. Data are expressed as ratios to control values. Note similar direction of changes in all behavioral states from one condition to the next.
DISCUSSION
The three major findings of this study are: i) our experimental paradigm was successful in conditioning an increase in generation of bursts of fast oscillations; ii) the increased generation of these conditioned bursts was associated with an enhanced synchrony of fast oscillations at different levels of the thalamocortical network; and iii) this increased thalamocortical synchrony acquired during the behavioral task also is expressed during subsequent quiet waking, NREM sleep, and REM sleep. Thus, the conditioning-reinforcement paradigm generates an animal model for the study of fast oscillations. Previous studies reported the induction of fast oscillations in behaving monkeys (11, 12) and in anesthetized cats (4) in areas functionally linked with the task or the stimulation. Our preparation produced 20- to 50-Hz bursts in a cortical area (motor area 4) that was not directly linked with the conditioned stimulus (visual) and in the absence of overt movements.
The intentional generation of fast oscillatory bursts was associated with an increased synchrony within corticothalamic networks. The motor area 4 is reciprocally linked with the association area 5 of the suprasylvian gyrus (13) and with the thalamic CL nucleus (14). It has been shown that the corticothalamic coherence in the range of fast oscillations requires reciprocal links between the recorded thalamic and cortical sites (2). The present data demonstrate an increased synchrony between specific (lateral geniculate) and nonspecific (CL) thalamic nuclei during a behavioral task, thus supporting the prediction in the model of Llinás et al. (15). Because there are no known direct projections between these nuclei (14), one has to rely on interposed cortical links or on a common input. The intermediate cortical link could be located in the VC, because CL neurons project to VC (16), and coherent fast activities were demonstrated between the lateral geniculate nucleus of the thalamus and association VC (2). Because the synchronization of fast activities is drastically reduced with distance (1), it is more likely that a common input to several thalamic nuclei would potentiate and synchronize 40-Hz activities. This common drive could arise in the cholinergic nuclei of the brainstem that project to virtually all dorsal thalamic nuclei (17, 18), in the reticular nucleus of the thalamus (19), or in the cortex itself. The local increase in generation of bursts of fast oscillations and in power density in the fast oscillation range further suggests that regional, cortical, and/or thalamic mechanisms are active. Glutamate, acting through various receptor subtypes, could be responsible for local changes in neuronal membrane potential, facilitating the occurrence of fast oscillations in thalamic and cortical networks (20–22). On a more global level, monoaminergic and cholinergic mechanisms (23) are known to shift the membrane potential into ranges that facilitate fast oscillations (1).
The generation of bursts of fast oscillations was not phase-locked to the conditioned stimulus (LED flash) and was not confined to the 2-s window where reward was available. The increase in synchrony was global and not phase-locked with the LED. The EEG power spectrum showed a progressive increase of about 10% through the conditioning sessions and reached its maximum during the extinction procedure (Fig. 4), whereas the synchrony and conditioned performance returned to control levels. All these results suggest that a diffuse process was developing underneath and simultaneously with learning. It is known that the sustained activity of ascending activating structures is responsible for the induction and maintenance of wakefulness (24). At the cellular level, brain-activated states are associated with tonic activity and more depolarized membrane potentials at which cortical and thalamic neurons tend to oscillate in the fast frequency range (1, 2, 6, 19, 25). At least in some recorded cells, the frequency of fast oscillations is directly dependent on the amount of depolarization (19, 25). It therefore could be inferred that a more activated corticothalamic network will more readily produce bursts of fast oscillations. However, the spatial specificity of the conditioned response, together with its extinction and simultaneous drop in synchrony (Figs. 2 and 3), suggests that the learning involved more than a diffuse increase in activation.
The increased synchrony of fast oscillations in corticothalamic systems also is expressed outside the behavioral task (Fig. 5), despite widely differing conditions in the thalamocortical network (quiet waking, NREM sleep, and REM sleep). Data from hippocampus (26, 27) show that cells firing together during behavioral tasks exhibit an increased tendency to fire together during subsequent sleep, in comparison to sleep episodes preceding the behavioral tasks. The reexpression of information acquired during active behavior during sleep has been postulated to be related to memory consolidation (27, 28).
Acknowledgments
We thank P. Giguère for technical assistance. F.A. and D.N. are postdoctoral fellows in this laboratory. The research was supported by the National Sciences and Engineering Research Council of Canada, the Medical Research Council of Canada, Fonds de Recherche en Santé du Québec, Human Frontier Research Program, and the Norwegian Research Council.
ABBREVIATIONS
- CL
centrolateral thalamic nucleus
- EEG
electroencephalogram
- LED
light-emitting diode
- MC
motor cortex
- VC
visual cortex
- REM
rapid eye movement
- NREM
nonrapid eye movement
References
- 1.Steriade M, Amzica F, Contreras D. J Neurosci. 1996;16:392–417. doi: 10.1523/JNEUROSCI.16-01-00392.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steriade M, Contreras D, Amzica F, Timofeev I. J Neurosci. 1996;16:2788–2808. doi: 10.1523/JNEUROSCI.16-08-02788.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, Reitboeck H J. Biol Cybern. 1988;60:121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- 4.Gray C M, Singer W. Proc Natl Acad Sci USA. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer W. Ann Rev Physiol. 1993;55:349–374. doi: 10.1146/annurev.ph.55.030193.002025. [DOI] [PubMed] [Google Scholar]
- 6.Steriade M, Curró Dossi R, Paré D, Oakson G. Proc Natl Acad Sci USA. 1991;88:4396–4400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribary U, Ioannides A A, Singh K D, Hasson R, Bolton J P R, Lado F, Mogilner A, Llinás R. Proc Natl Acad Sci USA. 1991;88:11037–11041. doi: 10.1073/pnas.88.24.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llinás R, Ribary U. Proc Natl Acad Sci USA. 1993;90:2078–2081. doi: 10.1073/pnas.90.5.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von der Malsburg C, Schneider W. Biol Cybern. 1986;54:29–40. doi: 10.1007/BF00337113. [DOI] [PubMed] [Google Scholar]
- 10.Steriade M, Glenn L L. J Neurophysiol. 1982;48:352–371. doi: 10.1152/jn.1982.48.2.352. [DOI] [PubMed] [Google Scholar]
- 11.Murthy V N, Fetz E E. Proc Natl Acad Sci USA. 1992;89:5670–5674. doi: 10.1073/pnas.89.12.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman W J, Van Dijk B W. Brain Res. 1987;422:267–276. doi: 10.1016/0006-8993(87)90933-4. [DOI] [PubMed] [Google Scholar]
- 13.Avendaño C, Rausell E, Perez-Aguilar D, Isorna S. J Comp Neurol. 1988;278:1–33. doi: 10.1002/cne.902780102. [DOI] [PubMed] [Google Scholar]
- 14.Jones E G. The Thalamus. New York: Plenum; 1985. [Google Scholar]
- 15.Llinás RR, Ribary U, Joliot M, Wang X-J. In: Temporal Coding in the Brain. Buzsáki G, Llinás R, Singer W, Berthoz A, Christen Y, editors. New York: Springer; 1994. pp. 251–272. [Google Scholar]
- 16.Cunningham E T, LeVay S. J Comp Neurol. 1986;254:65–77. doi: 10.1002/cne.902540106. [DOI] [PubMed] [Google Scholar]
- 17.Steriade M, Paré D, Parent A, Smith Y. Neuroscience. 1988;25:47–67. doi: 10.1016/0306-4522(88)90006-1. [DOI] [PubMed] [Google Scholar]
- 18.Paré D, Smith Y, Parent A, Steriade M. Neuroscience. 1988;25:69–86. doi: 10.1016/0306-4522(88)90007-3. [DOI] [PubMed] [Google Scholar]
- 19.Llinás R R, Grace A A, Yarom Y. Proc Natl Acad Sci USA. 1991;88:897–901. doi: 10.1073/pnas.88.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCormick D A, von Krosigk M. Proc Natl Acad Sci USA. 1992;89:2774–2778. doi: 10.1073/pnas.89.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCormick D A, Wang Z, Huguenard J R. Cereb Cortex. 1993;3:387–398. doi: 10.1093/cercor/3.5.387. [DOI] [PubMed] [Google Scholar]
- 22.Whittington M A, Traub R D, Jefferys J G. Nature (London) 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- 23.McCormick D A. Prog Neurobiol. 1992;39:337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- 24.Steriade M, McCarley R W. Brainstem Control of Wakefulness and Sleep. New York: Plenum; 1990. [Google Scholar]
- 25.Nuñez A, Amzica F, Steriade M. Neuroscience. 1992;51:7–10. doi: 10.1016/0306-4522(92)90464-d. [DOI] [PubMed] [Google Scholar]
- 26.Pavlides C, Winson J. J Neurosci. 1989;9:2907–2918. doi: 10.1523/JNEUROSCI.09-08-02907.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson M A, McNaughton B L. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 28.Buzsáki G. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]