Abstract
To assess the role of tyrosine phosphorylation/dephosphorylation balance in synaptic transmission, a set of studies was implemented at the squid giant synapse. Presynaptic induction of tyrosine phosphorylation, following administration of the tyrosine phosphatase inhibitor pervanadate, produced a sizable increase in presynaptic calcium current and a concomitant and paradoxical decrement of the postsynaptic potential amplitude. Presynaptic microinjection of an active protein tyrosine kinase dramatically increased calcium currents and incremented postsynaptic potential amplitude. By contrast, the same procedure at the postsynaptic terminal reduced the size of the postsynaptic potential. This differential effect may be prodromic to long-term plasticity, as postsynaptic sensitivity is momentarily deemphasized, whereas presynaptic second messenger cascades triggered by increased calcium currents are accentuated.
Keywords: calcium current, phosphorylation, synaptic transmission, protein tyrosine phosphatases, protein tyrosine kinase
Protein phosphorylation on tyrosine residues has been shown to modulate proliferation, differentiation, and mobility in eukaryotic cellular elements (1). Protein tyrosine kinases (PTKs) are also involved in secretory events in chromaffin cells, channel phosphorylation, and long-term facilitation and depression in central synapses (2–6). Here we address the possibility that such a phosphorylation/dephosphorylation ratio may play a role in synaptic release and/or on postsynaptic ligand sensitivity at the squid giant synapse. As tyrosine phosphorylation is regulated by the balance between tyrosine kinase(s) and protein tyrosine phosphatases (PTPases) alterations of their equilibrium, if present in the squid giant synapse, should result in a modification of some aspect of synaptic transmission. This balance was altered by using pervanadate, a membrane-permeant compound known to inhibit at submillimolar concentration PTPases, and by microinjection of an active protein tyrosine kinase (1, 7), thereby affecting tyrosine phosphorylation in the absence of other agonist(s).
MATERIALS AND METHODS
Electrophysiology.
Experiments were implemented at the Marine Biological Laboratory (Woods Hole, MA). The isolation of the stellate ganglion from squid (Loligo peallei) and the electrophysiological techniques used have been described (8, 9). The ganglion was perfused with artificial sea water buffered by Tris (423 mM NaCl/8.3 mM kCi/10 mM CaCl2/50 mM MgCl2/2 mM Tris, pH 7.2/0.001% H2O2) and maintained at room temperature (20°C). Two electrodes were used in the presynaptic terminal, one for pressure microinjection while the second monitored membrane potential. The pressure injection electrode also served as the current injection probe in the voltage clamp experiments (10). Microinjection was imaged using a fluorescent dye (0.001% dextran fluorescein). Sodium and potassium current were blocked by superfusion of 1 μM tetrodotoxin and 5 mM 3- or 4-aminopyridine, respectively, during voltage clamp experiments. Voltage clamped steps were delivered from a holding potential of −70 mV. The database consists of 25 experiments performed in 18 reparations.
Pharmacological Tools.
The tyrosine phosphatase inhibitor pervanadate (7) was used in nine experiments (in two injected presynaptically). It was prepared by mixing one part of 50 mM H2O2 with five parts of 10 mM sodium orthovanadate in artificial sea water (see above) and then incubated at room temperature for 10 min. The stock was diluted to a final concentration of 0.1 mM vanadate, 0.1 mM H2O2, and 500 units/ml catalase (Boehringer Mannheim). Genistein, a selective tyrosine phosphorylation inhibitor at the concentration indicated, was used in five experiments and was prepared in 5000× stock in dimethyl sulfoxide (from Research Biochemicals, Natick, MA). The PTK domain of fibroblast growth factor FGFR1K was baculovirus expressed via insect cells. FGFR1K was purified by affinity chromatography (10) as used in determining its x-ray crystallographic structure (11), kept at 0°C, and diluted to 5 μg/μl with regular intracellular solution (500 mM K acetate/100 mM Hepes, pH 7.2) before injection. Eleven FGFR1K experiments were performed.
RESULTS
Modulation of Presynaptic Calcium Currents (ICa) and Postsynaptic Potentials (PSPs) by the Tyrosine Phosphatase Inhibitor Pervanadate.
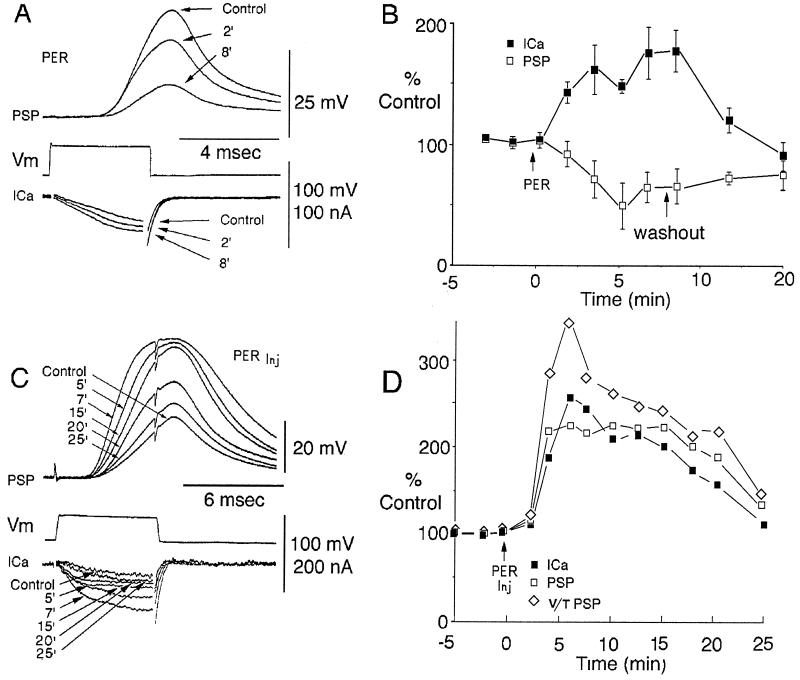
ICa were determined using clamped presynaptic voltage steps (8, 9, 12) that triggered postsynaptic responses in the absence of action potentials. Superfusion with micromolar doses of pervanadate produced a progressive increase in presynaptic ICa to 67% ± 17% of control (maximum effect), which was accompanied by a paradoxical and simultaneous decrease of PSP amplitude to 59.2 ± 16% (maximum effect) (Fig. 1 A and B). Following pervanadate wash out, both presynaptic ICa and PSP gradually returned to their control levels, indicating reversibility of the modulatory effect (Fig. 1B). To define more precisely its action site(s), presynaptic microinjections of pervanadate were implemented. These microinjections produced a similar increase of ICa as observed with superfusion; however, in this case PSP amplitude also increased (Fig. 1 C and D). Note that the amplitude of the PSP saturates above 50 mV, for which reason the normalized rate of rise of the PSP was also plotted against time in Fig. 1D. This dual increase returned to control levels within 20 min after the injection (Fig. 1B), confirming that the pervanadate effect is reversible as it is rapidly lost across the plasmalemma and diffuses intraaxonally away from the presynaptic active zone.
Figure 1.
Pervanadate effect on synaptic transmission. (A) Simultaneous recording of presynaptic ICa and of PSP evoked by a depolarizing presynaptic voltage clamp step (Vm). Before (control) and after pervanadate (PER) superfusion at 100 μM ICa increased and, simultaneously, PSP amplitude decreased. (B) Normalized time course of extracellular pervanadate (100 μM; arrow Per) effect on PSP (□) and ICa (▪) after 8 min the pervanadate was washed out (washout) in three different experiments (bars represent SE). (C) Pervanadate presynaptic microinjection (PER inj) using the same protocol as in A. Note that during the first 7 min pervanadate produced an increase of ICa and PSP amplitude and of the rate of rise (voltage/time), followed by a return toward control levels in 25 min. (D) Normalized time course of intracellular pervanadate effect on ICa (▪), PSP (□), and rate of rise [voltage/time (⋄)].
Presynaptic ICa and Postsynaptic Responses Are Modulated by the PTK Domain of Fibroblast Growth Factor Receptor 1 (FGFR1) and Reversibly Blocked by Genistein.
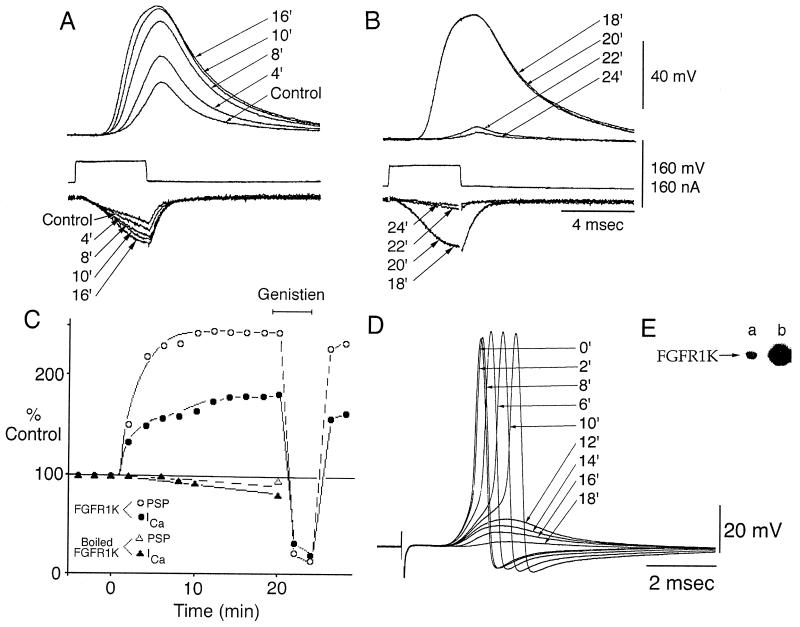
As PTPase inhibition modulated ICa and PSP, a pure PTK domain of FGFR1 endowed with intrinsic PTK activity FGFR1K (10) (Fig. 2E) was used to determine whether PTK also modulates ICa and PSP amplitude. FGFR1, one of a large family of membrane associated enzymes when activated by ligand (FGF) binding to its extracellular domain, induces receptor oligomerization, autophosphorylation and phosphorylation, and activation of intracellular target proteins (10, 11, 13). Following presynaptic microinjection of FGFR1K, a very clear potentiation of the ICa (to 83% of peak current), as well as of the PSP (to 140% of peak amplitude) was observed. A steady-state level was achieved within ≈20 min (Fig. 2 A and C) and in one case was followed for an additional 50 min after which transmission returned to control level. Similar results were obtained in three other synapses with an increase for ICa between 83% to 194% and of the PSP of 140% to 278%. Neither the ICa current nor PSP were potentiated with microinjection of heat-inactivated FGFR1K (Fig. 2C) or control solution.
Figure 2.
Presynaptic microinjection of FGFR1K. (A) Recordings of PSP and ICa (as in Fig. 1A) before (control) and after microinjection of FGFR1K. (B) Superfusion with genistein (10 μM) produced a significant reduction of both ICa and PSP. (C) Plot of two experiments showing the time course of FGFR1K presynaptic microinjection on ICa (○) and PSP (•) and their reversible block by genistein, as well as the lack of effect of a similar injection using heat-inactivated FGFR1K (▴). (D) PSP elicited by presynaptic stimulation, before (control) and its reduction after postsynaptic microinjection of FGFR1K. (E) (a) Coomasie blue-stained gel of purified FGFR1K. (b) FGFR1K was subjected to autophosphorylation and activation in the presence of [α-32P]ATP and Mg2+. Shown is the autophosphorylation of activated FGFR1K analyzed by SDS/PAGE and autoradiography.
Genistein, a PTK inhibitor, was used to demonstrate that the potentiation was due to the PTK activity of FGFR1K. Thus, as shown in Fig. 2 B and C, genistein blocked the FGFR1K-dependent increase of the pre- and postsynaptic responses. This genistein block of FGFR1K effect was repeated in four other synapses and in all cases was reversible within 3–5 min and in two synapses the experiment was repeated several times. Beyond reducing the FGFR1K, genistein reduced peak ICa to 40–75% of its initial value as did the PSP amplitude (Fig. 2 B and C).
Reduction of PSPs by PTK Activation.
Because pervanadate inhibition of PTPases modulates the PSP differently when superfused as opposed to when microinjected presynaptically, we tested whether PTK activation could modulate, directly, the postsynaptic response. Intracellular injection of the FGFR1K protein into the postsynaptic axon at the active zone produced a gradual inhibition of the PSP that declined with a time course comparable to that generated by pervanadate superfusion (Fig. 2D). This experiment was repeated in three synapses where similar results accrued. These observations indicate that tyrosine phosphorylation of postsynaptic proteins results in a decrement of synaptic transmission most likely due to inhibition of postsynaptic glutamate receptors.
Presynaptic Injection of FGFR1K Protein Produces Changes on Transmitter Depletion.
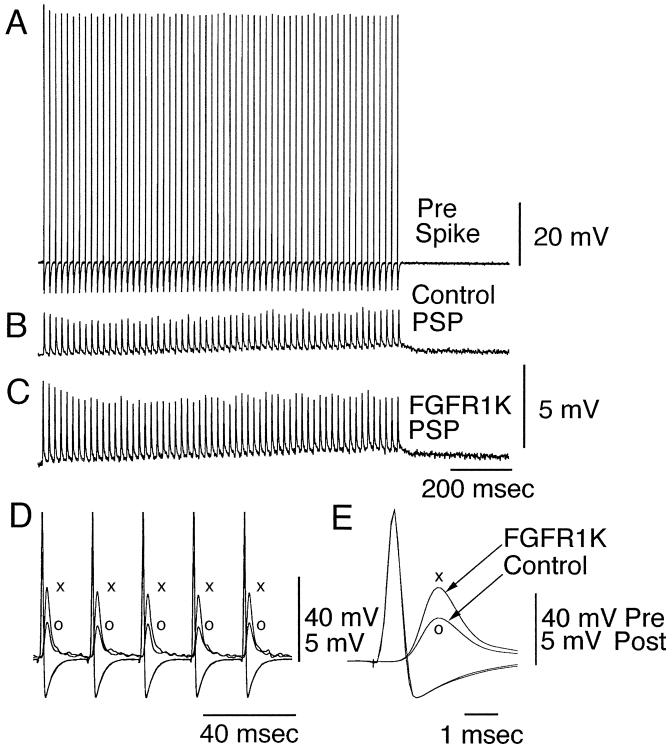
In the experiments described above synaptic transmission was studied by preterminal activation with a frequency of 2/min. Presynaptic high frequency stimulation (50 Hz, 60 stimuli every 2 min) reduces transmitter release over a period 15–30 min (14). In synapses injected with FGFR1K, after the postsynaptic response had reached a steady-state depression (15 min) (Fig. 3B), without modification of presynaptic spike properties (Fig. 3A) a clear increase in PSP amplitude was observed (Fig. 3 D and E). In the results illustrated in Fig. 3, the presynaptic microinjection of FGFR1K produced a close to 2-fold increase in the amplitude of the PSP (Fig. 3 C–E) without affecting the presynaptic spike (Fig. 3E). This potentiation was observed throughout the duration of the tetanic presynaptic stimulation (Fig. 3C), suggesting that tyrosine phosphorylation at presynaptic compartments may involve, in addition to calcium entry, other factors relating to neurotransmitter release and/or availability (14).
Figure 3.
Increased transmitter release by tetanic stimulation of a FGFR1K microinjected presynaptic terminal. Following protracted (15–30 min) tetanic stimulation of the presynaptic terminal (A), the amplitude of the PSP is substantially reduced (B). (C) After presynaptic injection of FGFR1K the amplitude of the presynaptic spike train is not modified, but the PSP demonstrates a doubling in amplitude. (D) Superimposed presynaptic potentials and PSP before (○) an after FGFR1K microinjection (×). Detailed amplitude and time course of the prespike and PSP can be observed in E.
DISCUSSION
Dynamic Modulation of Synaptic Function by Tyrosine Phosphorylation Levels.
The present set of experiments demonstrates that, in the squid giant synapse, tyrosine phosphorylation both enhanced presynaptic ICa and transmitter release and reduced PSP amplitude, thus providing evidence for a dynamic tyrosine phosphorylation/dephosphorylation cycle, allowing similar effects by either tyrosine kinase activation or tyrosine phosphatase inhibition.
In particular, while different calcium channel currents have been shown to be potentiated in many preparations (15–17), the level of ICa potentiation upon FGFR1K microinjection found in the present study is among the largest so far reported. As expected presynaptic ICa potentiation enhanced transmitter release but failed to increase transmission if tyrosine phosphorylation was induced postsynaptically, as with pervanadate superfusion.
Candidate Targets for Tyrosine Phosphorylation Events at the Squid Giant Synapse and Its Functional Consequences.
While present results demonstrate that PTK(s) is normally active in this synapse, the possible target(s) for the PTK at the pre- or postsynaptic axons are unknown. However, it is probable that the calcium channel, a multimeric protein, may be directly modulated by tyrosine phosphorylation at any of its subunits; in addition, similar phosphorylation could be occurring in any set of calcium channel modulating associated proteins. Likewise, postsynaptically, the most likely target is the ionotropic glutamate receptor and/or its associated proteins. Note, however, that the ICa in the squid presynaptic terminal corresponds (pharmacologically and electrophysiologically) to a P-type calcium channel (18, 19) for which no modulation by tyrosine phosphorylation has been reported so far.
Recently, a cDNA was cloned that encodes a putative molluscan peptide receptor (20) containing a conserved tyrosine kinase domain and a potential SH2 binding site, similar to those found in epidermal growth factor receptor. This finding opens the possibility that one type of these receptor(s), if localized at the pre- or postsynaptic terminal, could modulate synaptic transmission. This hypothesis is consistent with reports that peptide growth factors may not only be involved in growth and other long-term effects but also may modulate “faster physiological phenomena” as shown in mammalians and in invertebrates synapses (3, 17).
The importance of a tyrosine phosphorylation balance on the Mg2+ ATP stimulation of Na-Ca exchange was recently demonstrated in the squid axon (21). Furthermore, inhibition of tyrosine phosphatase(s) in other molluscan, such as the marine snail Bullagouldalina, blocks the eye circadian rhythm (22). Interestingly, circadian rhythms are influenced by neuropeptides such as FMRFamide (Phe-Met-Arg-Phe-NH2)(23) and some of the neuropeptide Y (NPY)-related peptides (24), which are important signaling molecules in the nervous system of vertebrates and invertebrates; for instance FMRFamide increases in flight and running responses in roaches (25). Members of the NPY-related peptides and FMRFamide have been identified in squid (26, 27), and FMRFamide has been shown to increase the neurotransmitter release and to reverse fatigue in squid synaptic transmission (27). The cloned NPY receptors showed the typical seven transmembrane domain structure, are coupled to Gi protein and elicited mitogen-activating protein kinase activation (28). It has also been established that in PC12 cells, Gi-coupled receptors can stimulate two PTKs (Pyk2 and Src) which results in the activation of mitogen-activating protein kinase (29).
In short, it is possible that in the squid tyrosine phosphorylation could be activated via a NPY-related peptide receptor or by tyrosine kinases. It is therefore feasible that the phosphorylation ratio for the target moieties of PTK may be peptide-modulated at many central nervous system sites across species. In particular, the fact that a newly described molluscan growth factor (17) can trigger a rapid increase in ICa further emphasizes the possibility that the differential effect of PTK may be prodromic to long-term plasticity, as calcium-triggered events such as the regulation of cyclic AMP-dependent kinases (30), which appear to be involved in activity-dependent neuronal reorganization.
Acknowledgments
We thank David H. Lau for his helpful comments on our manuscript. This work was supported by Grant NS13742 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, to R.L., H.M., and M.S., and by a grant from Sugen, Inc. to J.S.
ABBREVIATIONS
- PSP
postsynaptic potential
- ICa
calcium currents
- PTPases
protein tyrosine phosphatases
- FGF/FGFR
fibroblast growth factor/receptor
- PTK
protein tyrosine kinase
References
- 1.Hunter T. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- 2.Wang T, Salter M. Nature (London) 1994;369:233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- 3.Levine E, Dreyfus C, Black I, Plummer M. Proc Natl Acad Sci USA. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lev S, Moreno H, Plowman G D, Martines R, Peles E, Canoll P, Musacchio J N, Rudy B, Schlessinger J. Nature (London) 1995;376:737–744. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 5.Boxall A, Lancaster B, Garthwaite J. Neuron. 1996;16:805–813. doi: 10.1016/s0896-6273(00)80100-2. [DOI] [PubMed] [Google Scholar]
- 6.O’Dell T, Kandel E, Grant S. Nature (London) 1991;353:558–560. doi: 10.1038/353558a0. [DOI] [PubMed] [Google Scholar]
- 7.Pumiglia K, Lau L, Huang C, Feinstein M. Biochem J. 1992;286:441–449. doi: 10.1042/bj2860441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llinás R, Steinberg I, Walton K. Biophys J. 1981;33:323–352. doi: 10.1016/S0006-3495(81)84899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llinás R, Gruner J, Sugimori M, McGuinness T, Greengard P. J Physiol (London) 1991;436:257–282. doi: 10.1113/jphysiol.1991.sp018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohammadi M, Dikic I, Sorokin A, Burgess W H, Jaye M, Schlessinger J. Mol Cell Biol. 1996;16:977–989. doi: 10.1128/mcb.16.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohammadi M, Schlessinger J, Hubbard S. Cell. 1996;86:577–587. doi: 10.1016/s0092-8674(00)80131-2. [DOI] [PubMed] [Google Scholar]
- 12.Augustine G, Charlton M, Smith S. J Physiol (London) 1985;367:163–181. doi: 10.1113/jphysiol.1985.sp015819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlessinger J, Ullrich A. Neuron. 1992;9:383–391. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- 14.Llinás R, Sugimori M, Lang E J, Morita M, Fukuda M, Niinobe M, Mikoshiba M. Proc Natl Acad Sci USA. 1994;91:12990–12993. doi: 10.1073/pnas.91.26.12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolphin A. Exp Physiol. 1995;80:1–46. doi: 10.1113/expphysiol.1995.sp003825. [DOI] [PubMed] [Google Scholar]
- 16.Stea A, Soong T, Snutch T. Neuron. 1995;15:929–940. doi: 10.1016/0896-6273(95)90183-3. [DOI] [PubMed] [Google Scholar]
- 17.Fainzilber M, Smit A B, Syed N I, Wildering W C, Hermann P M, van der Schors R C, Jiménez C, Li K W, van Minnen J, Bulloch A G M, Ibáñez C F, Geraerts W P M. Science. 1996;274:1540–1543. doi: 10.1126/science.274.5292.1540. [DOI] [PubMed] [Google Scholar]
- 18.Llinás R, Sugimori M, Lin J-W, Cherksey B. Proc Natl Acad Sci USA. 1989;86:1689–1693. doi: 10.1073/pnas.86.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McFarlane M, Gilly W. Proc Natl Acad Sci USA. 1996;93:5067–5071. doi: 10.1073/pnas.93.10.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roovers E, Vincent M E, van Kester E, Geraerts W P M, Planta R J, Ureugdenhil E, van Heerikhuizen H. Gene. 1995;162:181–188. doi: 10.1016/0378-1119(95)00323-x. [DOI] [PubMed] [Google Scholar]
- 21.DiPolo, R. & Beauge, L. (1994) Am. Physiol. Soc., C1382–C1391. [DOI] [PubMed]
- 22.Roberts M, Towles J, Leader N. Brain Res. 1992;592:170–174. doi: 10.1016/0006-8993(92)91672-2. [DOI] [PubMed] [Google Scholar]
- 23.Colwell C, Khalsa S, Mulock G. J Comp Physiol A. 1992;170:211–215. doi: 10.1007/BF00196903. [DOI] [PubMed] [Google Scholar]
- 24.Heilig M, Widerlov E. Crit Rev Neurobiol. 1995;9:115–136. [PubMed] [Google Scholar]
- 25.Elia A, Money T, Orchard I. J Insect Physiol. 1995;41:565–570. [Google Scholar]
- 26.Smart D, Shaw C, Johnston C, Thim L, Halton D, Buchanan K. Biochem Biophys Res Commun. 1992;186:1616–1623. doi: 10.1016/s0006-291x(05)81593-1. [DOI] [PubMed] [Google Scholar]
- 27.Cottrell G A, Lin J-W, Llinás R, Price D A, Sugimori M, Stanley E F. Exp Physiol. 1992;77:881–889. doi: 10.1113/expphysiol.1992.sp003655. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura M, Sakanaka C, Aoki Y, Ogasawara H, Tsuiji T, Kodama H, Matzumolto T, Shimizu T, Noma M. J Biol Chem. 1995;270:30102–30110. doi: 10.1074/jbc.270.50.30102. [DOI] [PubMed] [Google Scholar]
- 29.Dikic I, Tokiwa G, Lev S, Courtneidge S A, Schlessinger J. Nature (London) 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 30.Carew T. Neuron. 1996;16:5–8. doi: 10.1016/s0896-6273(00)80016-1. [DOI] [PubMed] [Google Scholar]