Abstract
To gain insights into the significance of presenilins (PS) in the pathogenetic mechanisms of early-onset familial Alzheimer disease (FAD), we expressed cDNAs for wild-type PS2 and PS2 with the Volga German (N141I) mutation in cultured cells and then examined the metabolism of the transfected proteins and their effect on the C-terminal properties of secreted amyloid β protein (Aβ). PS2 was identified as a 50- to 55-kDa protein, which was cleaved to produce N-terminal fragments of 35–40 kDa and C-terminal fragments of 19–23 kDa. The Volga German (N141I) mutation did not cause any significant change in the metabolism of PS2. COS-1 cells doubly transfected with cDNAs for N141I mutant PS2 and human β-amyloid precursor protein (βAPP) or a C-terminal fragment thereof, as well as mouse Neuro2a neuroblastoma cells stably transfected with N141I mutant PS2 alone, secreted 1.5- to 10-fold more Aβ ending at residues 42 (or 43) [Aβ42(43)] compared with those expressing the wild-type PS2. These results strongly suggest that the PS2 mutation (N141I) linked to FAD alters the metabolism of Aβ/βAPP to foster the production of the form of Aβ that most readily deposits in amyloid plaques. Thus, mutant PS2 may lead to AD by altering the metabolism of Aβ/βAPP.
Alzheimer disease (AD) is a neurodegenerative disorder of the elderly characterized clinically by progressive dementia, and pathologically by neuronal loss in the cerebral cortex accompanied by massive accumulations of two types of abnormal fibrous proteins—i.e., amyloid β protein (Aβ) as amyloid deposits and hyperphosphorylated tau forming paired helical filaments (1). A significant proportion of early-onset AD (onset <60 years) is inherited as an autosomal dominant trait (2). Missense mutations of the β-amyloid precursor protein (βAPP) gene located on chromosome 21 were identified in a few families initially (3, 4). These mutations were shown to increase the secretion of total Aβ (5, 6) or that of the Aβ species ending at the 42nd (or 43rd) residues [Aβ42(43)] (7). Aβ42(43) is a relatively minor molecular species of the Aβ secreted from cells, but it has a much higher propensity to aggregate and form amyloid than other species of Aβ (8). These findings provide strong support for the hypothesis that the deposition of Aβ [and especially Aβ42(43)] is closely related to the pathogenesis of AD.
However, ≈60% of the pedigrees of early-onset familial AD (FAD) were subsequently found to be linked to chromosome 14 (9), and the presenilin 1 (PS1) gene was identified as the gene that harbors mutations that cause this type of FAD (termed AD3) (10). PS1 is a novel 467-aa long integral membrane protein localized to intracellular membranous compartments, especially the endoplasmic reticulum (11–13), that spans the membrane 7–9 times (10, 14, 15). More than 30 missense mutations (10, 14, 16) or amino acid deletions (17) have been identified so far in a number of AD3 pedigrees. Shortly after the discovery of PS1, the gene responsible for FAD in the so-called Volga German families was found on chromosome 1 (18). This gene is known as PS2 and it is highly homologous to PS1 (67% identical) (19). A point mutation in PS2 that results in the substitution of Asn-141 by Ile (N141I) was identified in affected patients from Volga German families (19), and another missense mutation (i.e., M239V) that causes FAD has been reported (20). However, information about the metabolism and function of PS1 and PS2 is still incomplete. Very recently, endoproteolytic processing of PS1 at its 6th loop domain was shown to generate an ≈27-kDa N-terminal and an ≈17-kDa C-terminal fragment (21), but it is still unknown whether PS2 undergoes similar endoproteolytic cleavage during normal metabolism. Moreover, the normal functions of PS1 and PS2 as well as the mechanisms that lead to AD as a result of missense mutations in these genes are still unknown.
One mechanism whereby these mutations in PS1 and PS2 could cause AD may be related to the deposition of Aβ. Indeed, recent data showing that the level of Aβ42(43) is increased in the plasma of FAD patients with mutations in PS1 or PS2 (22) strongly support the notion that these mutations alter the proteolytic processing of βAPP (especially at the C terminus of Aβ which is termed γ-cleavage), thereby fostering the production and deposition of Aβ42(43) as well as the clinical manifestations of AD. However, there is still little evidence that mutations in the PS1 or PS2 genes produce increased amounts of Aβ42(43). To address these issues, we performed transfection experiments in cultured cells using wild-type PS2 cDNAs as well as cDNAs with the N141I mutation found in FAD patients from Volga German families to examine the metabolism of PS2 as well as the levels and C-terminal properties of Aβ secreted from these cells.
EXPERIMENTAL PROCEDURES
Construction of PS2 Expression Vectors.
A full-length cDNA encoding human PS2 was generated by PCR using primers anchored with BamHI (5′ end) and XhoI (3′ end) sites from a normal human cDNA library (CLONTECH). A cDNA encoding human PS1 was similarly generated using primers anchored with a HindIII site at the 5′ end. The amplified cDNAs were subcloned into pBluescript (Stratagene) and sequenced using an automated sequencer (Li-Cor, Lincoln, NE). The coding region of PS2 or PS1 was then subcloned into a mammalian expression vector (pcDNA3; Invitrogen). The N141I mutation in PS2 was introduced by the dU-template method (23). All constructs were sequenced to verify the nucleic acid sequences as well as the identity and location of the desired mutation. The wild-type and 595/596 (KM-NL) mutant human βAPP695 cDNAs as well as a cDNA encoding the C-terminal 100 aa (C100) of βAPP were subcloned into an expression vector [p91023(E)] as described (24, 25).
Mammalian Cell Culture and Transfection.
Green monkey kidney cells (COS-1) and mouse Neuro2a neuroblastoma (N2a) cells were cultured in DMEM supplemented with 10% fetal calf serum. COS-1 cells were transfected with the appropriate pcDNA3 construct using the DEAE-dextran method as described (24, 25). For double transfection, COS-1 cells were transfected with cDNAs encoding PS2 in the pcDNA3 vector and βAPP in the p91023(E) vector. Stable N2a cells were generated by transfecting the cDNA in the pcDNA3 vector using a mammalian cell transfection kit (Stratagene) and selection in G418 (GIBCO) at 400 μg/ml.
Antibodies.
Polyclonal antibodies were raised in rabbits against synthetic peptides corresponding to the following predicted amino acid sequences of PS2 or PS1: anti-PS2L against residues 316–339 of PS2 [plus cysteine (Cys) at the N terminus], anti-PS1L against 309–336 of PS1 (plus Cys at the N and C termini), anti-PSC1/2 against 462–467 of PS1 (plus Cys at the N terminus), and anti-PS1N against 1–22 of PS1 (plus Cys at the C terminus). The immunogen peptides were conjugated to keyhole limpet hemocyanin and the antisera were affinity purified as described (26). Anti-PSC1/2 crossreacted with PS2, but did not react with PS1 on immunoblots (see Results); however, it labeled both PS1 and PS2 overexpressed in cells by immunofluorescence (data not shown). Polyclonal antiserum 2972 was raised in rabbits following immunization with affinity-purified human PS2 N-terminal glutathione S-transferase fusion protein (13). mAb 22C11 that recognize the N terminus of human βAPP was purchased from Boehringer Mannheim.
Protein Extraction and Immunoblot Analyses.
COS-1 cells were lysed 48 hr after transfection in 2% SDS buffer, briefly sonicated, and incubated at 4°C overnight. For the N2a cells, confluent cultures were homogenized in TSI buffer [50 mM Tris·HCl, pH 7.6/150 mM NaCl containing various protease inhibitors (27)] and centrifuged at 100,000 × g for 1 hr. The pellet was incubated in TSI buffer containing 1% Triton X-100 for 30 min at 4°C, centrifuged at 100,000 × g for 1 hr, and the resulting supernatants were collected. Next, aliquots of each of the different samples were separated by SDS/PAGE without prior heating, transferred to polyvinylidene difluoride membranes (Millipore), and probed with each of the anti-PS or anti-βAPP antibodies. The immunoblots were developed using a chemiluminescence kit according to the instructions of the vendor (ECL; Amersham).
Quantitation of Aβ by Two-Site ELISAs.
Two-site ELISAs that specifically detect the C terminus of Aβ (BAN50/BA27 or BC05, BNT77/BA27 or BC05) were used (7, 28, 29). BAN50 is a mAb raised against a synthetic peptide of human-type Aβ1–16; it preferentially reacts with the N-terminal portion of full-length human Aβ starting at Asp-1, but does not crossreact with N terminally truncated Aβ nor with rodent-type Aβ (which differs from human Aβ at positions 5, 10, and 13). Another mAb (BNT77) raised against human Aβ11–28, recognizes full-length as well as N terminally truncated Aβ, and it binds rodent-type Aβ with an affinity similar to that of human Aβ (H. Fukumoto and T.I., unpublished data), but it does not react with the 3-kDa fragment (p3) beginning at the Leu-17 residue of Aβ (29). BA27 and BC05 are mAbs that specifically recognize the C termini of Aβ40 and Aβ42(43), respectively, and these mAbs were conjugated with horseradish peroxidase and then used as detector antibodies. The specificity and sensitivity of these ELISAs have been characterized previously (7, 28, 29). Using COS-1 cells doubly transfected with cDNAs encoding human βAPPs and wild-type or N141I mutant PS2, we collected culture media after 60 hr of transfection, and we examined these samples with BAN50/BA27 or BC05, and BNT77/BA27 or BC05 ELISAs. We also studied confluent cultures of N2a cells that were stably transfected with human PS2 cDNAs and replated to 24-well microplates. Accordingly, culture media were collected after 24 hr from replating and subjected to BNT77/BA27 or BC05 ELISAs.
RESULTS
Characterization of PS2 Expressed in Cultured Cells.
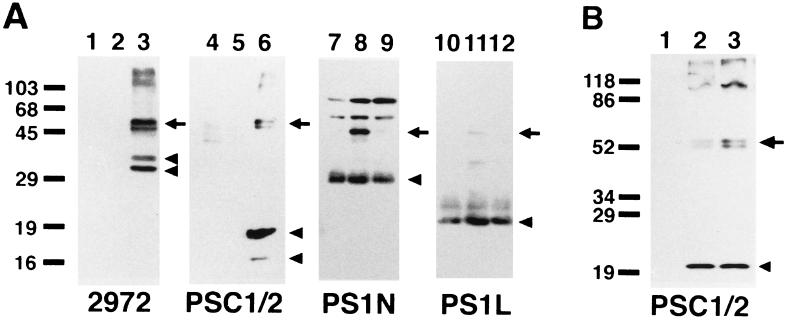
We transfected a cDNA encoding human PS2 into COS-1 cells and analyzed the cell lysates by Western blots using three PS2 antibodies (i.e., 2972, anti-PS2L, and anti-PSC1/2) that recognize the N terminus, 6th loop domain, and the C terminus of PS2, respectively. In cells transfected with a full-length wild-type PS2 cDNA, we detected 50- to 55-kDa polypeptides that run as closely migrating doublets and are reactive with the three PS2 antibodies (Fig. 1A, lanes 3 and 6, arrows). However, these bands were not observed in COS-1 cells transfected with the empty pcDNA3 expression vector (Fig. 1A, lanes 1 and 4). Because the position and banding patterns of these polypeptides were very similar to those reported for full-length PS2 tagged with FLAG in previous transfection experiments using COS-1 cells (11), we concluded that these polypeptides corresponded to full-length PS2. Moreover, 2972 against the N terminus of PS2 recognized two 35- to 40-kDa polypeptides (Fig. 1A, lane 3, arrowheads), whereas anti-PSC1/2 detected a 19-kDa polypeptide (Fig. 1A, lane 6, upper arrowhead), and occasionally, a faint ≈16-kDa band (Fig. 1A, lane 6, lower arrowhead), together with the 50- to 55-kDa polypeptides. Anti-PS2L recognized the 35- to 40-kDa polypeptides that were positive for 2972, as well as several minor fragments migrating at 25–35 kDa (data not shown). Because PS2 is a 448-aa protein that has a multiple membrane spanning structure similar to that of PS1 (19), we inferred that these 35- to 40-kDa and 19-kDa polypeptides represent proteolytic fragments of PS2 in transiently transfected COS-1 cells. However, in COS-1 cells transfected with the empty pcDNA3 expression vector (Fig. 1A, lanes 1 and 4), we could not consistently detect any of the 35- to 40-kDa or the 19-kDa polypeptides with our PS2 antibodies.
Figure 1.
PS2 expression and metabolism in transiently transfected COS-1 cells. (A) Western blot analysis of human PS2 expression in comparison with that of PS1 in transiently transfected COS-1 cells. Cell lysates (10 μg protein) from COS-1 cells transfected with empty pcDNA3 vector (lanes 1, 4, 7, 10), with wild-type human PS1 (lanes 2, 5, 8, and 11), or PS2 (lanes 3, 6, 9, and 12) cDNAs were fractionated by SDS/PAGE and analyzed by immunoblotting with 2972 (raised against the N terminus of PS2) (lanes 1–3), anti-PSC1/2 (lanes 4–6), anti-PS1N (lanes 7–9), and anti-PS1L (lanes 10–12) antibodies. Full-length PS proteins are marked by arrows, and fragments thereof by arrowheads. Molecular mass standards are shown in kilodaltons. (B) Comparison of proteolytic processing of wild-type and N141I mutant PS2. Cell lysates from nontransfected COS-1 cells (lane 1), cells transfected with wild-type human PS2 (lane 2), or N141I mutant PS2 (lane 3) cDNAs were probed with anti-PSC1/2.
We then transfected the cDNA encoding the N141I mutant PS2 (i.e., the one linked to FAD in Volga German families) into COS-1 cells to see if this would result in any differences in the amount or size of the PS2 polypeptides as well as in the proteolytic fragments thereof. However, the Western blot profiles of the N141I mutant and wild-type PS2 proteins were almost identical (Fig. 1B, lanes 2 and 3).
We next compared the processing of PS2 to that of PS1 by transfecting the cDNA encoding human wild-type PS1 into COS-1 cells and probing Western blots of homogenates of these cells with anti-PS1 antisera. In contrast with PS2, we detected an ≈30-kDa polypeptide with anti-PS1N specific for the N terminus of PS1, and an ≈20-kDa polypeptide with anti-PS1L in COS-1 cells transfected with the PS1 cDNA (Fig. 1A, lanes 8 and 11, arrowheads) as well as in cells with empty vectors (Fig. 1A, lanes 7 and 10, arrowheads), although anti-PSC1/2 did not react with the ≈20-kDa C-terminal fragment of PS1 for some unknown reason (Fig. 1A, lane 5). In addition, we also detected full-length PS1 (Fig. 1A, lanes 8 and 11, arrows) which migrated a bit faster than full-length PS2 (Fig. 1A, lanes 3 and 6, arrows).
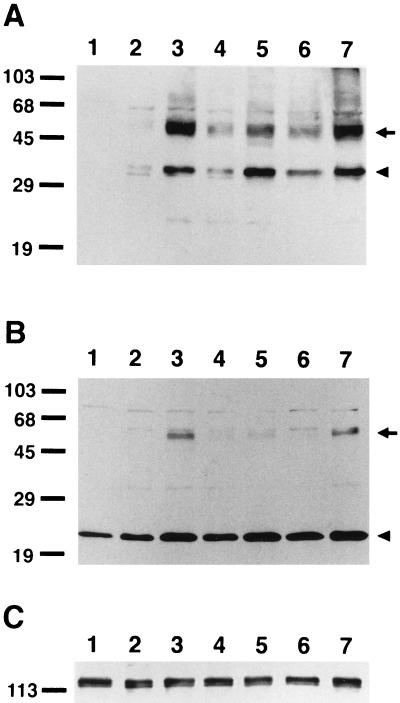
We then studied the expression of PS2 in N2a cells stably transfected with wild-type or N141I mutant PS2. Antiserum 2972 (Fig. 2A) and anti-PS2L (Fig. 2B) specifically detected the 50- to 55-kDa full-length PS2 immunobands closely migrating as doublets in all of the cell lines expressing wild-type (Fig. 2 A and B, lanes 2–4, arrow) or N141I mutant (Fig. 2 A and B, lanes 5–7, arrow) PS2, with some variations in the levels of expression among cell lines. However, these polypeptides were not observed in N2a cells transfected with empty pcDNA3 vector (Fig. 2 A and B, lane 1). Furthermore, 2972 detected a 35-kDa polypeptide (Fig. 2A, lanes 1–7, arrowhead), whereas anti-PS2L recognized a 23-kDa band (Fig. 2B, lanes 1–7, arrowhead) in N2a cells stably transfected with PS2 (Fig. 2 A and B, lanes 2–7). The amounts of these fragments correlated with the levels of full-length PS2. Moreover, a small amount of these fragments, especially the 23-kDa species, were detected also in cells transfected with empty vector (Fig. 2B, lane 1). The Western blot profiles of wild-type (Fig. 2 A and B, lanes 2–4) and the N141I mutant (Fig. 2 A and B, lanes 5–7) PS2 proteins were again almost identical.
Figure 2.
Expression of PS2 and βAPP in stable N2a cells. Western blot analysis of stable N2a cells transfected with human PS2 cDNAs. Triton X-100 soluble fractions from lysates of N2a cells (10 μg protein) transfected with an empty vector (lane 1), cDNAs encoding wild-type (lanes 2–4, corresponding to clones PS2.a–c in Fig. 3B, respectively), or N141I mutant human PS2 (lanes 5–7, corresponding to clones PS2N141I.a–c in Fig. 3B, respectively) were fractionated by SDS/PAGE and analyzed by immunoblotting with 2972 (raised against the N terminus of PS2) (A), anti-PS2L (B), and 22C11 (C). Immunobands representing full-length PS2 and fragments thereof are marked by arrows and arrowheads, respectively, in A and B. Molecular mass standards are shown in kilodaltons.
Characterization of Aβ Secreted from Cells Expressing Wild-Type or N141I Mutant PS2 Genes.
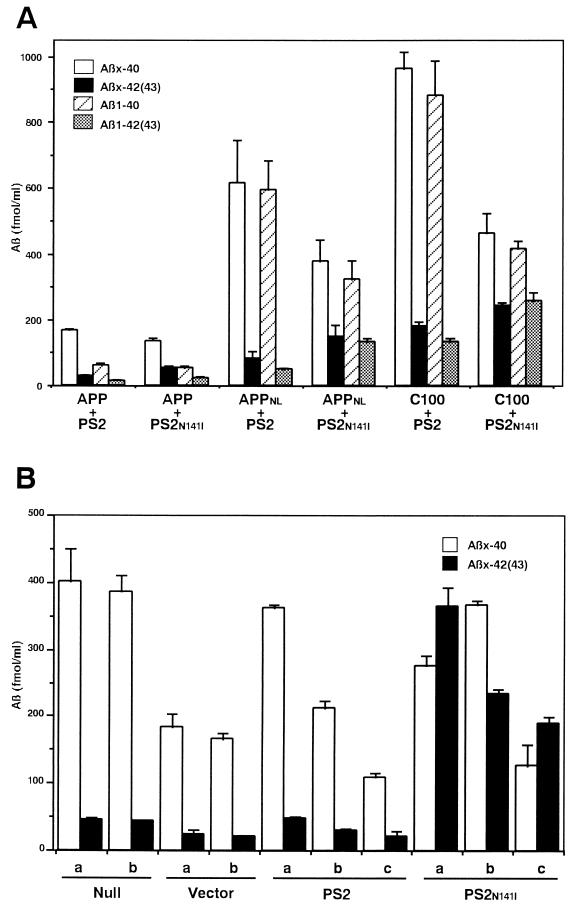
To examine the effects of the N141I mutation in PS2 on the metabolism of βAPP/Aβ as well as on the C-terminal properties of secreted Aβ, we used two different experimental systems. First, we doubly transfected COS-1 cells with a cDNA encoding either the wild-type human βAPP695, or the same construct with the 595/596 (KM-NL) double mutation (Swedish type) or with a C-terminal fragment of human βAPP695 starting from βAPP597 (C100), together with either the wild-type PS2 or the N141I mutant form of PS2. Although double immunofluorescence studies showed that the percentage of cells expressing PS2 was 2–4 times higher (20–40% of total cells) than the percentages of cells expressing βAPP, ≈80% of cells expressing βAPP695 also were positive for PS2 (data not shown). Because COS-1 cells transfected only with cDNAs encoding PS2 did not secrete detectable amounts of Aβ, the changes in the properties of secreted Aβ should largely derive from cells doubly transfected with βAPP and PS2. In double transfection experiments, the N141I mutant PS2 invariably increased the levels of secreted Aβ42(43) by 1.5- to 2-fold compared with those with wild-type PS2, whereas the secretion of Aβ40 was decreased by 20–50% (Fig. 3A). These effects were observed consistently irrespective of the type of βAPP695 cDNAs, although the levels of secreted Aβ were higher with the 595/596 (KM-NL) βAPP695 mutant and the C100 cDNAs (Fig. 3A). Finally, the PS2 mutant resulted in a 1.5- to 3.8-fold increase in the percentage of total Aβ [= Aβ40 + Aβ42(43)] comprised by Aβ42(43) in cells transfected with the N141I mutant PS2 (Table 1).
Figure 3.
Secreted Aβ40 and Aβ42(43) from cells expressing wild-type or N141I mutant PS2 genes. (A) Levels of Aβx-40, Aβx-42(43), Aβ1–40, and Aβ1–42(43) secreted from COS-1 cells doubly transfected with human βAPP and PS2 genes quantitated by two-site ELISAs. Mean values ± SE in three independent experiments are shown. Combinations of the transfected cDNAs are indicated below the columns: PS2, wild-type PS2; PS2N141I, N141I mutant PS2; APP, wild-type human βAPP695; APPNL, 595/596 (KM-NL) mutant human βAPP695; C100, C-terminal fragment of human βAPP. (B) Levels of Aβx-40 and Aβx-42(43) secreted from N2a cells stably transfected with PS2 cDNAs. Mean values ± SE (n = 4) from three independent cell lines (PS2.a–c transfected with wild-type PS2 and PS2N141I.a–c with N141I mutant PS2 cDNAs) are shown together with those from two nontransfected cell lines (Null.a and b) and cells transfected with empty vector (Vector.a and b).
Table 1.
Percentage of total Aß comprised by Aß42(43) secreted from cells expressing wild-type or N141I mutant PS2 genes
| COS-1 Cells
|
Neuro2a Cells
|
||||
|---|---|---|---|---|---|
| cDNAs | %Aßx-42(43) | %Aß1-42(43) | Clone | %Aßx-42(43) | Average |
| Null | 16.2 | ND | Null.a | 10.2 | 10.2 |
| PS2 | ND | ND | Null.b | 10.1 | |
| PS2N141I | ND | ND | Vector.a | 11.9 | 11.5 |
| APP | 13.3 | 14.7 | Vector.b | 11.1 | |
| APP + PS2 | 14.9 | 19.9 | PS2.a | 11.6 | |
| APP + PS2N141I | 27.9 | 30.7 | PS2.b | 12.7 | 13.8 |
| APPNL | 8.7 | 6.8 | PS2.c | 17.0 | |
| APPNL + PS2 | 12.1 | 7.7 | PS2N141I.a | 57.0 | |
| APPNL + PS2N141I | 28.8 | 29.4 | PS2N141I.b | 39.0 | 52.0 |
| C100 | 10.4 | 15.4 | PS2N141I.c | 59.9 | |
| C100 + PS2 | 15.8 | 13.2 | |||
| C100 + PS2N141I | 34.6 | 38.5 | |||
Percentages of total Aß [= Aßx-40 + Aßx-42(43) or Aß1-40 + Aß1-42(43)] comprised by Aßx-42(43) or Aß1-42(43), respectively, are shown. Abbreviations for cDNAs transfected into COS-1 cells and the names of N2a clones are as in Fig. 3. The “+” means double transfection. Null, nontransfected; vector, transfected with empty pcDNA3 vector; ND, not detected.
We then examined Aβ secreted from N2a cells stably transfected with the cDNA encoding the N141I mutant PS2. It has been shown that N2a cells constitutively secrete rodent-type Aβ species that are detectable with the BNT77/BA27 or BC05 system, and that the percentage of Aβ42(43) that comprises the total Aβ is ≈10% (29). Transfection of the empty pcDNA3 vector or wild-type PS2 decreased the secretion of total Aβ by 40–50% (Fig. 3B), whereas it did not change the percentage of Aβ42(43) (Table 1). The total amount of cellular βAPP was at similar levels among N2a cells transfected with empty pcDNA3 vector (Fig. 2C, lane 1), wild-type PS2 (lanes 2–4) or N141I mutant PS2 (lanes 5–7). However, when the N141I mutant PS2 was transfected, the total level of secreted Aβ42(43) was dramatically increased by 5–10 times compared with those in cells transfected with wild-type PS2, whereas the secretion of Aβ40 remained at similar levels (Fig. 3B). These changes were consistently observed in three independent cell lines expressing the N141I mutant PS2 (Fig. 3B), and they resulted in a 3.8-fold increase in the percentage of secreted Aβ42(43) from 13.8% (mean of wild-type PS2) to 52.0% (mean of N141I mutant PS2) (Table 1).
DISCUSSION
In this study, we have clearly shown that (i) human PS2 is a polypeptide with molecular weight of 50–55 kDa, which is cleaved to form N-terminal fragments of 35–40 kDa and a C-terminal fragment of 19 kDa (in COS-1 cells) or 23 kDa (in N2a cells); (ii) the expression and processing of PS2 are similar in cells transfected with wild-type and N141I mutant PS2 cDNAs; and (iii) the N141I mutation in PS2 that is linked to FAD (Volga German families) increases the secretion of Aβ42(43) in mammalian cells transiently or stably transfected with this mutant gene.
Thinakaran et al. (21) showed that PS1 undergoes endoproteolytic processing in vivo to form ≈27 kDa N-terminal and ≈17 kDa C-terminal fragments. Notably, our observations on the fragmentation of PS2 were very similar to those described for PS1, with some exceptions. (i) Antibody 2972 raised against the N terminus of PS2 recognized the 35- to 40-kDa fragments of PS2 in transiently transfected COS-1 cells, whereas the N-terminal fragment of PS2 in stable N2a cells migrated at a slightly faster position at 35 kDa. Furthermore, the C-terminal fragment of PS2 in transiently transfected COS-1 cells migrated at 19 kDa, whereas a C-terminal fragment of 23 kDa was detected with an antibody against the loop domain of PS2 in N2a cells. Thus, the mode of PS2 processing could be somewhat heterogenous between different cell types as well as depending on the methods of transfection. (ii) We failed to detect the N- and C-terminal fragments of PS2 in COS-1 cells transfected with empty vectors, although N- and C-terminal fragments of PS1 were observed in the same samples. In contrast, we could detect a small amount of the N- and C-terminal fragments of PS2 in N2a cells transfected with an empty vector alone, and the amount of PS2 fragments seemed to increase upon transfection and correlate with the levels of full-length PS2. These results suggest that the amount of endogenous PS2 fragments is relatively small because of the lower levels of expression (20), or rapid degradation, compared with those of PS1. Furthermore, wild-type PS2 and N141I mutant PS2 exhibited very similar expression and processing patterns in transiently transfected COS-1 cells as well as in stable N2a cells. This strongly suggests that the N141I mutation does not lead to AD through the changes in the proteolytic processing of PS2.
The most intriguing finding obtained in this study was the consistent increase in the secretion of Aβ42(43) from cells transfected with a cDNA encoding N141I mutant PS2 linked to FAD (Volga German families). This effect was constantly observed not only in a transient double-expression system in COS-1 cells, regardless of the types of cotransfected βAPP695 cDNAs, but also in stably transfected mouse N2a cells where rodent-type βAPP serves as the substrate for Aβ production. To our knowledge, these data provide the first direct evidence that the N141I mutation of PS2 alters the generation of the C terminus of Aβ (i.e., γ-cleavage) in a manner to foster the production and deposition of Aβ42(43). Very recently, it was shown that PS1 mutations linked to AD3 also increase the levels of Aβ42(43) secreted from transfected cells (30) as well as in the brains of transgenic mice (30, 31). However, the elevation in the ratio of secreted Aβ42(43) in N2a cells expressing mutant PS1 was relatively modest (9.8% in cells expressing wild-type PS1 vs. 13.5–17.0% in cells expressing mutant PS1) (30) compared with our N2a cells expressing PS2 (13.8% in cells expressing wild-type PS2 vs. 52.0% in cells expressing N141I mutant PS2). These data are not apparently consistent with the milder clinical course and less abundant Aβ42(43) burden in patients with the Volga German families compared with those in patients with PS1 mutations (32, 33). The reason for these discrepancies is not known at present. One possibility would be that exogenously introduced mutant PS2 protein could have stronger effects than PS1, because the levels of endogenous PS2 (including those of fragments) are lower than those of PS1. In any event, it is highly conceivable that mutant forms of PS linked to FAD may interact with βAPP or with γ-secretases in intracellular membranous compartments, and alter the metabolism of Aβ/βAPP by shifting production and/or secretion of Aβ species from the normally predominant Aβ40 to Aβ42(43), thereby leading to AD. Indeed, these data are in agreement with our in vivo observations on the postmortem brains from patients with FAD linked to mutations of PS1 and PS2 showing that Aβ42(43) also was the predominant Aβ species deposited in these brains (32, 33).
Further studies are needed to elucidate the mechanisms that account for the effects of PS mutations on Aβ generation and the development of AD. Nonetheless, the present findings provide strong support for the hypothesis that the production and deposition of Aβ, especially Aβ42(43), plays a crucial pathogenetic role in AD.
Acknowledgments
We thank H. Kuzume, R. Hosoda, T. Kosaka, and T. Hashimoto for skillful technical assistance, J. Q. Trojanowski and D. J. Selkoe for helpful comments, and Takeda Chemical Industries for continuous support. This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture, Japan, the Mitsubishi Foundation, the Kato Memorial Trust for Nanbyo Research, and grants from the Deutsche Forschungsgemeinschaft (SFB 317) and from Boehringer Ingelheim KG.
ABBREVIATIONS
- Aβ
amyloid β protein
- AD
Alzheimer disease
- FAD
familial AD
- βAPP
β-amyloid precursor protein
- N2a
mouse Neuro2a neuroblastoma
- PS
presenilin
References
- 1.Selkoe D J. J Biol Chem. 1996;271:18295–18298. doi: 10.1074/jbc.271.31.18295. [DOI] [PubMed] [Google Scholar]
- 2.Schellenberg G D. Proc Natl Acad Sci USA. 1995;92:8552–8559. doi: 10.1073/pnas.92.19.8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goate A, Chartier-Harlin M-C, Mullan M, Brown J, Crawford F, et al. Nature (London) 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 4.Mullan M, Crawford F, Axelman K, Houldin H, Lilius L, Winblad B, Lannfelt L. Nat Genet. 1992;1:345–347. doi: 10.1038/ng0892-345. [DOI] [PubMed] [Google Scholar]
- 5.Citron M, Oltersdolf T, Haass C, McConlogue L, Hung A Y, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe D J. Nature (London) 1992;360:672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- 6.Cai X-D, Golde T E, Younkin S G. Science. 1993;259:514–516. doi: 10.1126/science.8424174. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki N, Cheung T T, Cai X-D, Odaka A, Otvos L, Jr, Eckman C, Golde T E, Younkin S G. Science. 1994;264:1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- 8.Jarrett J T, Berger E P, Lansbury P T., Jr Biochemistry. 1993;32:4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- 9.St George-Hyslop P H, Haines J, Rogaev E, Mortilla M, Vaula G, et al. Nat Genet. 1992;2:330–334. doi: 10.1038/ng1292-330. [DOI] [PubMed] [Google Scholar]
- 10.Sherrington R, Rogaev E I, Liang Y, Rogaeva E A, Levesque G, et al. Nature (London) 1995;375:754–760. [Google Scholar]
- 11.Kovacs D M, Fausett H J, Page K J, Kim T W, Moir R D, Merriam D E, Hollister R D, Hallmark O G, Mancini R, Felsenstein K M, Hyman B T, Tanzi R E, Wasco W. Nat Med. 1996;2:224–229. doi: 10.1038/nm0296-224. [DOI] [PubMed] [Google Scholar]
- 12.Cook D G, Sung J C, Golde T E, Felsenstein K M, Wojczyk B S, Tanzi R E, Trojanowski J Q, Lee V M-Y, Doms R W. Proc Natl Acad Sci USA. 1996;93:9223–9228. doi: 10.1073/pnas.93.17.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walter J, Capell A, Grünberg J, Pesold B, Schindzielorz A, Prior R, Podlisny M B, Fraser P, St George-Hyslop P, Selkoe D J, Haass C. Mol Med. 1996;2:673–691. [PMC free article] [PubMed] [Google Scholar]
- 14.Alzheimer’s Disease Collaborative Group. Nat Genet. 1995;11:219–222. doi: 10.1038/ng1095-219. [DOI] [PubMed] [Google Scholar]
- 15.Doan A, Thinakaran G, Borchelt D R, Slunt H H, Ratovitsky T, Podlisny M, Selkoe D J, Seeger M, Gandy S E, Price D L, Sisodia S S. Neuron. 1996;17:1023–1030. doi: 10.1016/s0896-6273(00)80232-9. [DOI] [PubMed] [Google Scholar]
- 16.Wasco W, Pettingell W P, Jondro P D, Schmidt S D, Gurubhagavatula S, Rodes L, DiBlasi T, Romano D M, Guenette S Y, Kovacs D M, Growdon J H, Tanzi R E. Nat Med. 1995;1:848. doi: 10.1038/nm0995-848a. [DOI] [PubMed] [Google Scholar]
- 17.Perez-Tur J, Froelich S, Prihar G, Crook R, Baker M, et al. NeuroReport. 1995;7:297–301. [PubMed] [Google Scholar]
- 18.Levy-Lahad E, Wijsman E M, Nemens E, Anderson L, Goddard K A, Weber J L, Bird T D, Schellenberg G D. Science. 1995;269:970–973. doi: 10.1126/science.7638621. [DOI] [PubMed] [Google Scholar]
- 19.Levy-Lahad E, Wasco W, Poorkaj P, Romano D M, Oshima J, Pettingell W H, Yu C-e, Jondro P D, Schmidt S D, Wang K, Crowley A C, Fu Y-H, Guenette S Y, Galas D, Nemens E, Wijsman E M, Bird T D, Schellenberg G D, Tanzi R E. Science. 1995;269:973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- 20.Rogaev E I, Sherrington R, Rogaeva E A, Levesque G, Ikeda M, et al. Nature (London) 1995;376:775–778. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- 21.Thinakaran G, Borchelt D R, Lee M K, Slunt H H, Spitzer L, Kim G, Ratovitsky T, Davenport F, Nordstedt C, Seeger M, Hardy J, Levey A I, Gandy S E, Jenkins N A, Copeland N G, Price D L, Sisodia S S. Neuron. 1996;17:181–190. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 22.Scheuner D, Eckman C, Jensen M, Song X, Citron M, et al. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 23.Kunkel T A. Proc Natl Acad Sci USA. 1985;82:488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maruyama K, Tagawa K, Kawamura Y, Asada H, Ishiura S, Obata K. Biochem Biophys Res Commun. 1995;207:971–977. doi: 10.1006/bbrc.1995.1280. [DOI] [PubMed] [Google Scholar]
- 25.Maruyama K, Terakado K, Usami M, Yoshikawa K. Nature (London) 1990;347:566–569. doi: 10.1038/347566a0. [DOI] [PubMed] [Google Scholar]
- 26.Saido T C, Yokota M, Maruyama K, Yamao-Harigaya W, Tani E, Ihara Y, Kawashima S. J Biol Chem. 1994;269:15253–15257. [PubMed] [Google Scholar]
- 27.Iwatsubo T, Yamaguchi H, Fujimuro M, Yokosawa H, Ihara Y, Trojanowski J Q, Lee V M-Y. Am J Pathol. 1996;148:1517–1529. [PMC free article] [PubMed] [Google Scholar]
- 28.Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 29.Asami-Odaka A, Ishibashi Y, Kikuchi T, Kitada C, Suzuki N. Biochemistry. 1995;34:10272–10278. doi: 10.1021/bi00032a022. [DOI] [PubMed] [Google Scholar]
- 30.Borchelt D R, Thinakaran G, Eckman C B, Lee M K, Davenport F, et al. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 31.Duff K, Eckman C, Zehr C, Yu X, Prada C-M, Perez-tur J, Hutton M, Buee L, Harigaya Y, Yager D, Morgan D, Gordon M N, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S. Nature (London) 1996;383:710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- 32.Mann D M A, Iwatsubo T, Cairns N J, Lantos P L, Nochlin D, Sumi S M, Bird T D, Poorkaj P, Hardy J, Hutton M, Prihar G, Crook R, Rossor M N, Haltia M. Ann Neurol. 1996;40:149–156. doi: 10.1002/ana.410400205. [DOI] [PubMed] [Google Scholar]
- 33.Mann D M A, Iwatsubo T, Nochlin D, Sumi S M, Levy-Lahad E, Bird T D. Ann Neurol. 1997;41:52–57. doi: 10.1002/ana.410410110. [DOI] [PubMed] [Google Scholar]