Abstract
Though l-amino acids predominate in living organisms, substantial levels of free d-serine and d-aspartate occur in mammals, especially in nervous and endocrine tissues. Using an antibody specific for glutaraldehyde-fixed d-aspartate, we have localized d-aspartate in rat tissues. In the brain we observe discrete neuronal localizations of d-aspartate, especially in the external plexiform layer of the olfactory bulb, hypothalamic supraoptic and paraventricular nuclei, the medial habenula, and certain brainstem nuclei. In rats 3–4 weeks old, we observe d-aspartate in septal nuclei and in a subset of stellate and basket cells of the cerebellum. d-aspartate is also concentrated in glands, including the epinephrine cells of the adrenal medulla, the posterior pituitary, and the pineal gland. Levels in the pineal gland are the highest of any mammalian tissue. d-aspartate oxidase, visualized by enzyme histochemistry, is concentrated in neurons of the hippocampus, cerebral cortex, and olfactory epithelium, as well as choroid plexus and ependyma. Localizations of d-aspartate oxidase are reciprocal to d-aspartate, suggesting that the enzyme depletes endogenous stores of the amino acid and might inactivate synaptically released d-aspartate.
Keywords: d-amino acid, d-aspartate oxidase, pituitary, pineal, adrenal
Although d-amino acids are well known in bacterial physiology, only recently have they been found in mammals (1–3). Substantial levels of d-serine occur in brain tissue with a regional distribution resembling the the N-methyl-d-aspartate (NMDA) subtype of glutamate receptors (4). By immunohistochemistry we have shown that d-serine is selectively localized to a subpopulation of astrocytes in close proximity to NMDA receptors and released by glutamate stimulation (5, 6). Since d-serine potently activates the glycine site on NMDA receptors, these findings indicate that d-serine is a novel messenger molecule that serves as an endogenous ligand for this site.
Lajtha and coworkers (1) discovered very high levels of d-aspartate in the brain and other tissues of mammals. d-aspartate levels are highest in neonatal tissues, attaining millimolar concentrations in newborn rat cerebral cortex, pituitary gland, and 3-week-old adrenal gland (7, 8). Using immunohistochemistry, we now describe selective neuronal localizations of d-aspartate in discrete brain areas and endocrine structures. d-aspartate oxidase (DAPOX), visualized by a novel histochemical technique, is localized inversely to endogenous d-aspartate.
MATERIALS AND METHODS
Materials.
Sprague–Dawley rats were purchased from Sasco (Wilmington, MA). Hypophysectomized rats were from Charles River Breeding Laboratories. Mice (129/SvEv Agouti strain) were from Taconic Farms. Antibody to l-aspartate (9) was from Chemicon. Glutaraldehyde (GA) was from Electron Microscopy Sciences (Fort Washington, PA). The peroxidase Elite staining kit was from Vector Laboratories. All other reagents were from Sigma.
Antibody Production and Purification.
d-aspartate was coupled to BSA with GA and then reduced with NaBH4 (10). Rabbits were immunized using a colloidal gold technique (5, 11), and stereoselective, high-affinity antiserum was produced after 7 weeks. Before use, batches of d-aspartate antiserum were diluted 1:10 with 0.05% NaN3 and 10 mM Tris (pH 7.4). Diluted serum (10 ml) was mixed with 1 ml packed agarose beads that had been coupled to GA-BSA at a concentration of 4.3 mg/ml with respect to BSA. Antiserum and beads were incubated for 2 hr at room temperature on a rotating wheel. This incubation was followed by another against l-glutamate-GA-beads for 2 hr. These steps removed antibodies against carrier protein and fixative. Polyclonal antiserum against l-aspartate-thyroglobulin-GA conjugates was negatively purified against GA-treated thyroglobulin beads and used at a 1:500 dilution in the presence of 0.2 mM d-aspartate-GA conjugate.
Immunohistochemistry.
Sprague–Dawley rats or Agouti mice were anesthetized with an overdose of sodium pentobarbital and perfused through the aorta for 30 sec with 37°C oxygenated Krebs–Henseleit buffer and then switched to 37°C 5% glutaraldehyde/0.5% paraformaldehyde containing 0.2% Na2S2O5 in 0.1 M sodium phosphate (pH 7.4). The perfusion flow rates and volumes were adjusted for the size of the animal, ranging from 20 ml/min and 150 ml for mice (20-gauge needle) to 60 ml/min and 450 ml for adult rats (cannula). After a 20-min delay, brains were removed, trimmed, and postfixed in the same buffer for 2 hr at room temperature. After cryoprotection for 2 days at 4°C in 20% glycerol, 1% NaCl, 0.01% thimerosal, and 50 mM sodium phosphate (pH 7.4), brain sections (40 μm) were cut on a sliding microtome. Floating brain sections were stained using an avidin-biotin peroxidase technique, as described (5). Because of the high concentrations of l-aspartate in brain, we carried out all d-aspartate antibody incubations (1:2000 dilution) in the presence of 0.2 mM l-aspartate-GA liquid-phase conjugate (5, 12).
DAPOX and Norepinephrine Histochemistry.
Sodium pentobarbital-anesthetized rats were perfused with 37°C Krebs–Henseleit buffer and then 4°C 2% paraformaldehyde in 0.1 sodium phosphate or 4°C 2% glutaraldehyde/0.5% paraformaldehyde in 0.1 M sodium phosphate (pH 7.4). After a 20-min delay, tissue was removed, trimmed, and postfixed on ice for 2 hr. Sections (40 μm) were cryoprotected overnight, frozen, and then cut on a sliding microtome. To visualize DAPOX, we modified a technique for d-amino acid oxidase (13). Floating brain sections were incubated in the dark at 37° in 0.1% horseradish peroxidase, 0.01% diaminobenzidine, 0.02% NiCl2, 0.065% NaN3, 0.02 mM FAD, and 100 mM Tris preset buffer (pH 8.3), with 20 mM NMDA as a substrate. Specific staining developed after 2–18 hr of incubation, and no staining was observed if l-aspartate was added instead. GA-fixed norepinephrine was visualized as described (14).
Analysis of d-Amino Acids.
Free amino acids were extracted from tissues, derivatized with fluorescent chiral reagents, and quantified by HPLC (15).
RESULTS
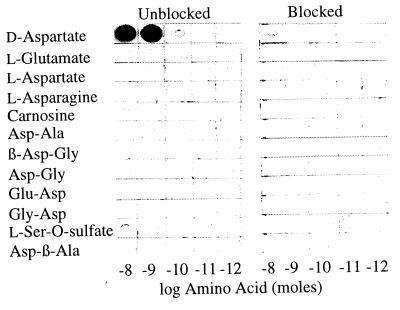
We developed antiserum to d-aspartate as it would occur in fixed tissues by immunizing rabbits with a GA conjugate of BSA and d-aspartate coated onto colloidal gold particles. We examined the specificity and sensitivity by coupling various amino acids and peptides to ovalbumin with GA and spotting preparations onto nitrocellulose. Dot blots were probed with a 1:5000 dilution of the antiserum (Fig. 1). The antibody detected 0.1 nmol of d-aspartate conjugate, while no reactivity was apparent with l-aspartate, l-glutamate, or a variety of aspartate-containing peptides. Slight cross-reactivity (1%) was observed with l-serine-O-sulfate. Preabsorption of the antibody with 0.2 mM d-aspartate-GA conjugate abolished staining. In tissue sections the conjugate also eliminated immunoreactivity (not shown).
Figure 1.
Antibody specificity test. Ovalbumin was coupled to various amino acids and peptides with GA, reduced with NaBH4, and then spotted onto nitrocellulose in serial 1:10 dilutions. (Left) Nitrocellulose was probed with a 1:5000 dilution of purified antiserum in the presence of 0.2 mM l-aspartate-GA conjugate. (Right) Blocked incubations included an additional 0.2 mM d-aspartate-GA conjugate. Immunoreactivity was visualized with an alkaline phosphatase secondary antibody. All concentrations are with respect to the amino acid or peptide.
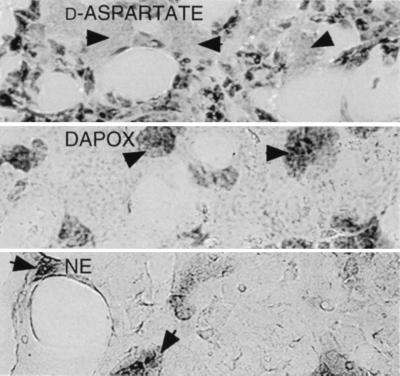
We first examined the adrenal glands of 3-week-old rats, which are known to contain high levels of d-aspartate (7). The medulla stained intensely, but the cortex was unstained (Fig. 2 Top). Staining in the medulla was associated with some but not all chromaffin cells. To discriminate chromaffin cell subtypes, we stained sections with a histochemical method for norepinephrine (Fig. 2 Bottom). Norepinephrine occurred in ≈20% of chromaffin cells and appeared as round clusters (14). d-aspartate was selectively excluded from norepinephrine cells and instead concentrated in the remaining 80% of the cells, which make epinephrine (Fig. 2 Top). By contrast, DAPOX staining was selectively concentrated in norepinephrine chromaffin cells, suggesting that DAPOX destroys endogenous d-aspartate in these cells.
Figure 2.
Localization of d-aspartate (Top), DAPOX (Middle), or norepinephrine (NE) (Bottom) in the adrenal gland. For the d-aspartate localization, 3-week-old animals were used. Arrowheads point to NE-producing chromaffin cells, which appear in round clusters and are completely unstained for d-aspartate, but densely stained for DAPOX and NE.
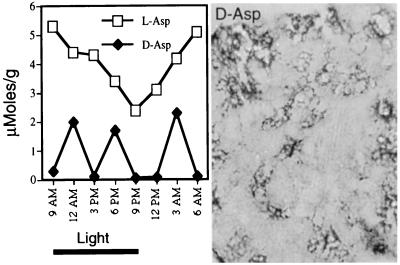
Using an HPLC technique, we measured d-aspartate in the pineal. d-aspartate levels in many pineal glands were higher than any rat tissue previously reported, with an average value of 1.2 ± 1.6 mM (n = 12), while levels of l-aspartate were 3.65 ± 0.8 mM. Because the pineal displayed diurnal rhythms in numerous substances, we trained male littermates on a 12-hr light/dark cycle for 3 weeks and then sacrificed an animal every 3 hr throughout a diurnal cycle. We found 20-fold variations in d-aspartate (♦), but there was no diurnal rhythm (Fig. 3 Left). Conceivably the variations reflect changes on a shorter ultradian cycle or simply random fluctuations. By contrast, l-aspartate levels (□) displayed diurnal rhythmicity, with levels doubling during the dark cycle, and decreasing during light. The pineal gland stained intensely for d-aspartate. In some rats staining occurred in all pineal cells; in other animals staining concentrated in islands of cells near blood vessels (Fig. 3 Right). We found no DAPOX staining in the pineal.
Figure 3.
Disposition of d- and l-aspartate in the pineal gland. (Left) Male rat littermates were trained on a 12-hr light/dark cycle for 3 weeks. One pineal gland was harvested every 3 hr of the cycle and analyzed by HPLC for amino acids. (Right) Immunohistochemical localization of d-aspartate in the pineal. Some glands exhibited the scattered pattern depicted; in other glands, every cell was intensely labeled.
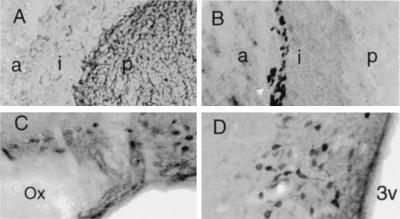
In the pituitary gland, d-aspartate was concentrated in the posterior lobe, with a few stained cells widely scattered in the intermediate and anterior lobes (Fig. 4A). In the posterior lobe, extremely intense staining was observed in nerve processes and terminals, which derive primarily from cells in the supraoptic and paraventricular nuclei of the hypothalamus. Indeed, intense and highly localized staining for d-aspartate occurred in most magnocellular neurons of both nuclei (Fig. 4 C and D). By contrast, these nuclei stained only faintly with an antibody to l-aspartate and had little DAPOX activity (not shown). The median eminence, which contains axons of magnocellular neurons, was also enriched in d-aspartate and devoid of DAPOX. In the pituitary, DAPOX activity occurred exclusively in the intermediate lobe, with staining concentrated in the outermost cells, adjacent to the anterior lobe (Fig. 4B).
Figure 4.
d-aspartate and DAPOX visualized in the pituitary and hypothalamic nuclei. (A) Endogenous d-aspartate in the pituitary is intensely concentrated in the posterior lobe (p), while the intermediate (i) and anterior (a) lobe exhibit very low staining in a few widely scattered cells. (B) DAPOX in the pituitary is restricted to the intermediate lobe (i), with activity concentrated in a narrow band of cells immediately adjacent to the anterior lobe (a). (C) d-aspartate is concentrated in magnocellular neurons of the supraoptic nucleus of the hypothalamus, near the optic nerve (Ox). (D) d-aspartate is concentrated in magnocellular neurons of the paraventricular nucleus, near the third ventricle (3v).
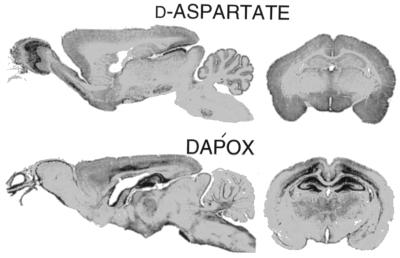
In the brain (Fig. 5 Top), d-aspartate staining was most intense in the external plexiform layer of the olfactory bulb, accessory olfactory nucleus, superior colliculus, medial habenula, and in scattered brainstem nuclei. High densities were also evident in the hypothalamus around the third ventricle, while no staining occurred in the adjacent thalamus. d-aspartate occurred in the molecular layer but not the granular or white matter layers of the cerebellum. The localization of DAPOX was almost exactly inverse to that of d-aspartate (Fig. 5 Bottom). For instance, the hippocampus displayed the weakest staining for d-aspartate and the most intense staining for DAPOX in the brain.
Figure 5.
Inverse brain localizations of d-aspartate and DAPOX. d-aspartate is concentrated in the olfactory bulb mitral cell layer, hypothalamus, anterior olfactory bulb, superior colliculus, the molecular layer of the cerebellum, and scattered nuclei in the brainstem. By contrast, DAPOX is concentrated in olfactory receptor neuron layer and glomeruli, hippocampus, cerebral cortex, thalamus, and cerebellar granule cells.
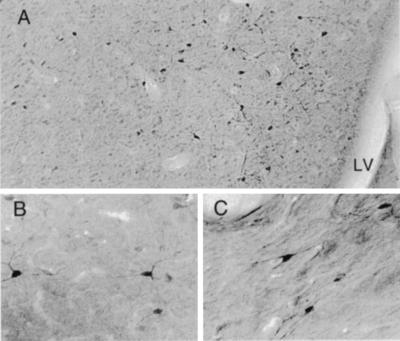
Under higher power examination, highly selective enrichment of d-aspartate but not l-aspartate was evident in large neurons located in lateral septal, triangular septal, and septofimbrial nuclei of 23-day-old male rats (Fig. 6). Staining was also evident in neurons within the fimbria (Fig. 7C). These nuclei receive their major inputs from the hippocampus and are thought to produce γ-aminobutyric acid (16). The triangular septal and septofimbrial nuclei provide 90% of all projections to the medial habenula via the stria medullaris, while the lateral septal cells are interneurons. By contrast, the medial septal nuclei and the bed nucleus of the stria terminalis, groups that project to the hippocampus, were unstained for d-aspartate.
Figure 6.
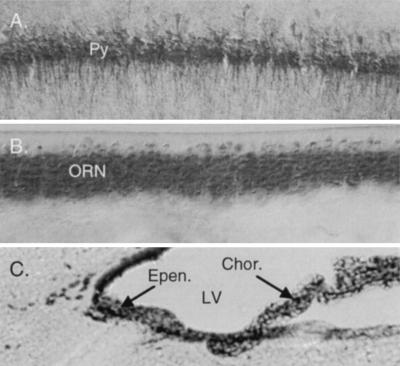
Cellular localization of DAPOX. Intense DAPOX activity occurs in all hippocampal pyramidal cells (Py) (A), olfactory receptor neurons (ORN) (B), and ependymal cells (Epen.) and choroid plexus (Chor.) (C). LV, lateral ventricle.
Figure 7.
Localization of d-aspartate in P23 septal neurons. (A) d-aspartate occurs in lateral septal nuclei near the lateral ventricle (LV). (B) Higher-power view of multipolar stained neurons in the septum. (C) Localization of d-aspartate in septofimbrial neurons of the fornix.
Relative to stained cells in the olfactory bulb, hypothalmus, and septum, neurons in the brainstem were less densely stained; however, certain nuclei exhibited staining above background. These consisted mainly of magnocellular groups, the interpeduncular nucleus, inferior olive, cochlear nuclei, gigantocellular cells of the reticular nucleus, and cranial nuclei, especially the hypoglossal and facial. Many of these groups also stained intensely for l-aspartate and/or are known to contain aspartate aminotransferase (17).
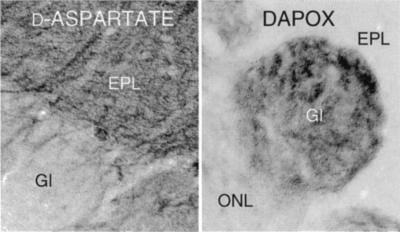
Substantial DAPOX activity occurs in most brain areas (18). The most intense staining occurred in the choroid plexus and ependyma (Fig. 6C), which did not stain for d-aspartate but stains intensely for l-aspartate (19). At high magnification, DAPOX was concentrated in most pyramidal neurons in the cerebral cortex and hippocampus (Fig. 6A) as well as granule cells of the dentate gyrus. Olfactory receptor neuron cell bodies in the olfactory epithelium neurons also displayed intense DAPOX activity (Fig. 6B). Within the olfactory bulb, extremely intense DAPOX activity was observed in the glomeruli (Fig. 8 Right), which contained terminals of the olfactory neurons, suggesting that DAPOX occurred throughout these cells. By contrast, high densities of d-aspartate occurred in the adjacent external plexiform layer (Fig. 8 Left).
Figure 8.
Inverse localizations of d-aspartate (Left) and DAPOX (Right) in the olfactory bulb. EPL, external plexiform layer; Gl, glomeruli; ONL, olfactory nerve layer.
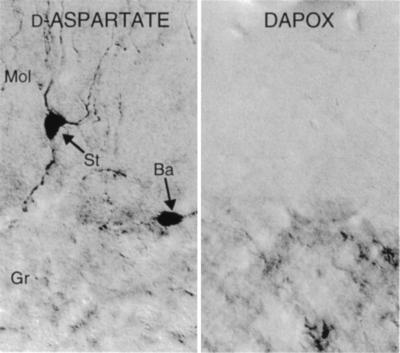
A reciprocal relationship between d-aspartate and DAPOX was also evident in the cerebellum (Fig. 9). d-aspartate was localized to a subpopulation of basket and stellate cells (Fig. 9 Left), but was not contained in Purkinje cells or glia. This staining was most prominent in 3-week-old rats, and could be further enhanced by removal of the pituitary. Four weeks following removal of the pituitary gland from 4-week-old animals, basket and stellate cells were intensely stained for d-aspartate, especially cells with processes in close vicinity to the pia. By contrast, sham-operated animals exhibited no staining (not shown). No d-aspartate was evident in the granule cell layer, whereas DAPOX staining was observed in the granule cell layer and white matter, but not the molecular layer (Fig. 9 Right).
Figure 9.
Inverse localizations of d-aspartate and DAPOX in P23 cerebellum. (Left) d-aspartate is present in a subset of basket (Ba) and stellate (St) neurons of the cerebellum. Cells in the granular layer (Gr) are unstained for d-aspartate, but contain DAPOX (Right).
Biochemical studies have detected substantial d-aspartate in adult mouse cerebral cortex (3), but not rat (8). We confirmed this species difference biochemically and immunohistochemically. Whereas rat levels in cortex were below 50 nmol/g, mouse levels were about 300 nmols/g. Mouse cerebral cortex and hippocampus stained much more intensely for d-aspartate than rat. Staining in the cortex occurred mainly in pyramidal neurons, especially in superficial layers of piriform cortex. The most dramatic species difference occurred in the hippocampus, where mice exhibited intense staining for d-aspartate in neurons of the CA3 region and hilus (not shown).
DISCUSSION
The principal finding of this study was the selective localization of d-aspartate in discrete neuronal populations in the brain as well as various endocrine organs. L-aspartate also displays discrete localizations to neuronal populations (9, 19, 20). Low levels of d-aspartate staining occurred throughout the brain in a neuronal pattern closely resembling that reported for l-aspartate. Either the antibody had a slight cross-reactivity with l-aspartate in tissue sections, or endogenous d-aspartate existed at low levels in many of the same neurons enriched in the l-isomer. Two regions with intense staining for d-aspartate, the external plexiform layer of the olfactory bulb, and the medial habenula, are known to contain high densities of l-aspartate (9, 19, 21).
In most brain regions, d-aspartate localizations appeared to reflect those of l-aspartate minus areas with high densities of DAPOX. This fits with the notion that DAPOX degrades endogenous d-aspartate. Selected brain regions stained much more intensely with the d-aspartate antibody than with the antibody to l-aspartate, and lacked DAPOX activity. These include the supraoptic and paraventricular nuclei of the hypothalamus, septal nuclei, and cerebellar basket/stellate cells of 3-week-old animals. d-aspartate and DAPOX localizations in mouse and rat brain were similar, but the density of d-aspartate staining was much greater in mouse cerebral cortex and hippocampus than in rat.
d-aspartate is well known to activate NMDA receptors. Conceivably d-aspartate functions as a neurotransmitter at NMDA or other receptors. If d-aspartate is a neurotransmitter, one would expect depolarization to stimulate its release. Detailed release studies have not yet been conducted. In preliminary experiments, spreading depression elicited by potassium injection into the cerebral cortex of mice caused a pronounced increase in d-aspartate staining in the choroid plexus (M.J.S., M. Eliasson, S.H.S., and M. Moskowitz, unpublished work). This suggests that depolarization elicited by potassium releases d-aspartate, which reaches ventricular fluid and accumulates in choroid plexus to levels great enough to exceed the DAPOX activity.
DAPOX was first described in mammalian tissues more than 45 yr ago, but its biological function is unclear (22). DAPOX is a peroxisomal enzyme (23). In the brain, peroxisomes are concentrated in oligodendrocytes in white matter and contain enzymes required for the synthesis of myelin lipids (24). The localizations of DAPOX appear to reflect a unique population of peroxisomes enriched in neurons. In some brain regions, such as the olfactory glomeruli and hippocampus, DAPOX appears concentrated in nerve terminals, suggesting a synaptic function. A synaptic role has been suggested for platelet activating factor, a lipid made exclusively in peroxisomes (25); this lipid could conceivably be made by peroxisomes expressing DAPOX. The reciprocal localizations of DAPOX and d-aspartate are analagous to the reciprocal distributions of d-serine and d-amino acid oxidase, which degrades endogenous d-serine (26). Besides depleting endogenous d-aspartate, DAPOX might inactivate the synaptically released amino acid. For example, hippocampal neurons have intense DAPOX activity and express abundant glutamate transporters, which also carry d-aspartate. Any d-aspartate released synaptically near these cells would be rapidly taken up and degraded.
The anatomical relationships between neuronal groups containing d-aspartate and those containing DAPOX suggest that another role for DAPOX is to degrade d-aspartate in brain regions that might otherwise accumulate d-aspartate via retrograde axonal transport. d-[3H]aspartate is a well studied retrograde label for glutamatergic pathways (27–29). We observed three prominent localizations suggesting that the retrograde transport of d-aspartate occurs endogenously. In the olfactory bulb, d-aspartate is concentrated in the dendrites of mitral and tufted cells, while DAPOX is localized to primary olfactory neurons and their terminals in glomeruli. d-aspartate taken up into olfactory receptor neuron terminals would accumulate in these cells if it were not degraded by their intense DAPOX activity. Likewise, hippocampal axons project to the septofimbrial and triangular septal nuclei, which are intensely stained for d-aspartate; any d-aspartate taken up into hippocampal neurons would be degraded by their intense DAPOX activity. A third example occurs in the cerebellum, where basket and stellate cells contain d-aspartate. Parallel fibers, the axons of granule cells located in the molecular layer, could take up released d-aspartate and transport it retrogradely to their cell bodies in the granular layer, which contain DAPOX.
d-aspartate localizations in various glands suggest a role in neuroendocrine modulation. In the adrenal gland d-aspartate is selectively concentrated in chromaffin cells that make epinephrine, while DAPOX appears in norepinephrine cells. In the hypothalamus, it is unclear whether d-aspartate occurs primarily in oxytocin or vasopressin cells. In preliminary experiments we injected 23-day-old rats i.v. with d-aspartate (20 mg/kg) and stained them 15 min or 8.5 hr later. At early time points, we observed a marked enhancement in staining of the pituitary and median eminence but not in the hypothalamus, whereas at 8.5 hr, staining had increased dramatically in the paraventricular nucleus. This suggests that circulating d-aspartate is selectively accumulated by nerve terminals of the posterior pituitary and retrogradely transported to cell bodies in the paraventricular nucleus.
The very high levels of d-aspartate in the pineal gland varied markedly in an unclear pattern. The variations might reflect an ultradian rhythm or pulsatile alterations in pituitary hormone release. However, hypophysectomy did not alter pineal d-aspartate (M.J.S. and S.H.S., unpublished observations). The pineal gland is a circumventricular organ (30). These highly vascularized structures, which are outside the blood–brain barrier, concentrate i.v.-administered l-aspartate (31, 32). In rats injected i.v. with d-aspartate, intense staining in the pineal was evident in 15 min. The medial habenula, which contains neurons that project to the pineal gland (33, 34), displayed a prominent increase in d-aspartate staining 8 hr after an i.v. injection of d-aspartate, while the lateral habenula was unstained (M.J.S. and S.H.S., unpublished observations). Thus, habenular d-aspartate might derive in part from retrograde transport. Habenular d-aspartate could also accumulate via uptake following release from septal nuclei, which contain d-aspartate and comprise the major input to the medial habenula.
Other selected circumventricular organs are greatly enriched in d-aspartate. The median eminence is intensely stained, but the area postrema is not. Intravenous injections of d-aspartate markedly augment staining in the median eminence. The selectivity of the d-aspartate uptake system is suggested by the lack of staining in the area postrema, another circumventricular organ. By contrast, l-aspartate and l-glutamate accumulate in all circumventricular organs following i.v. administration (31, 32, 35).
In summary, we have localized d-aspartate in rat tissues and find it selectively enriched in neurons and glands, where it might function as a novel messenger. The target for d-aspartate is unclear. Although most glutamate receptor subtypes respond to d-aspartate, brain areas enriched in glutamate receptors, such as the hippocampus, have very low levels of d-aspartate; moreover, a number of neuronal groups enriched in d-aspartate, such as septal neurons and cerebellar basket and stellate cells, produce γ-aminobutyric acid. While the transport systems for most amino acids strongly prefer the l-isomer, those for aspartate are stereoblind, suggesting that uptake of d-aspartate into cells plays an important role in its function. Endocrine systems are attractive candidates for modulation by d-aspartate, since the pituitary, adrenal, and pineal glands contain the highest levels of d-aspartate. Consistent with this notion, a recent study demonstrated that d-aspartate can stereospecifically regulate the production of sex steroids (36). Sex steroids serve important functions in brain as well, and steroid biosynthesis is one possible target for neuronal d-apartate.
Acknowledgments
We thank Roscoe Brady, Jr., Mikael Eliasson, Mark Molliver, Jean-Pierre Mothet, and Hui Wang for helpful discussions. This work was supported by Public Health Service Grant MH-18501, Research Scientist Award DA-00074 to S.H.S., and a gift from the Theodore and Vada Stanley Foundation.
ABBREVIATIONS
- DAPOX
d-aspartate oxidase
- GA
glutaraldehyde
- NMDA
N-methyl-d-aspartate
References
- 1.Dunlop D S, Neidle A, McHale D, Dunlop D M, Lajtha A. Biochem Biophys Res Commun. 1986;141:27–32. doi: 10.1016/s0006-291x(86)80329-1. [DOI] [PubMed] [Google Scholar]
- 2.Nagata Y, Yamamoto K, Shimojo T, Konno R, Yasumura Y, Akino T. Biochim Biophys Acta. 1992;1115:208–211. doi: 10.1016/0304-4165(92)90055-y. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto A, Nishikawa T, Konno R, Niwa A, Yasumura Y, Oka T, Takahashi K. Neurosci Lett. 1993;152:33–36. doi: 10.1016/0304-3940(93)90476-2. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto A, Nishikawa T, Oka T, Takahashi K. J Neurochem. 1993;60:783–786. doi: 10.1111/j.1471-4159.1993.tb03219.x. [DOI] [PubMed] [Google Scholar]
- 5.Schell M J, Molliver M E, Snyder S H. Proc Natl Acad Sci USA. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schell, M. J., Brady, R. O., Jr., Molliver, M. E. & Snyder, S. H. (1997) J. Neurosci., in press. [DOI] [PMC free article] [PubMed]
- 7.Hashimoto A, Nishikawa T, Oka T, Hayashi T, Takahashi K. FEBS Lett. 1993;331:4–8. doi: 10.1016/0014-5793(93)80286-4. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto A, Oka T, Nishikawa T. Eur J Neurosci. 1995;7:1657–1663. doi: 10.1111/j.1460-9568.1995.tb00687.x. [DOI] [PubMed] [Google Scholar]
- 9.Campistron G, Buijs R M, Geffard M. Brain Res. 1986;365:179–184. doi: 10.1016/0006-8993(86)90737-7. [DOI] [PubMed] [Google Scholar]
- 10.Campistron G, Buijs R M, Geffard M. Brain Res. 1986;376:400–405. doi: 10.1016/0006-8993(86)90208-8. [DOI] [PubMed] [Google Scholar]
- 11.Pow D V, Crook D K. J Neurosci Methods. 1993;48:51–63. doi: 10.1016/s0165-0270(05)80007-x. [DOI] [PubMed] [Google Scholar]
- 12.Ottersen O P, Storm-Mathisen J, Madsen S, Skumlien S, Stromhaug J. Med Biol. 1986;64:147–158. [PubMed] [Google Scholar]
- 13.Horiike K, Arai R, Tojo H, Yamano T, Nozaki M, Maeda T. Acta Histochem Cytochem. 1985;18:539–550. [Google Scholar]
- 14.Coupland R E, Hopwood D. J Anat. 1966;100:227–243. [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto A, Nishikawa T, Oka T, Takahashi K, Hayashi T. J Chromatogr. 1992;582:41–48. doi: 10.1016/0378-4347(92)80300-f. [DOI] [PubMed] [Google Scholar]
- 16.Panula P, Revuelta A V, Cheney D L, Wu J Y, Costa E. J Comp Neurol. 1984;222:69–80. doi: 10.1002/cne.902220107. [DOI] [PubMed] [Google Scholar]
- 17.Wenthold R J, Altschuler R A. In: Glutamine, Glutamate, and GABA in the Central Nervous System. Hertz L, Kbamme E, McGeer E G, Schousboe A, editors. New York: Liss; 1983. pp. 33–50. [Google Scholar]
- 18.Yusko S C, Neims A H. J Neurochem. 1973;21:1037–1039. doi: 10.1111/j.1471-4159.1973.tb07555.x. [DOI] [PubMed] [Google Scholar]
- 19.Aoki E, Semba R, Kato K, Kashiwamata S. Neuroscience. 1987;21:755–765. doi: 10.1016/0306-4522(87)90035-2. [DOI] [PubMed] [Google Scholar]
- 20.Ottersen O P, Storm-Mathisen J. Neuroscience. 1985;16:589–606. doi: 10.1016/0306-4522(85)90194-0. [DOI] [PubMed] [Google Scholar]
- 21.Saito N, Kumoi K, Tanaka C. Neurosci Lett. 1986;65:89–93. doi: 10.1016/0304-3940(86)90125-4. [DOI] [PubMed] [Google Scholar]
- 22.Still J L, Buell M V, Knox E W, Green D E. J Biol Chem. 1949;179:831–837. [PubMed] [Google Scholar]
- 23.Van Veldhoven P P, Brees C, Mannaerts P. Biochim Biophys Acta. 1991;1073:203–208. doi: 10.1016/0304-4165(91)90203-s. [DOI] [PubMed] [Google Scholar]
- 24.McKenna O, Arnold G, Holtzman E. Brain Res. 1976;117:181–194. doi: 10.1016/0006-8993(76)90729-0. [DOI] [PubMed] [Google Scholar]
- 25.Wieraszko A, Li G, Kornecki E, Hogan M V, Ehrlich Y H. Neuron. 1993;10:553–557. doi: 10.1016/0896-6273(93)90342-o. [DOI] [PubMed] [Google Scholar]
- 26.Nagata Y. Experientia. 1992;48:753–755. doi: 10.1007/BF02124295. [DOI] [PubMed] [Google Scholar]
- 27.Taxt T, Storm-Mathisen J. Neuroscience. 1984;11:79–100. doi: 10.1016/0306-4522(84)90215-x. [DOI] [PubMed] [Google Scholar]
- 28.Matute C, Wiklund L, Streit P, Cuenod M. Exp Brain Res. 1987;66:445–447. doi: 10.1007/BF00243320. [DOI] [PubMed] [Google Scholar]
- 29.Ottersen O P, Fischer B O, Storm-Mathisen J. Neurosci Lett. 1983;42:19–24. doi: 10.1016/0304-3940(83)90415-9. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto P H. Prog Brain Res. 1992;91:439–443. doi: 10.1016/s0079-6123(08)62364-x. [DOI] [PubMed] [Google Scholar]
- 31.Price M T, Pusateri M E, Crow S E, Buchsbaum S, Olney J W, Lowry O H. J Neurochem. 1984;42:740–744. doi: 10.1111/j.1471-4159.1984.tb02745.x. [DOI] [PubMed] [Google Scholar]
- 32.Price M T, Olney J W, Lowry O H, Buchsbaum S. J Neurochem. 1981;36:1774–1780. doi: 10.1111/j.1471-4159.1981.tb00430.x. [DOI] [PubMed] [Google Scholar]
- 33.Ronnekleiv O K, Kelly M J, Wuttke W. Exp Brain Res. 1980;39:187–192. doi: 10.1007/BF00237549. [DOI] [PubMed] [Google Scholar]
- 34.Ronnekleiv O K, Moller M. Exp Brain Res. 1979;37:551–562. doi: 10.1007/BF00236823. [DOI] [PubMed] [Google Scholar]
- 35.Hawkins R A, DeJoseph M R, Hawkins P A. Cell Tissue Res. 1995;281:207–214. doi: 10.1007/BF00583389. [DOI] [PubMed] [Google Scholar]
- 36.Daniello A, Dicosmo A, Dicristo C, Annunziato L, Petrucelli L, Fisher G. Life Sci. 1996;59:97–104. doi: 10.1016/0024-3205(96)00266-4. [DOI] [PubMed] [Google Scholar]