Abstract
The interleukin 1β converting enzyme (ICE) family plays a pivotal role in programmed cell death and has been implicated in stroke and neurodegenerative diseases. During reperfusion after filamentous middle cerebral artery occlusion, ICE-like cleavage products and tissue immunoreactive interleukin 1β (IL-1β) levels increased in ischemic mouse brain. Ischemic injury decreased after intracerebroventricular injections of ICE-like protease inhibitors, N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (z-VAD.FMK), acetyl-Tyr-Val-Ala-Asp-chloromethylketone, or a relatively selective inhibitor of CPP32-like caspases, N-benzyloxycarbonyl-Asp-Glu-Val-Asp-fluoromethylketone, but not a cathepsin B inhibitor, N-benzyloxycarbonyl-Phe-Ala-fluoromethylketone. z-VAD.FMK decreased ICE-like cleavage products and tissue immunoreactive IL-1β levels in ischemic mouse brain and reduced tissue damage when administered to rats as well. Only z-VAD.FMK and acetyl-Tyr-Val-Ala-Asp-chloromethylketone reduced brain swelling, and N-benzyloxycarbonyl-Asp-Glu-Val-Asp-fluoromethylketone did not attenuate the ischemia-induced increase in tissue IL-1β levels. The three cysteine protease inhibitors significantly improved behavioral deficits, thereby showing that functional recovery of ischemic neuronal tissue can follow blockade of enzymes associated with apoptotic cell death. Finally, we examined the effect of z-VAD.FMK on excitotoxicity and found that it protected against α-amino-3-hydroxy-5-methyl-4-isoxazole propionate-induced or to a lesser extent N-methyl-d-aspartate-induced excitotoxic brain damage. Thus, ICE-like and CPP32-like caspases contribute to mechanisms of cell death in ischemic and excitotoxic brain injury and provide therapeutic targets for stroke and neurodegenerative brain damage.
Keywords: infarction, neurological deficit, caspase inhibitors, transient focal ischemia
Apoptosis is a cell suicide mechanism under active cell control. In the nematode Caenorhabditis elegans, the product of the ced-3 gene is essential for programmed cell death (1, 2). Members of the mammalian interleukin 1β (IL-1β) converting enzyme (ICE) family are homologs of C. elegans Ced-3 (3). Apoptosis has been observed in striatal and cortical neurons in animal models of stroke (4, 5) and may play a role in neuronal injury induced by ischemia. ICE-like proteases promote neuronal cell death induced by trophic factor deprivation in vitro (6, 7). Blocking ICE-like protease activity delays motoneuron death induced in vitro by trophic factor deprivation and in vivo during development (8).
Although resistance to ischemia in transgenic mice overexpressing Bcl-2 or deficient in p53 provides evidence for the importance of gene regulation to brain injury and repair, the molecular mechanisms of apoptosis after ischemic brain injury are unknown (9, 10). A role for apoptosis in ischemic injury was proposed based on morphological (terminal deoxynucleotidyltransferase-mediated UTP end labeling staining) and biochemical (DNA fragmentation) evidence (4, 5, 11). Moreover, IL-1β mRNA and protein expression increases in ischemic tissue during permanent focal (12, 13) and global ischemia (14, 15). Endogenously produced mature IL-1β plays an important role in hypoxia-mediated apoptosis in vitro (16). Furthermore, IL-1 receptor antagonist administration inhibits ischemic and excitotoxic neuronal damage in the rat, a fact that implicates IL-1β in ischemic pathophysiology (17, 18).
The ICE family now consists of 11 members (19–33) that can be divided into three subfamilies, the ICE subfamily, the CPP32 subfamily, and the Ich-1 subfamily, which have recently been named caspases (34). Each of the 11 family members contains a cysteine residue and the highly conserved pentapeptide sequence QACRG at the active site. Peptide (acyloxy) methylketones, which are active site inhibitors of ICE-like and CPP32-like caspases, have recently been developed (35). N-benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluoromethylketone (z-VAD.FMK) is a relatively nonselective inhibitor that blocks both ICE-like and CPP32-like caspase activity. Acetyl-Tyr-Val-Ala-Asp-chloromethylketone (YVAD.CMK) is more selective for ICE, whereas N-benzyloxycarbonyl-Asp(OMe)-Glu(OMe)-Val-Asp(OMe)-fluoromethylketone (z-DEVD.FMK), in part due to an aspartate residue in the P4 position, inhibits CPP32-like caspases more selectively (36). We examined the effects of these three competitive irreversible synthetic peptide inhibitors on brain injury as well as behavioral deficits after middle cerebral artery (MCA) occlusion to determine whether inhibition of proteins associated with programmed cell death can lead to functional neurological recovery. We also examined whether histological damage due to intrastriatal microinjection of N-methyl-D-aspartate (NMDA) or α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA), a model of excitotoxic brain damage in stroke and neurodegenerative disorders, is reduced by an inhibitor of ICE and related family members. Our findings have important therapeutic implications for the treatment of stroke and neurodegeneration.
MATERIALS AND METHODS
Drugs.
z-VAD.FMK, z-DEVD.FMK, and N-benzyloxycarbonyl-Phe-Ala-fluoromethylketone (z-FA.FMK) were obtained from Enzyme Systems Products (Dublin, CA), and YVAD.CMK was obtained from Bachem. Compounds were dissolved in 0.2–0.6% dimethyl sulfoxide (MCB Chemical, prepared with 0.1 M PBS, pH 7.4) and made fresh daily. AMPA and NMDA were obtained from Sigma and dissolved in 0.1 M PBS.
Physiology.
Regional cerebral blood flow (rCBF) was determined by laser-Doppler flowmetry (PF2B; Perimed, Stockholm) using a flexible 0.5-mm fiber optic extension to the master probe affixed over the ischemic cortex (2 mm posterior and 6 mm lateral from the bregma) on the intact skull. rCBF, blood pressure, and heart rate were monitored as described (37), and heart rate was monitored from the arterial blood pressure pulse. Arterial blood samples (30 μl) were analyzed for oxygen (PaO2) and carbon dioxide (PaCO2) before and during ischemia and 30 min after reperfusion using a blood gas/pH analyzer (Corning 178; Ciba Corning Diagnostics, Medford, MA). Core temperature was maintained at approximately 37°C with a thermostat (FHC, Brunswick, ME) and a heating lamp (Skytron, Daiichi Shomei, Tokyo) until 1 hr after reperfusion. In the rat experiments, rCBF, blood pressure, and heart rate were determined as described above, and the rCBF probe was affixed to the intact skull over the ischemic cortex at the bregma and 8 mm from midline.
Ischemia Model: Mouse.
Spontaneously ventilating adult male SV-129 mice (n = 173; 19–23 g; Taconic Farms) were initially anesthetized with 1.0% and maintained on 0.4–0.8% halothane in 70% N2O and 30% O2 using a Fluotec 3 vaporizer (Colonial Medical, Amherst, NH). The left MCA was occluded with an 8–0 nylon monofilament (Ethicon, Somerville, NJ) coated with a mixture of silicone resin (Xantopren; Bayer Dental, Osaka) and a hardener (Elastomer Activator; Bayer Dental) as described (37, 38). The procedure lasted 15 min, and the anesthesia was discontinued. Two hours later, animals were briefly re-anesthetized with halothane, and the filament was withdrawn. Eighteen hours after reperfusion, the forebrains were divided into five coronal (2-mm) sections using a mouse brain matrix (RBM-2000C; Activational Systems, Warren, MI), and the sections were stained with 2% 2,3,5-triphenyltetrazolium chloride (Sigma). The infarcted areas were quantitated by an image-analysis system (Bioquant IV; R & M Biometrics, Nashville, TN) and calculated by summing the volumes of each section determined directly (39) or indirectly by the following formula: contralateral hemisphere (mm3) − ipsilateral undamaged volume (mm3; ref. 40). Although statistical significance was achieved by both methods of analysis, only data from the direct method are presented. Brain swelling was calculated according to the following formula: [(infarct volume + ipsilateral undamaged volume − contralateral volume) × 100/contralateral volume (%)]. For histological evaluation of neuronal damage at 18 hr and 3 days after ischemia, sections (12 μm) were stained with hematoxylin/eosin.
z-VAD.FMK (13.5, 40, or 120 ng), z-FA.FMK (120 ng), and z-DEVD.FMK (40 or 120 ng) were injected intracerebroventricularly (i.c.v.) twice (2 μl per dose; bregma: 0.9 mm lateral, 0.1 mm posterior, 3.1 mm deep) 15 min before ischemia and immediately after reperfusion. YVAD.CMK (100 or 200 ng) was injected i.c.v. 45 min before ischemia and immediately after reperfusion. In separate experiments, z-VAD.FMK (a single 80-ng dose) was injected i.c.v. either immediately or 1 hr after reperfusion, and the animal killed 18 hr later.
Ischemia Model: Rat.
Adult male Sprague–Dawley rats (n = 39; 280–330 g; Charles River Breeding Laboratories) were initially anesthetized with 2.0% and maintained by 1.0% halothane in 70% N2O and 30% O2 using a Fluotec 3 vaporizer. The left MCA was occluded with a 3–0 nylon monofilament (Ethicon) with its tip rounded by heating near a flame. The filament (23 mm long) was inserted from the left external carotid artery and advanced into the internal carotid artery. The distance from the suture tip to the left common carotid artery bifurcation was approximately 20 mm. Two hours after ischemia, animals were briefly re-anesthetized with halothane, and the filament was withdrawn. Twenty-two hours later, the brains were stained with 2,3,5-triphenyltetrazolium chloride for morphometric analysis. z-VAD.FMK (8, 27, or 80 ng) was injected i.c.v. 15 min before ischemia and 10 min after reperfusion in 2 equal doses (4 μl per dose; 1.5 mm lateral, 0.8 mm posterior from bregma, 4.0 mm below bone surface).
Neurological Deficit.
Mice and rats were tested for neurological deficits and scored as described by Bederson et al. (41) with the following minor modification: 0, no observable neurological deficits (normal); 1, failure to extend right forepaw (mild); 2, circling to the contralateral side (moderate); 3, loss of walking or righting reflex (severe). The rater was naive to the treatment group, and assessments were made at 10 min and 2 hr during ischemia, and again 18 hr after reperfusion.
Western Blot Analysis.
Mouse brain tissue was lysed in a RIPA buffer (0.15 M NaCl/0.05 M Tris·HCl, pH 7.2/1% Triton X-100/1% sodium deoxycholate/0.2% SDS) in the presence of a protease inhibitor cocktail (leupeptin, phenylmethylsulfonyl fluoride, pepstatin, and aprotinin) for 45 min at 4°C. After ultracentrifugation at 4°C (TLA 45 type rotor at 35,000 × g), supernatants were diluted, boiled for 3 min, and loaded on an SDS/PAGE gel (12%; 10 μg per lane). An affinity purified polyclonal antibody against ICE (M8; 2 mg/ml) was incubated with transferred membranes for 2 hr at room temperature. This antibody was raised in rabbits by injection of a p45 full-length ICE protein fused to a His tag. Resultant antiserum was evaluated by Western blotting or immunoprecipitation and found to recognize endogenous ICE. It did not cross-react with ICH-1, ICH-3, or members of the CPP32 family (unpublished data), although it recognized an ICE-like protein in brain and other tissues of an ICE knockout mouse.
IL-1β Immunoassay.
Immunoreactive IL-1β was determined using an ELISA kit (Genzyme; lot B6499F). Male SV-129 mice (18–23 g) were injected twice i.c.v. with z-VAD.FMK (120 ng in 1 μl per dose) 15 min before occlusion and immediately after reperfusion. Each hemisphere was homogenized for 15 sec in PBS (0.1 M; pH 7.4; 4°C) containing 2 mM phenylmethylsulfonyl fluoride (stock dissolved in dimethyl sulfoxide, diluted 1:100 in PBS), 1 μg/ml leupeptin, 1 μg/ml antipain, 1 μg/ml aprotinin, 1 μg/ml pepstatin, 0.05% (wt/vol) sodium azide, and 4 mM ethylenediaminetetraacetic acid. The homogenates were centrifuged (30 min at 50,000 × g), and 100 μl of the supernatant was used for each determination. Immunoreactive IL-1β data are expressed as the difference between ischemic and contralateral hemispheres.
Neurotoxicity.
Adult male SV-129 mice (20–28 g) were anesthetized with halothane (2.5% for induction, 1–1.5% for maintenance), and the head was fixed in a stereotaxic frame (Kopf Instruments, Tujunga, CA). AMPA (20 mM, 6 nmol) or NMDA (67 mM, 20 nmol) was injected (26-gauge Hamilton syringe) at 0.5 mm anterior and 2 mm lateral to the bregma, and 2.5 mm below the dura in 0.3 μl. Rectal temperature was monitored during this 10-min procedure. To assess the effects of ICE-like inhibition, AMPA plus z-VAD.FMK were co-injected intrastriatally followed 3 hr later by an injection of z-VAD.FMK alone (0.3 μl each). z-VAD.FMK was also administered i.c.v. 15 min before and 3 hr after NMDA injection (2 μl each). Mice were killed after 48 hr, and tissue sections (20 μm) were stained by hematoxylin/eosin. The lesion was measured by an image analysis system (M4; Image Research, St. Catharine’s, ON, Canada), and sections were integrated to calculate volume.
Statistical Analysis.
Data are presented as the mean ± SE. Statistical comparisons were made by one- or two-way ANOVA followed by Student’s t test, Dunnett’s test, or Mann–Whitney U test using the software super anova or stat view, version 4.5 (Abacus Concepts, Berkeley, CA). P < 0.05 was considered statistically significant.
RESULTS
Reduction of Ischemic Infarction by the Inhibitors of the ICE Family.
To determine the roles of the ICE-like proteases in ischemic brain injury and possible therapeutic value of ICE family inhibitors in stroke, we determined the ability of three peptide inhibitors of the ICE-like proteases to reduce brain injury in a mouse model of stroke. When the peptide inhibitors were injected into ventricle 15 min before and upon reperfusion, a significant reduction in infarct size was noted. z-VAD.FMK, a nonselective inhibitor of the ICE family and perhaps some serine proteases as well, reduced infarction volume by approximately 40–50% when injected at 80 and 240 ng (Fig. 1). The extent of the decrease was similar after 80 and 240 ng. Infarct size ranged from 76 to 128 mm3 in vehicle-treated brains, whereas values ranged from 31 to 80 mm3 after treatment with 240 ng of z-VAD.FMK. Infarct sparing was particularly noted in posterior forebrain (coronal sections from 6 to 10 mm). Neurological deficits were significantly reduced after 18 hr but not immediately upon reperfusion. The neurological score was somewhat better after the injection of 240 ng rather than 80 ng, although the differences were not statistically significant.
Figure 1.
Effects of z-VAD.FMK and cathepsin B inhibitor (z-FA.FMK) on infarct volume (A) and area (B), and neurological deficits (C) after transient focal ischemia in SV-129 mice. Animals were subjected to MCA filament occlusion for 2 hr and reperfused for 18 hr as described. Vehicle (○), z-VAD.FMK (27 ng; •), z-VAD.FMK (80 ng; ▵), z-VAD.FMK (240 ng; ▴), and z-FA.FMK (240 ng; □) were injected i.c.v. 15 min before ischemia and immediately after reperfusion. Infarct area was determined in each of five coronal sections (2 mm) from anterior (2 mm from anterior pole) to posterior (10 mm; B). z-VAD.FMK (80 and 240 ng) decreased infarct volume and neurological deficits, whereas z-FA.FMK (240 ng) did not. After treatment with z-FA.FMK and z-VAD.FMK (27 ng), infarct areas did not differ from vehicle in the 5 coronal sections (data not shown). Data are presented as means ± SE (n = 5–12). ∗, P < 0.05; ∗∗, P < 0.01 vs. vehicle.
Neuroprotection sustained for at least 3 days [152 ± 5 (n = 4) vs. 75 ± 30 mm3 (n = 5) for vehicle-treated and z-VAD.FMK-treated (80 ng) groups, respectively]. Some of the damage at 18 hr and 3 days was attributed to brain swelling which was detected in the control group. z-VAD.FMK significantly decreased brain swelling at 18 hr [12.9 ± 2.7% (n = 12) vs. 2.9 ± 1.4% (n = 8) for vehicle and z-VAD.FMK, respectively] and 3 days as well.
To determine if more specific inhibitors of the ICE family may also reduce ischemic brain injury, we examined whether YVAD.CMK, which is specific for the ICE-like caspases, can also reduce ischemic injury in our model. YVAD.CMK was injected i.c.v. 45 min before MCA occlusion and upon reperfusion after 2 hr of occlusion. YVAD.CMK (400 ng but not 200 ng) decreased infarction volume by 64%. Significant decreases were measurable in each of the five coronal sections except in the most anterior 2 mm. Brain swelling at 18 hr was statistically less after injection of YVAD.CMK [14.0 ± 1.4% (n = 8) vehicle vs. 7.6 ± 1.2% (n = 6) YVAD.CMK, 400 ng (P < 0.05)]. YVAD.CMK (400 ng) also ameliorated the behavioral deficits assessed during reperfusion (Table 1).
Table 1.
Effects of ICE-like caspase inhibitor (YVAD.CMK) and a relatively selective CPP32-like caspase inhibitor (z-DEVD.FMK) on infarct volume and neurological deficits after temporary MCA occlusion (2 hr) and reperfusion (18 hr)
| Treatments | n | Infarct volume, mm3 | Neurological deficits |
|---|---|---|---|
| Vehicle | 8 | 100 ± 9 | 1.8 ± 0.2 |
| YVAD.CMK (200 ng) | 6 | 96 ± 13 | 1.5 ± 0.3 |
| YVAD.CMK (400 ng) | 6 | 36 ± 18** | 0.8 ± 0.4* |
| Vehicle | 10 | 90 ± 6 | 2.1 ± 0.1 |
| z-DEVD.FMK (80 ng) | 10 | 83 ± 7 | 1.6 ± 0.2 |
| z-DEVD.FMK (240 ng) | 9 | 66 ± 8* | 1.1 ± 0.3** |
Neurological deficits were decreased by YVAD.CMK and z-DEVD.FMK after 18 hr, although no significant group differences were detected at 10 min or 2 hr of ischemia (data not shown). Data are presented as means ± SE. ∗, P < 0.05; ∗∗, P < 0.01 vs. vehicle.
To assess the contribution of the subfamily of CPP32-like caspases to ischemic brain injury, we evaluated the ability of z-DEVD.FMK to inhibit ischemic brain injury. z-DEVD.FMK (240 ng but not 80 ng) reduced infarct volume by 27%, whereas infarct volume ranged from 72 to 129 mm3 in the vehicle groups (n = 10), and from 9 to 89 mm3 after the higher dose of z-DEVD.FMK (n = 9). Injury was reduced only in section 6 mm (4–6 mm from anterior pole) that contained the largest infarct area. Lower values were also measured in sections 8 and 10 mm, but these did not reach statistical significance. z-DEVD.FMK did not decrease brain swelling but reduced behavioral deficits as effectively as YVAD.CMK or z-VAD.FMK.
To determine if the ability to reduce ischemic brain injury was specific for the protease inhibitors of the ICE family, we administered z-FA.FMK (240 ng), a cathepsin B inhibitor, as a control peptide to test for possible effects of the fluoromethylketone moiety, although the degree of cathepsin B inhibition was not determined at the dosage tested. This drug did not reduce infarct volume or neurological deficits (Fig. 1). Thus, we conclude that the ability to reduce ischemic injury is most likely due to specific inhibition of the ICE family proteases.
To determine if administering an ICE family inhibitor upon recirculation has therapeutic value, we injected z-VAD.FMK upon reperfusion after 2 hr of occlusion. z-VAD.FMK (80 ng), administered as a single dose, diminished infarct size [114 ± 7 (n = 11) and 79 ± 10 mm3 (n = 8, P < 0.05) for vehicle and z-VAD.FMK, respectively]. Sparing was observed in the three coronal sections [4–6 mm, 10.7 ± 1.1 and 6.9 ± 1.3 mm2; 6–8 mm, 18.6 ± 0.7 and 16.3 ± 1.0 mm2; and 8–10 mm, 20.5 ± 1.7 and 13.3 ± 3.1 mm2, for vehicle (n = 11) and z-VAD.FMK (n = 8), respectively]. The protection was less than when z-VAD.FMK (40 ng × 2) was administered before and upon reperfusion. Neurological deficits, although improved, did not reach statistical significance 18 hr later [2.1 ± 0.2 (n = 11) vs. 1.5 ± 0.3 (n = 8) for the vehicle- and z-VAD.FMK-treated groups, respectively]. When z-VAD.FMK (80 ng) was injected 1 hr after reperfusion, the decrease in infarct volume did not reach significance [114 ± 7 (n = 11) and 87 ± 10 mm3 (n = 5) for vehicle and z-VAD.FMK, respectively], although a significant reduction was measured in the most anterior coronal section [4 mm; 10.7 ± 1.1 (n = 11) and 6.2 ± 1.8 mm2 (n = 5, P < 0.05) for vehicle and z-VAD.FMK, respectively].
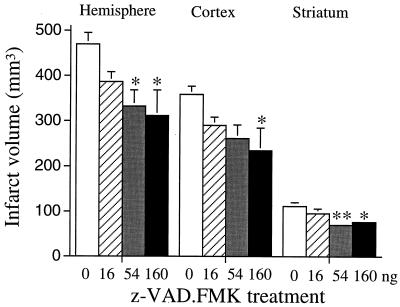
To test effects of z-VAD.FMK in a second species, the drug was injected into the lateral ventricle of Sprague–Dawley rats 15 min before ischemia (2 hr of MCA occlusion) and upon reperfusion. z-VAD.FMK (54 and 160 ng) significantly reduced infarct volume in rat cortex and striatum (Fig. 2) and decreased neurological deficits [2.4 ± 0.2 (n = 9) vs. 1.4 ± 0.4 (n = 8) for vehicle- and z-VAD.FMK (160 ng)-treated groups, respectively; P < 0.05], which indicates that inhibition of ICE family members ameliorates injury in more than a single species.
Figure 2.
Effects of z-VAD.FMK on infarct size after transient focal ischemia in rats. Animals were subjected to filament MCA occlusion for 2 hr and reperfused for 22 hr. z-VAD.FMK was injected i.c.v. 15 min before ischemia and 10 min after reperfusion. Open bar, vehicle; hatched bar, z-VAD.FMK (16 ng); shaded bar, z-VAD.FMK (54 ng); solid bar, z-VAD.FMK (160 ng). Data are represented as means ± SE (n = 7–9). ∗, P < 0.05; ∗∗, P < 0.01 vs. vehicle.
We monitored physiology in some of the mice before, during, and after operation. rCBF decreased to approximately 20% of baseline immediately after MCA occlusion and sustained during 2 hr of ischemia in both mice and rats. After reperfusion, rCBF increased immediately to 90–100% of baseline, and hyperemia (approximately 30% above the baseline) was observed ipsilaterally over the subsequent 10 min in both vehicle- and z-VAD.FMK-treated groups. There were no significant blood flow differences between vehicle- and drug-treated groups at any time point, nor were there differences in mean arterial blood pressure, heart rate, rectal (core) temperature, and blood gases detected between groups after drug administration to mice (Table 2) or rats (data not shown). Thus, reduction of ischemic infarct by the inhibitors of the ICE family does not result from hemodynamic effects on cerebral blood flow, blood pressure, or heart rate. In our experiments, less than 5% of mice died after drug treatment or MCA occlusion. In preliminary experiments, 3 of 6 mice died after large doses (800 ng) of YVAD.CMK, presumably due to a low toxic/therapeutic ratio (2:1).
Table 2.
Physiological variables before and during ischemia, and during reperfusion in SV-129 mice under halothane anesthesia
| Parameters | Treatments | Ischemia
|
Reperfusion | |
|---|---|---|---|---|
| Before | During | |||
| rCBF | ||||
| Ipsilateral | Vehicle | 100 | 19 ± 5 | 125 ± 20 |
| Drug | 100 | 18 ± 5 | 113 ± 11 | |
| MABP | Vehicle | 86 ± 5 | 93 ± 6 | 80 ± 7 |
| Drug | 92 ± 3 | 95 ± 4 | 87 ± 4 | |
| Heart rate | Vehicle | 502 ± 13 | 482 ± 26 | 509 ± 48 |
| Drug | 505 ± 18 | 484 ± 27 | 477 ± 19 | |
| Core temperature | Vehicle | 36.4 ± 0.2 | 36.7 ± 0.3 | 36.3 ± 0.3 |
| Drug | 36.9 ± 0.2 | 36.8 ± 0.1 | 36.8 ± 0.2 | |
| Blood gases | ||||
| Arterial pH | Vehicle | 7.31 ± 0.02 | 7.32 ± 0.02 | 7.33 ± 0.02 |
| Drug | 7.31 ± 0.003 | 7.29 ± 0.01 | 7.30 ± 0.01 | |
| Arterial PaCO2 | Vehicle | 47 ± 4 | 48 ± 4 | 44 ± 3 |
| Drug | 47 ± 2 | 52 ± 1 | 49 ± 2 | |
| Arterial PaO2 | Vehicle | 130 ± 16 | 133 ± 10 | 155 ± 9 |
| Drug | 123 ± 7 | 125 ± 7 | 151 ± 9 | |
z-VAD.FMK (120 ng) was injected i.c.v. 15 min before ischemia. rCBF (% of baseline), MABP (mean arterial blood pressure; millimeters of mercury; 1 mmHg = 133 Pa), heart rate (beats per min), core temperature (°C), and arterial blood gases [pH, PaCO2 (torr), PaO2 (torr)] were measured before occlusion, during ischemia (2 hr), and during up to 30 min reperfusion. Data are presented as the mean ± SE (n = 5 per group). There are no significant differences between groups. Values were averaged over a 15-min time period immediately before ischemia, during ischemia (45–60 min), and after reperfusion (15–30 min).
ICE-Like Proteases in Ischemic Brain.
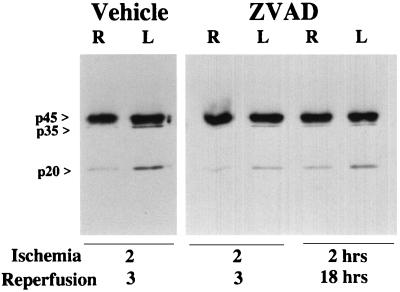
To examine if ischemic injury is accompanied by activation of ICE-like proteases, we determined whether immunoreactive ICE cleavage products appear on a Western blot in ischemic brain using polyclonal antisera. ICE cleavage is associated with the appearance of p20 and p10 bands (42). Ischemia increased the ICE-like cleavage product (p20) on immunoblots at 2 and 18 hr after reperfusion (Fig. 3). An unidentified band corresponding to p35 was detected and became considerably more pronounced on the ipsilateral side after ischemia and reperfusion. This additional band may represent the activation of ICE-like proteases in ischemic brain. z-VAD.FMK (40 ng × 2) administered before ischemia and upon recirculation decreased p20 and p35 bands 3 hr and 18 hr after reperfusion (Fig. 3).
Figure 3.
Effects of z-VAD.FMK on ICE-like protease expression in brain after left MCA filament occlusion and reperfusion. Immunoreactive products p35 and p20 increased in the ipsilateral hemisphere at 3 hr after reperfusion. z-VAD.FMK (80 ng) administered 15 min before ischemia and immediately upon reperfusion decreased immunoreactive products p35 and p20 in the ipsilateral hemisphere at 3 and 18 hr after reperfusion. There was no significant difference between normal brain (sham) and the nonischemic contralateral hemisphere (data not shown). L, Left (ischemic); R, right (nonischemic) hemisphere.
Enhanced IL-1β Level in Ischemic Brain.
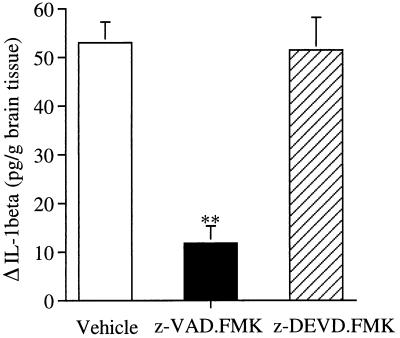
Processing of pro-IL-1β to generate mature IL-1β is a specific function of ICE in vitro and in vivo, and thus, secretion of mature IL-1β is a specific indication of ICE activation (16). To examine whether ischemic injury induces IL-1β secretion, we measured the brain IL-1β levels using an ELISA kit that detects mature IL-1β. We found that brain IL-1β levels reached a peak 30 min to 1 hr after reperfusion, and then decreased, suggesting that ICE is transiently activated upon reperfusion. We assessed the relative specificity of z-VAD.FMK and z-DEVD.FMK on IL-1β production. Since z-VAD.FMK is an inhibitor of the ICE family, whereas z-DEVD.FMK prefers the CPP32 subfamily, we anticipated that z-VAD.FMK but not z-DEVD.FMK would reduce the augmentation of IL-1β. We found that z-VAD.FMK (240 ng) reduced IL-1β by 76% vs. control, whereas z-DEVD.FMK treatment (240 ng) did not prevent the rise at 30 min after reperfusion (Fig. 4). No differences were measured between ipsilateral and contralateral hemispheres in sham animals, or between control groups.
Figure 4.
Effect of z-VAD.FMK and z-DEVD.FMK on ischemia-induced immunoreactive IL-1β levels in brain. Treatment with z-VAD.FMK (120 ng × 2) but not z-DEVD.FMK (120 ng × 2) 15 min before ischemia and at reperfusion reduced the expected augmentation of IL-1β ipsilaterally after 30 min of reperfusion after 2 hr of MCA occlusion. Control animals were treated with the same volume of vehicle. Data are presented as the mean ± SEM (n = 6 or 7). ∗∗, P < 0.01 vs. vehicle.
The Effects of ICE Inhibitor on Glutamate Receptor-Mediated Neurotoxicity.
The importance of glutamate neurotoxicity to cerebral ischemia has been well documented (43). NMDA and AMPA cause tissue injury when added to neuronal cultures or when injected into the striatum, and this can be blocked by NMDA and AMPA receptor antagonists. NMDA or AMPA was microinjected under stereotaxic control into the striatum in anesthetized mice. Two days later, we determined that intrastriatal co-injection of z-VAD.FMK (24 ng) attenuated AMPA-induced neuronal damage [32.2 ± 2.5 mm3 (n = 8) vehicle vs. 24.5 ± 1.8 mm3 (n = 8) z-VAD.FMK; P < 0.05]. However, z-VAD.FMK attenuated the NMDA-induced neuronal damage only when administered at a higher dose (80 ng) and directly into the ventricle [32.1 ± 1.6 mm3 (n = 5) vehicle vs. 24.1 ± 2.1 mm3 (n = 6) z-VAD.FMK; P < 0.05]. When z-VAD.FMK was co-injected intrastriatally with NMDA, there was no difference from control [22.7 ± 1.0 mm3 (n = 5) vehicle vs. 24.3 ± 1.5 mm3 (n = 5) z-VAD.FMK]. We conclude that the nonselective inhibitor of ICE-like caspases z-VAD.FMK can reduce toxicity by AMPA and to a lesser extent NMDA, suggesting the importance of ICE family members to glutamate neurotoxicity.
DISCUSSION
We demonstrated here that lipid soluble cysteine protease inhibitors of the ICE family reduced tissue injury caused by temporary MCA occlusion in mice. Decreased infarct volume was observed 18 hr after reperfusion and sustained for at least 72 hr after z-VAD.FMK treatment, and was consistently present in the coronal section containing frontal cortex, striatum, and anterior hippocampus. rCBF, arterial blood pressure, heart rate, core temperature, blood gases, or arterial pH did not change with treatment, suggesting that the observed protection reflects increased resistance of ischemic tissue. Moreover, neurological deficits also improved. In nearly every experiment showing a significant reduction in infarct volume, motor deficits were noticeably better. Hence, deterioration of neurological function after acute injury may be reduced by administering cysteine protease inhibitors. Inhibition of ICE-like caspases attenuated ischemic cell death in rat cortex and striatum, as described in a preliminary report after permanent ischemia (44). Our findings were associated with behavioral improvement as well. Taken together, these data are consistent with our recent preliminary result showing that a transgenic mouse expressing an endogenous ICE inhibitor is protected from ischemic damage after temporary MCA occlusion (45).
z-VAD.FMK decreased brain swelling, at least in part due to reduced formation of the proinflammatory cytokine IL-1β. Active ICE is a tetramer composed of two subunits, p20 and p10, processed from p45 precursor peptide (42). The subunits are cleaved from a single proenzyme, p45 (42). Ischemia promoted activation of ICE or ICE-like proteins, as evidenced by increased ICE-like cleavage products (p35 and p20) on immunoblots at 3 hr and 18 hr (Fig. 3) after reperfusion. Furthermore, the appearance of cleavage products was decreased by z-VAD.FMK as was brain IL-1β levels 30 min after reperfusion. Treatments that reduce or antagonize the action of IL-1β (e.g., recombinant IL-1β antibodies or zinc protoporphyrin administration) decrease brain water content in ischemic brain (46).
We tested the effects of two other irreversible inhibitors. YVAD.CMK is more selective for ICE-like than for CPP32-like caspases (47), and this drug decreased infarct size, edema formation, and behavioral deficits. z-DEVD.FMK shows greater selectivity for CPP32-like than for ICE-like caspases (48). A related compound, Ac-DEVD.CHO, inhibits cleavage of a CPP32 substrate, poly (ADP ribose) polymerase 10,000 times more potently than Ac-YVAD.CHO (47). Moreover, z-DEVD.FMK did not reduce IL-1β levels in ischemic brain (Fig. 4), thereby suggesting that the cleavage of ICE or pro-IL-1β may not be an important action of CPP32-like caspases in vivo under the stated condition. The most potent (and least selective) among the three inhibitors was z-VAD.FMK. z-VAD.FMK inhibits anti-Fas antibody-induced apoptosis in Jurkat cells by blocking activation of CPP32-like caspases indirectly (49). Both z-VAD.FMK and YVAD.CMK afforded similar protection (57% vs. 64% decrease in volume, respectively), although the dose of z-VAD.FMK required was approximately 4-fold lower (0.17 nmol vs. 0.74 nmol, respectively). The data suggest that the development of drugs that are nonselective cysteine protease inhibitors may offer advantages over those showing greater selectivity.
We are not aware of published studies addressing whether CPP32 is activated during ischemia, although this would not be unexpected since ischemia activates ICE, and ICE cleaves CPP32 (47). We observed that z-DEVD.FMK reproducibly reduced both infarct size and behavioral deficits, although z-DEVD.FMK appeared weaker (27% protection at 0.36 nmol) than either z-VAD.FMK or YVAD.CMK. z-DEVD.FMK treatment did not block edema formation, suggesting mechanistic differences between inhibitors of CPP32-like and ICE-like caspases and IL-1β synthesis as noted above. Moreover, improved neurological function after z-DEVD.FMK treatment suggested that an effect on brain swelling alone did not explain fully the neurological improvement after z-VAD.FMK and YVAD.CMK administration. Inhibitors of both ICE-like and CPP32-like caspases confer ischemic brain protection, although their mechanism of action seems to differ. Inhibition of ICE-like caspases and IL-1β production can reduce the appearance of inflammation and apoptosis as a consequence of ischemia, whereas inhibition of CPP32-like caspases may be more specifically related to blockade of apoptosis.
An inhibitor of ICE and ICE-like activity (z-VAD.FMK, 80 ng) successfully reduced tissue injury when given immediately as a single dose upon reperfusion. These findings suggest the therapeutic potential for combining ICE family protease inhibition with recombinant tissue plasminogen activator during clot lysis and subsequent reperfusion. However, the therapeutic window for this peptide inhibitor appears relatively short as the data after z-VAD.FMK was administered 1 hr after recirculation did not reach statistical significance.
Excitotoxins cause apoptosis in cell culture (50) or after intrastriatal injection (51), particularly when the injury is mild (50). Neuronal damage due to AMPA was attenuated when z-VAD.FMK and AMPA were co-injected intrastriatally. The same protocol, however, was ineffective after NMDA injection despite the presence of a smaller (submaximal) lesion. MK-801, a selective NMDA receptor antagonist, however, blocked lesion development completely (data not shown). When administered at a higher dose and directly into the ventricle, the lipid protease inhibitor attenuated NMDA-induced neuronal damage. Whether this means that NMDA is more closely associated with necrotic mechanisms of cell death compared with AMPA neurotoxicity is unknown at this time, but it suggests important mechanistic differences between the two excitotoxins (43). Nevertheless, the protection we observed in the presence of excitotoxin injection is consistent with the results after administering an endogenous IL-1 receptor antagonist (17, 18), and this further implicates the ICE family proteases in events related to ischemic pathophysiology.
In conclusion, our results demonstrated that inhibitors of the ICE family proteases prevent ischemic and excitotoxic neuronal damage and, by so doing, preserve and protect neurological function. Inhibition of ICE-like and/or other caspase family members, particularly by non-peptide inhibitors that cross the blood–brain barrier and easily penetrate neurons and glia, could provide novel treatments for stroke and other forms of brain and spinal cord injury in humans.
Acknowledgments
We thank Drs. Seth P. Finkelstein and Bradley T. Hyman (Massachusetts General Hospital) and Dr. Pierre A. Henkart (National Cancer Institute, National Institutes of Health, Bethesda) for their advice and Drs. Gamze Ayata and Albert Kim for helpful technical assistance. Our studies were supported by Massachusetts General Hospital Interdepartmental Stroke Project Grant NS10828 and by an unrestricted award in Neuroscience from Bristol-Myers Squibb (to M.A.M.). K.F. was supported by the Deutsche Forschungsgemeinschaft (Fi600/2-1). J.Y. was supported by the National Institute of Neurological Disorders and Stroke, American Heart Association Established Investigatorship Award and by Bristol–Myers Squibb. R.M.F. was supported by a postdoctoral training fellowship from the National Institutes of Health and by an Upjohn award from the joint section on Cerebrovascular Surgery, the Congress of Neurological Surgeons, and the American Association of Neurological Surgeons.
ABBREVIATIONS
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole propionate
- i.c.v.
intracerebroventricular(ly)
- IL-1β
interleukin 1β
- ICE
IL-1β converting enzyme
- MCA
middle cerebral artery
- NMDA
N-methyl-d-aspartate
- rCBF
regional cerebral blood flow
- YVAD.CMK
acetyl-Tyr-Val-Ala-Asp-chloromethylketone
- z-VAD.FMK
N-benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluoromethylketone
- z-DEVD.FMK
N-benzyloxycarbonyl-Asp(OMe)-Glu(OMe)-Val-Asp(OMe)-fluoromethylketone
- z-FA.FMK
N-benzyloxycarbonyl-Phe-Ala-fluoromethylketone
References
- 1.Ellis R E, Yuan J, Horvitz H R. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- 2.Yuan J, Horvitz H R. Dev Biol. 1990;138:33–41. doi: 10.1016/0012-1606(90)90174-h. [DOI] [PubMed] [Google Scholar]
- 3.Yuan J, Shaham S, Ledoux S, Ellis H M, Horvitz H R. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Chopp M, Jiang N, Yao F, Zaloga C. J Cereb Blood Flow Metab. 1995;15:389–397. doi: 10.1038/jcbfm.1995.49. [DOI] [PubMed] [Google Scholar]
- 5.Charriaut-Marlangue C, Margaill I, Represa A, Popovici T, Plotkine M, Ben-Ari Y. J Cereb Blood Flow Metab. 1996;16:186–194. doi: 10.1097/00004647-199603000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Gagliardini V, Fernandez P-A, Lee R K K, Drexler H C A, Rotello R, Hartwieg E A, Yuan J. Science. 1994;263:826–828. doi: 10.1126/science.8303301. [DOI] [PubMed] [Google Scholar]
- 7.Li W, Fishman M C, Yuan J. Cell Death Differ. 1995;3:105–112. [PubMed] [Google Scholar]
- 8.Milligan C E, Prevette D, Yaginuma H, Homma S, Cardwell C, Fritz L C, Tomaselli K J, Oppenheim R W, Schwartz L M. Neuron. 1995;15:385–393. doi: 10.1016/0896-6273(95)90042-x. [DOI] [PubMed] [Google Scholar]
- 9.Martinon J-C, Dubois-Dauphin M, Staple J K, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C, Huarte J. Neuron. 1994;13:1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 10.Crumrine R C, Thomas A L, Morgan P F. J Cereb Blood Flow Metab. 1994;14:887–891. doi: 10.1038/jcbfm.1994.119. [DOI] [PubMed] [Google Scholar]
- 11.Johnson E J, Greenlund L J, Akins P T, Hsu C Y. J Neurotrauma. 1995;12:843–852. doi: 10.1089/neu.1995.12.843. [DOI] [PubMed] [Google Scholar]
- 12.Liu T, McDonnell P C, Young P R, White R F, Siren A L, Hallenbeck J M, Barone F C, Feuerstein G Z. Stroke. 1993;24:1746–1751. doi: 10.1161/01.str.24.11.1746. [DOI] [PubMed] [Google Scholar]
- 13.Buttini M, Sauter A, Boddeke H W G M. Mol Brain Res. 1994;23:126–134. doi: 10.1016/0169-328x(94)90218-6. [DOI] [PubMed] [Google Scholar]
- 14.Minami M, Kuraishi Y, Yabuuchi K, Yamazaki A, Satoh M. J Neurochem. 1992;58:390–392. doi: 10.1111/j.1471-4159.1992.tb09324.x. [DOI] [PubMed] [Google Scholar]
- 15.Bhat R V, DiRocco R, Marcy V R, Flood D G, Zhu Y, Dobrzanski P, Siman R, Scott R, Contreras P C, Miller M. J Neurosci. 1996;16:4146–4154. doi: 10.1523/JNEUROSCI.16-13-04146.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedlander R M, Gagliardini V, Rotello R J, Yuan J. J Exp Med. 1996;184:717–724. doi: 10.1084/jem.184.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothwell N J, Relton J K. Neurosci Biobehav Rev. 1993;17:217–227. doi: 10.1016/s0149-7634(05)80152-6. [DOI] [PubMed] [Google Scholar]
- 18.Relton J K, Rothwell N J. Brain Res Bull. 1992;29:243–246. doi: 10.1016/0361-9230(92)90033-t. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S, Kinoshita M, Noda M, Copeland N G, Jenkins N A. Genes Dev. 1994;8:1613–1626. doi: 10.1101/gad.8.14.1613. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Alnemri T, Litwack G, Alnemri E S. Cancer Res. 1995;55:2737–2742. [PubMed] [Google Scholar]
- 21.Fernandes-Alnemri T, Takahashi A, Armstrong R, Krebs J, Fritz L, Tomaselli K, Wang L, Yu Z, Croce C M, Earnshaw W C, Litwack G, Alnemri E S. Cancer Res. 1995;55:6045–6052. [PubMed] [Google Scholar]
- 22.Fernandes-Alnemri T, Armstrong R C, Krebs J, Srinivasula S M, Wang L, Bullrich F, Fritz L C, Trapani J A, Tomaselli K J, Litwack G, Alnemri E S. Proc Natl Acad Sci USA. 1996;93:7464–7469. doi: 10.1073/pnas.93.15.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Miura M, Bergeron L, Zhu H, Yuan J. Cell. 1994;78:739–750. doi: 10.1016/s0092-8674(94)90422-7. [DOI] [PubMed] [Google Scholar]
- 24.Faucheu C, Diu A, Chan A W, Blanchet A-M, Miossec C, Herve F, Collard-Dutilleul V, Gu Y, Aldape R A, Lippke J A, Rocher C, Su M S-S, Livingston D J, Hercend T, Lalanne J-L. EMBO J. 1995;14:1914–1922. doi: 10.1002/j.1460-2075.1995.tb07183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tewari M, Dixit V M. J Biol Chem. 1995;270:3255–3260. doi: 10.1074/jbc.270.7.3255. [DOI] [PubMed] [Google Scholar]
- 26.Kamens J, Paskind M, Hugunin M, Talanian R V, Allen H, Banach D, Bump N, Hackett M, Johnston C G, Li P, Mankovich J A, Terranova M, Ghayur T. J Biol Chem. 1995;270:15250–15256. doi: 10.1074/jbc.270.25.15250. [DOI] [PubMed] [Google Scholar]
- 27.Munday N A, Vaillancourt J P, Ali A, Casano F J, Miller D K, Molineaux S M, Yamin T-T, Yu V L, Nicholson D W. J Biol Chem. 1995;270:15870–15876. doi: 10.1074/jbc.270.26.15870. [DOI] [PubMed] [Google Scholar]
- 28.Wang S, Miura M, Jung Y, Zhu H, Gagliardini V, Shi L, Greenberg A H, Yuan J. J Biol Chem. 1996;271:20580–20587. doi: 10.1074/jbc.271.34.20580. [DOI] [PubMed] [Google Scholar]
- 29.Lippke J A, Gu Y, Sarnecki C, Caron P R, Su M S-S. J Biol Chem. 1996;271:1825–1828. doi: 10.1074/jbc.271.4.1825. [DOI] [PubMed] [Google Scholar]
- 30.Duan H J, Chinnaiy A M, Hudson P L, Wing J P, He W W, Dixit V M. J Biol Chem. 1996;271:1621–1625. doi: 10.1074/jbc.271.3.1621. [DOI] [PubMed] [Google Scholar]
- 31.Duan H, Orth K, Chinnaiyan A M, Poirier G G, Froelich C J, He W, Dixit V. J Biol Chem. 1996;271:16720–16724. doi: 10.1074/jbc.271.28.16720. [DOI] [PubMed] [Google Scholar]
- 32.Muzio M, Chinnaiyan A M, Kischkel F C, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, Mann M, Kreammer P H, Peter M E, Dixit V M. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 33.Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 34.Alnemri E S, Livingston D J, Nicholson D W, Salvesen G, Thornberry N A, Wong W W, Yuan J. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 35.Thornberry N A, Peterson E P, Zhao J J, Howard A D, Griffin P R, Chapman K T. Biochemistry. 1994;33:3934–3940. doi: 10.1021/bi00179a020. [DOI] [PubMed] [Google Scholar]
- 36.Cain K, Inayat-Hussain S H, Couet C, Cohen G M. Biochem J. 1996;314:27–32. doi: 10.1042/bj3140027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hara H, Huang P L, Panahian N, Fishman M C, Moskowitz M A. J Cereb Blood Flow Metab. 1996;16:605–611. doi: 10.1097/00004647-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Yang G Y, Chen S F, Kinouchi H, Chan P H, Weistein P R. Stroke. 1992;23:1331–1336. doi: 10.1161/01.str.23.9.1331. [DOI] [PubMed] [Google Scholar]
- 39.Huang Z, Huang P L, Panahian N, Dalkara T, Fishman M C, Moskowitz M A. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 40.Swanson R A, Morton M T, Tsao-Wu G, Savalos R A, Davidson C, Sharp F R. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 41.Bederson J B, Pitts L H, Tsuji M, Nishimura M C, Davis R L, Bartkowski H M. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 42.Thornberry N A, Bull H G, Calaycay J R, Chapman K T, Howard A D, et al. Nature (London) 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 43.Choi D W. J Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 44.Loddick S A, MacKenzie A, Rothwell N J. NeuroReport. 1996;7:1465–1468. doi: 10.1097/00001756-199606170-00004. [DOI] [PubMed] [Google Scholar]
- 45.Hara H, Friedlander R M, Gagliardini V, Ayata C, Ayata G, Yuan J, Moskowitz M A. Circulation Suppl. 1996;94:2283. (abstr.). [Google Scholar]
- 46.Yamasaki Y, Matsuura N, Shozuhara H, Onodera H, Itoyama Y, Kogure K. Stroke. 1995;26:676–681. doi: 10.1161/01.str.26.4.676. [DOI] [PubMed] [Google Scholar]
- 47.Nicholson D W, Ali A, Thornberry N A, Vaillancourt J P, Ding C K, Gallant M, Gareau Y, Griffin P R, Labelle M, Lazebnik Y A, Munday N A, Raju S M, Smulson M E, Yamin T T, Yu V L, Miller D K. Nature (London) 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 48.Sarin A, Wu M-L, Henkart P A. J Exp Med. 1996;185:2445–2450. doi: 10.1084/jem.184.6.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slee E A, Zhu H, Chow S C, MacFarlane M, Nicholson D W, Cohen G M. Biochem J. 1996;315:21–24. doi: 10.1042/bj3150021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton S A. Proc Natl Acad Sci USA. 1995;92:7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferrer I, Martin F, Serrano T, Reiriz J, Perez-Navarro E, Alberch J, Macaya A, Planas A M. Acta Neuropathol. 1995;90:504–510. doi: 10.1007/BF00294812. [DOI] [PubMed] [Google Scholar]