Abstract
In mice, the mdr1a and mdr1b genes encode drug-transporting proteins that can cause multidrug resistance in tumor cells by lowering intracellular drug levels. These P-glycoproteins are also found in various normal tissues such as the intestine. Because mdr1b P-glycoprotein is not detectable in the intestine, mice with a homozygously disrupted mdr1a gene [mdr1a(−/−) mice] do not contain functional P-glycoprotein in this organ. We have used these mdr1a(−/−) mice to study the effect of gut P-glycoprotein on the pharmacokinetics of paclitaxel. The area under the plasma concentration-time curves was 2- and 6-fold higher in mdr1a(−/−) mice than in wild-type (wt) mice after i.v. and oral drug administration, respectively. Consequently, the oral bioavailability in mice receiving 10 mg paclitaxel per kg body weight increased from only 11% in wt mice to 35% in mdr1a(−/−) mice. The cumulative fecal excretion (0–96 hr) was markedly reduced from 40% (after i.v. administration) and 87% (after oral administration) of the administered dose in wt mice to below 3% in mdr1a(−/−) mice. Biliary excretion was not significantly different in wt and mdr1a(−/−) mice. Interestingly, after i.v. drug administration of paclitaxel (10 mg/kg) to mice with a cannulated gall bladder, 11% of the dose was recovered within 90 min in the intestinal contents of wt mice vs. <3% in mdr1a(−/−) mice. We conclude that P-glycoprotein limits the oral uptake of paclitaxel and mediates direct excretion of the drug from the systemic circulation into the intestinal lumen.
P-glycoproteins, encoded by the human MDR1 or the murine mdr1a (also called mdr3) and mdr1b (also called mdr1) genes are drug efflux pumps localized in the plasma membrane. They can reduce the intracellular accumulation of a wide range of compounds, including cytotoxic drugs such as vinca alkaloids and anthracyclines (1–3). Although initially discovered by their ability to cause multidrug resistance in mammalian tumor cells, these P-glycoproteins are also present in many normal tissues (4–13).
The normal physiological function of these P-glycoproteins is still a matter of conjecture, but the idea that they serve to protect the organism against toxins is supported by several studies (4–6). Drug-transporting P-glycoproteins are found in particular in organs that have an excretory function, with P-glycoprotein being localized at the apical side of the epithelial layer (e.g., the bile canalicular membrane and the renal proximal tubules) (4, 7–9). Furthermore, P-glycoproteins are found in tissues that act as a barrier, such as the brain capillary endothelial cells that form the blood-brain barrier and the brush border of the intestine (5, 10–13). The latter is of particular interest because food ingestion exposes the body to a wide variety of potentially harmful compounds (14). P-glycoprotein in the gut epithelium could therefore protect against these xenotoxins, and indirect evidence for this hypothesis has been reported (15–18).
Recently, Schinkel et al. (10) generated mice with a disruption of the mdr1a gene [mdr1a(−/−) mice] to study the physiological and pharmacological role of P-glycoproteins. The extreme sensitivity of these mdr1a(−/−) mice for the central neurotoxic pesticide ivermectine has clearly demonstrated the important protective function of P-glycoprotein in the blood-brain barrier (10). Further studies with vinblastine in these mice demonstrated a lower body clearance and a reduced fecal excretion (19), which suggested that P-glycoprotein may contribute to drug elimination by mediating biliary excretion and/or limiting (re-)uptake from the intestinal lumen after hepatobiliary excretion. We have studied this possibility in more detail with the cytotoxic agent paclitaxel (Taxol). (The United States Adopted Name Council has given the name paclitaxel to refer to the registered trade name Taxol; Bristol–Myers Squibb.) Paclitaxel has become a very important drug in the treatment of cancer (20, 21), and it is also a very good substrate of P-glycoprotein, as was shown by in vitro transport studies in polarized monolayers of an MDR1-transfected pig kidney epithelial cell line (LLC-PK1; unpublished results). Our pilot experiments confirmed an earlier study, reporting that the concentration of paclitaxel in plasma of mice remained very low after oral drug administration (22). These observations raised the possibility that this low bioavailability may result from P-glycoprotein activity in the gut. We have, therefore, tested the fate of orally and i.v.-administered paclitaxel in wild-type (wt) and mdr1a(−/−) mice. Our results show that P-glycoprotein in the intestine limits the uptake of orally administered paclitaxel and suggest that P-glycoprotein may serve to protect the body against a broad range of orally ingested xenotoxins that are substrates of this transport protein.
MATERIALS AND METHODS
Chemicals.
Paclitaxel (batch 80617492D), 2′-methylpaclitaxel, and paclitaxel for injection (Taxol) supplied as a concentrated sterile solution with 6 mg/ml in a 5 ml vial in Cremophor EL and dehydrated ethanol (United States Pharmacopeia; 1:1, vol/vol) originated from Bristol–Myers Squibb. [3H]Paclitaxel (batches 114-195-014 and 125-075-0116; specific activities 16.4 and 14.5 mCi/mg; radiochemical purity >99%) was supplied by Moravek Biochemicals (Brea, CA). The major part of the tritium label is at the m- and p-positions in the aromatic rings, whereas a minor fraction is either at the 2- or 10-position in the taxane ring. Metabolite I (3′-p-hydroxypaclitaxel) and metabolite II (6α-hydroxypaclitaxel) were isolated from patient feces samples, as described in detail elsewhere (23). Hypnorm was from Janssen (Tilburg, The Netherlands) and Dormicum from Roche Nederland (Mijdrecht, The Netherlands). Lyophilized BSA was obtained from Organon Teknika–Cappel. Acetonitrile and methanol were of Lichrosolv gradient grade (Merck). All other chemicals were of the highest grade available commercially and were used as received. Water was deionized by the Milli-Q Plus system (Millipore). Blank human plasma was obtained from healthy volunteers.
Drug Analyses.
Paclitaxel and its hydroxylated metabolites were determined by liquid–liquid and solid-phase extraction, followed by reversed-phase HPLC with UV detection as described (24). Aliquots of 10–200 μl of plasma, bile, and homogenates of intestinal contents were also added to 3 ml of Ultima Gold scintillation fluid (Packard), and radioactivity was determined by liquid scintillation counting in a Tri-Carb Series 4000 Minaxi model B4430 counter (Packard) using quench correction by external standardization. Cremophor EL levels were determined by a novel HPLC method described in detail elsewhere (25).
Pharmacokinetic Studies.
Animals were housed and handled according to institutional guidelines complying with Dutch legislation. Female FVB wt and mdr1a(−/−) mice weighing 25 ± 5 g (10–14 weeks of age) were used in all experiments. They were given food (Hope Farms, Woerden, The Netherlands) and acidified water ad libitum. Plasma pharmacokinetic studies were carried out with animals receiving 10 or 5 mg/kg (body weight) of unlabeled paclitaxel, while other experiments were performed with a drug solution supplemented with [3H]paclitaxel (≈1 μCi per animal; 1 Ci = 37 GBq).
For oral administration, the commercially available paclitaxel formulation was used. This solution was diluted 10- or 20-fold with saline for the 10 and 5 mg/kg experiments, respectively. The solution used for the 5 mg/kg experiment was supplemented with Cremophor EL and ethanol to achieve the same vehicle exposure in both dose groups. Volumes of 16.7 ml/kg (body weight) of these solutions were administered by direct injection into the stomach using a blunt-ended needle inserted via the esophagus, resulting in dose levels of 10 and 5 mg/kg (body weight).
For i.v. administration, paclitaxel was formulated in polysorbate-80 and ethanol (1:1, vol/vol). This solution was diluted 2- or 4-fold with saline for the 10 and 5 mg/kg experiments, respectively. The required injection volume for both dose levels was 3.3 ml/kg and was administered into a tail vein. Blood samples for plasma pharmacokinetic studies were collected under diethyl ether anaesthesia. Animals receiving 10 mg/kg (body weight) of paclitaxel were sacrificed at 5, 15, 30, and 45 min, and at 1, 1.5, 2, 4, 6, and 8 hr after treatment, with 2–10 animals per time point. After oral administration of 5 mg/kg (body weight) blood samples were taken at 1, 2, 4, 6, and 8 hr, with 4 animals per time point. Samples were collected in heparinized tubes and centrifuged at 1500 × g for 10 min. Plasma was separated and stored at −20°C until analysis. Urine and feces were collected from wt and mdr1a(−/−) mice receiving 10 mg/kg (body weight) of paclitaxel by i.v. or oral administration (n = 4–6 per group). They were kept in Ruco type M/1 stainless-steel metabolic cages (Valkenswaard, The Netherlands), and specimens were collected during time intervals of 0–8, 8–24, 24–48, 48–72, and 72–96 hr.
To monitor the biliary excretion of the drug, mice were anaesthetized with a mixture of Hypnorm (fentanyl 0.2 mg/ml, fluanisone 10 mg/ml), Dormicum (midazolam 5 mg/ml), and water (1:1:2, vol/vol/vol). The volume of the mixture injected i.p. was 5–7 ml/kg body weight. After median laparotomy and ligation of the common bile duct, the gall bladder was cannulated using a polyethylene tube (Portex, Hythe, U.K.) with a 0.28 mm internal diameter. The tube was fixed in the gall bladder with additional ligation to prevent leakage. The temperature of the animals was monitored with a rectal probe and maintained at 36 ± 1°C using a standard heating pad and a lamp. The tissues at the open surface of the abdominal cavity were moistened with saline. Additional anaesthesia (≈30 μl) was also given directly in the opening of the abdominal cavity, if needed. Bile was collected for 90 min after drug administration (n = 4 per group). Next, blood was obtained from the axillary venous plexus and intestinal contents were removed. Intestinal contents and feces were homogenized in 4% (wt/vol) BSA and frozen at −20°C until analysis.
Pharmacokinetic Analysis.
The area under the plasma concentration-time curves (plasma AUC) after oral (AUCoral) and i.v. (AUCi.v.) administration was calculated by the linear trapezoidal rule, without extrapolation to infinity. The standard error of the AUC was calculated with the law of propagation of errors. The t1/2 after i.v. injection was calculated by linear regression analysis of the log-linear part of the plasma concentration-time curves. The clearance (Cl) was calculated by the formula Cl = dose/AUC and the oral bioavailability (F) by the formula F = AUCoral/AUCi.v. × 100%. The two-sided unpaired Student’s t-test was used for statistical analysis.
RESULTS
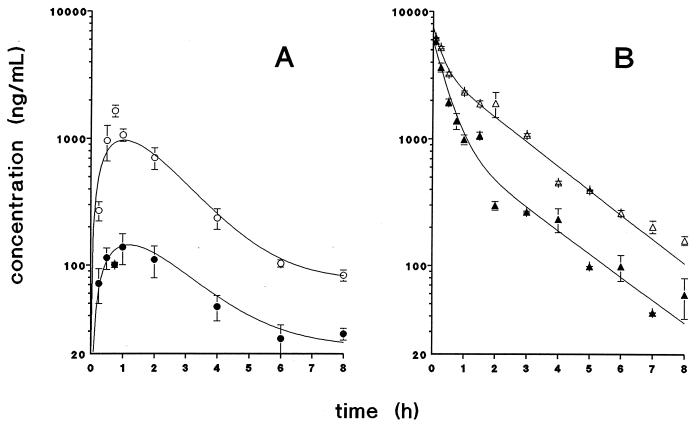
If P-glycoprotein in the intestinal mucosa of mice would limit the bioavailability of paclitaxel, this should result in higher plasma levels in mdr1a(−/−) mice than in wt mice after oral drug administration. This is borne out by the results shown in Fig. 1A. After administration of 10 mg paclitaxel/kg body weight, the plasma AUC is about 6-fold higher in mdr1a(−/−) mice than in wt mice. This difference in plasma AUC is reflected by the maximum plasma concentration, which is ≈10-fold higher in mdr1a(−/−) mice (Table 1). During the elimination phase the plasma levels remain ≈3-fold higher in mdr1a(−/−) mice.
Figure 1.
Plasma concentration-time curves of paclitaxel in female FVB wt (•/▴) and mdr1a(−/−) (○/▵) mice after oral (A) and i.v. (B) administration of paclitaxel (10 mg/kg). Data are shown as the mean concentration, and error bars represent the SEM (n = 2–10 per time point).
Table 1.
Plasma pharmacokinetic parameters after i.v. or oral administration of paclitaxel at 10 mg/kg to mice
| i.v. administration
|
Oral administration
|
|||
|---|---|---|---|---|
| Wild type | mdr1a(−/−) | Wild type | mdr1a(−/−) | |
| AUC(0–8), mg/(l·hr) | 4.45 ± 0.15 | 9.10 ± 0.33* | 0.50 ± 0.05 | 3.20 ± 0.25* |
| Cl, l/hr/kg | 2.24 ± 0.08 | 1.10 ± 0.04* | — | — |
| t½, hr | 1.66 ± 0.31 | 1.71 ± 0.08 | — | — |
| Cmax, mg/l | 5.84 ± 0.24 | 6.23 ± 0.02 | 0.14 ± 0.04 | 1.6 ± 0.17* |
| F, % | — | — | 11.2 | 35.2 |
AUC(0–8), area under the plasma concentration-time curve up to 8 hr; Cmax, maximum plasma levels; Cl, clearance; F, oral bioavailability.
P < 0.05.
Because the plasma AUC is determined by both uptake and elimination, we also treated mice with paclitaxel given by i.v. injection. After i.v. administration, maximum plasma levels of paclitaxel were equal in wt and mdr1a(−/−) mice (Fig. 1B). The plasma concentration-time curves coincide but diverge ≈30 min after drug administration, with a more rapid decay in wt animals. During the elimination phase the plasma levels are about 5-fold higher in mdr1a(−/−) mice. The difference in plasma concentration-time profiles after i.v. administration results in an approximately 2-fold higher AUC in mdr1a(−/−) mice (Table 1). Relative to their respective plasma AUC values after i.v. drug administration, the estimated bioavailability after oral administration (AUCoral/AUCi.v. × 100%) is 11.2% for wt and 35.2% for mdr1a(−/−) mice. Macroscopic signs of toxicity were absent in both mdr1a(−/−) and wt mice and thus cannot explain the increased bioavailability. Drug uptake after oral administration appears to be a nonlinear process in both wt and mdr1a(−/−) mice. When the dose was reduced by 50% to 5 mg/kg (body weight), the plasma AUC was about 4-fold lower [namely, 0.88 ± 0.04 mg/(l·hr)] in the mdr1a(−/−) mice. In wt mice the plasma AUC could not be calculated after oral administration of 5 mg/kg because the plasma levels hardly exceeded the lower limit of quantitation (25 ng/ml) of the HPLC assay.
In mice, excretion of paclitaxel via the feces is an important route of drug elimination (26). To test whether the pattern of drug excretion had been altered in mdr1a(−/−) mice, we studied the cumulative excretion in feces and urine over a period of 96 hr after i.v. administration of [3H]paclitaxel (Table 2), analyzing both total radioactivity and unchanged drug. In wt mice, ≈90% of the radioactivity was recovered in the feces, much of it as unchanged drug (40%) or as hydroxylated metabolites (15%). The remaining fraction of the administered radioactivity left the body via the feces or the urine as unknown breakdown products. In mdr1a(−/−) mice receiving paclitaxel by i.v. injection, the fecal excretion of unaltered drug was markedly reduced to only 1.5% of the dose. In contrast, however, the fecal excretion of the hydroxylated metabolites was increased. In this group of animals a major part of the radioactivity (≈45%) was excreted in the urine, but as in wt animals, only a very minor fraction of this was unchanged drug or one of the known hydroxylated metabolites.
Table 2.
Amounts of paclitaxel and metabolites excreted by intact mice within 96 hr after i.v. or oral administration of 10 mg/kg (body weight) of [3H]paclitaxel
| Biological matrix | Compound | i.v. administration
|
Oral administration
|
||
|---|---|---|---|---|---|
| Wild type | mdr1a(−/−) | Wild type | mdr1a(−/−) | ||
| Feces | Paclitaxel | 39.8 ± 2.6 | 1.5 ± 0.2* | 87.1 ± 8.8 | 2.2 ± 0.30* |
| 3′-p-hydroxypaclitaxel | 8.6 ± 0.5 | 16.1 ± 0.8 | 2.4 ± 0.2 | 17.6 ± 1.9* | |
| 6α-hydroxypaclitaxel | 6.3 ± 0.4 | 13.8 ± 0.4 | 1.9 ± 0.5 | 11.3 ± 1.2* | |
| 3H label | 90.4 ± 5.5 | 34.3 ± 1.2* | NA | NA | |
| Urine | Paclitaxel | 0.97 ± 0.10 | 0.78 ± 0.11 | 0.09 ± 0.03 | 0.07 ± 0.03 |
| 3′-p-hydroxypaclitaxel | <0.01 | <0.01 | ND | ND | |
| 6α-hydroxypaclitaxel | <0.01 | <0.01 | ND | ND | |
| 3H label | 15.9 ± 1.7 | 44.6 ± 4.7* | NA | NA | |
Excretion is given as percentage of the dose (mean ± SEM; n = 4–6) NA, Not available; ND, not detectable.
P < 0.05.
In line with the higher bioavailability in mdr1a(−/−) mice, a large difference in fecal excretion was also observed after oral administration of paclitaxel. In mdr1a(−/−) mice, only 2% of the orally delivered dose was recovered in the feces as unchanged drug, whereas in wt mice almost 90% was excreted unchanged. In mdr1a(−/−) mice, the amounts of hydroxylated metabolites recovered in the feces were not dependent on the route of administration (oral or i.v.). In wt mice, however, fecal excretion of hydroxylated metabolites after oral administration of paclitaxel was about 3-fold lower than after i.v. administration (Table 2).
To test whether diminished biliary excretion or increased (re-)uptake from the intestinal lumen was responsible for the reduced fecal excretion of paclitaxel in the mdr1a(−/−) mice, we analyzed the excretion of drug in mice in which the common bile duct was ligated and the bile was collected via a cannula located in the gall bladder (Table 3). After i.v. administration of [3H]paclitaxel (10 mg/kg body weight), about one-quarter of the recovered radioactivity was unchanged drug. Furthermore, the biliary excretion of unchanged drug and of hydroxylated metabolites was not significantly different in mdr1a(−/−) and wt mice (P = 0.13). These findings suggest that the higher bioavailability in the mdr1a(−/−) mice results from increased (re-)uptake in the intestine. However, the anaesthesia and surgical procedures in combination with the administered dose of 10 mg/kg influenced the condition of the animals. They developed respiratory and cardiac problems (transient bradycardia) immediately after injection of the drug solution. The paclitaxel plasma levels observed in these mice were >2-fold higher than those observed in “intact” wt animals (Fig. 1). We therefore repeated the same experiment with animals receiving half the dose of paclitaxel (namely, 5 mg/kg). Major toxicities were no longer observed in this set of animals. The plasma levels found in these animals were similar to those found in intact animals given the same dose. At this lower dose level the biliary excretion of unchanged paclitaxel was also similar in wt and mdr1a(−/−) mice.
Table 3.
Amounts of paclitaxel and metabolites excreted by mice with cannulated gall bladder within 90 min after i.v. administration of [3H]paclitaxel
| Sample | Compound | Dose, 10 mg/kg
|
Dose, 5 mg/kg
|
||
|---|---|---|---|---|---|
| Wild type | mdr1a(−/−) | Wild type | mdr1a(−/−) | ||
| Plasma (level, ng/ml) | Paclitaxel | 2020 ± 420 | 2370 ± 280 | 289 ± 38 | 327 ± 44 |
| Bile | Paclitaxel | 4.81 ± 0.27 | 2.56 ± 0.58 | 5.94 ± 1.70 | 4.10 ± 0.54 |
| 3′-p-hydroxypaclitaxel | 2.86 ± 0.28 | 2.48 ± 0.26 | 4.74 ± 0.69 | 4.10 ± 0.18 | |
| 6α-hydroxypaclitaxel | 2.00 ± 0.25 | 1.29 ± 0.24 | 3.25 ± 0.51 | 1.98 ± 0.22 | |
| 3H label | 17.9 ± 0.69 | 15.0 ± 1.5 | 25.7 ± 4.5 | 26.6 ± 2.90 | |
| Intestinal contents | Paclitaxel | 10.77 ± 0.25 | 2.49 ± 0.86* | 3.53 ± 0.38 | 0.98 ± 0.28* |
| 3-p-hydroxypaclitaxel† | |||||
| 6α-hydroxypaclitaxel | 0.37 ± 0.10 | 0.37 ± 0.16 | 0.16 ± 0.05 | <0.1 | |
| 3H label | 12.22 ± 0.28 | 3.79 ± 1.11* | 4.63 ± 0.49 | 1.52 ± 0.05 | |
Excretion is given as percentage of the dose (mean ± SEM; n = 4). ND, Not detectable.
P < 0.05.
This compound could not be accurately quantified in the intestinal contents due to the presence of an interfering substance.
A remarkable effect was noticed in wt mice with a cannulated gall bladder. Although the excretion of bile into the intestinal lumen is blocked in these mice, ≈11% of the dose of 10 mg/kg was recovered as unchanged drug in the intestinal lumen within 90 min after i.v. drug administration. In mdr1a(−/−) mice, however, this direct excretion into the intestinal lumen was <3% of the dose (Table 3). At the 5 mg/kg dose level the direct intestinal excretion in wt mice was reduced to 3.5% of the dose, but was still significantly higher than in mdr1a(−/−) mice (P = 0.02).
DISCUSSION
Our results show that the disruption of the mdr1a P-glycoprotein gene in mice results in a drastic alteration in the pharmacokinetics of paclitaxel, a cytotoxic drug increasingly used in the treatment of cancer patients. P-glycoprotein at the lumenal side of the intestinal epithelium appears to be an important component of the defense against substrate drugs such as paclitaxel. The disruption of this defense leads to a substantial increase in bioavailability after oral administration (35.2 vs. 11.2%) in the mdr1a(−/−) mice. The ability to transfer paclitaxel from the circulation into the gut is greatly reduced, establishing a major role for P-glycoprotein in this process. The overall result is a 6-fold increase in the area under the paclitaxel plasma AUC in the mdr1a(−/−) mice after oral administration, and a 2-fold increase after i.v. administration. Clinical trials are underway in which amphipathic drugs, such as paclitaxel, are combined with inhibitors of P-glycoprotein. These combinations have led to diminished drug clearances (27–29) similar to our observations in mdr1a(−/−) mice.
The murine pharmacokinetics of paclitaxel have been studied extensively by us using sensitive and selective HPLC methods (24, 26, 30). Because the normal pharmaceutical formulation contains Cremophor EL, which causes nonlinear pharmacokinetic behavior of paclitaxel (30), we have used an i.v. formulation that does not contain this additive. For experiments with animals receiving paclitaxel orally, we have used the commercially available formulation of paclitaxel in Cremophor EL: ethanol (1:1, vol/vol; Taxol). Nonlinear pharmacokinetic behavior of paclitaxel after oral administration due to Cremophor EL is not likely to occur because the levels of Cremophor EL in plasma remain very low [<0.1% (vol/vol)]. Furthermore, our recent experiments with different amounts of Cremophor EL demonstrated that the oral bioavailability was unaffected (results not shown).
The mdr1a(−/−) mice are particularly useful for studying the role of P-glycoprotein in the intestine, because the mdr1a gene is the only murine P-glycoprotein expressed in this tissue (10). The biliary output of paclitaxel after i.v. administration was not reduced in mdr1a(−/−) mice, which may seem unexpected because the mdr1a gene is expressed in the liver. Although the mdr1b gene is expressed in the liver as well, and Schinkel et al. (10) have shown that the mdr1b expression is elevated in the mdr1a(−/−) mice, recent experiments in mice with both disrupted mdr1a and mdr1b genes showed an unaltered biliary excretion too (unpublished results). This result implies that alternative transport systems for paclitaxel are present in the liver.
Because biliary excretion is not different in mdr1a(−/−) and (+/+) mice, the low bioavailability in wt mice should result from the presence of P-glycoprotein in the intestine. Our finding that the amount of unchanged drug recovered in the feces of mdr1a(−/−) mice after oral administration is very low and similar to that after i.v administration, whereas in wt mice almost the complete dose of orally administered paclitaxel is recovered in the feces, indicates that (re-)absorption of drug from the intestinal lumen is very efficient if not prevented by P-glycoprotein. The fact that the bioavailability of paclitaxel does not equal 100%, in spite of the almost complete (re-)absorption from the intestinal lumen, is probably due to first-pass hepatic extraction and/or metabolism in the liver and intestinal mucosa. The nonlinear increase of the plasma AUC at higher dose levels may be due to saturation of the enzymes involved.
Whereas in wt mice a substantial fraction of the dose (40%) is excreted as unchanged drug in the feces, the elimination of paclitaxel in mdr1a(−/−) mice occurs almost exclusively through metabolic breakdown. In wt mice, 3′-p-hydroxypaclitaxel and 6α-hydroxypaclitaxel, which are probably formed in the liver by the cytochrome P450 enzymes (31, 32), are the two principal metabolites identified thus far. Because these compounds could not be detected in plasma or tissues except for small quantities in the liver and the intestines, it is very likely that they are efficiently excreted via the bile into the gut, and that reabsorption is low (26). In mdr1a(−/−) mice the excretion of these known metabolites increased 2-fold. Furthermore, a large amount (45%) of the administered radioactivity was recovered in the urine of these mice. The identity of this metabolic breakdown product (or products) is unknown. Because the 3H label is mainly located (>95%) in the aromatic side chains of the molecule, it is not certain whether this urinary excretion product contains the basic taxane skeleton, or whether this part of the molecule is still excreted into the feces.
A remarkable finding is the substantial fraction of the dose recovered in the intestinal contents of wt animals with cannulated gall bladder receiving paclitaxel by i.v. administration. This fraction must be excreted directly through the mucosal cells into the intestinal lumen. Excretion is drastically reduced in mdr1a(−/−) mice, implicating P-glycoprotein as an essential component in this excretion process. The reduced excretion at the lower dose level suggests that this P-glycoprotein-mediated transport occurs only when the plasma level is sufficiently high. The basis of this threshold effect is unknown. Our findings clearly show, however, that direct excretion of compounds through the gut wall can be an important pathway of drug elimination, as also indicated by earlier observations (33–35).
We expect that the results presented here for paclitaxel are representative for other amphipathic drugs transported by P-glycoprotein. This is supported by our recent demonstration that the predominantly fecal excretion of the cardiac glycoside digoxin in wt mice shifted to predominantly urinary excretion in mdr1a(−/−) mice. Moreover, after interruption of biliary excretion, we found substantial excretion of digoxin into the gut of wt mice, but not in mdr1a(−/−) mice (36).
In conclusion, P-glycoprotein in the epithelium of the gut limits the bioavailability of orally administered paclitaxel, and it is likely that a similar protection is afforded to many other orally ingested toxic substances transported by P-glycoprotein. Intestinal P-glycoprotein also contributes to the elimination of parenterally administered substrate drugs by a direct secretion of drug into the intestinal lumen (37). These findings provide a rationale for attempts to improve the low and variable oral bioavailability of paclitaxel and other substrate drugs (e.g., etoposide) by concomitant administration of P-glycoprotein blockers.
Acknowledgments
We thank Dr. M. J. Ratain (Department of Medicine, University of Chicago) and Dr. B. I. Sikic (Oncology Division, Stanford University, School of Medicine) for their helpful comments on the manuscript. This work was supported in part by Grant NKI 92-41 from the Dutch Cancer Society to P.B.
ABBREVIATIONS
- wt
wild type
- plasma AUC
area under the plasma concentration-time curves
References
- 1.Danø K. Cancer Chemother Rep. 1972;56:701–708. [PubMed] [Google Scholar]
- 2.Riordan J R, Ling V. J Biol Chem. 1979;254:12701–12705. [PubMed] [Google Scholar]
- 3.Ueda K, Cardarelli C, Gottesman M M, Pastan I. Proc Natl Acad Sci USA. 1987;84:3004–3008. doi: 10.1073/pnas.84.9.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thiebaut F, Tsuruo T, Hamada H, Gottesman M M, Pastan I, Willingham M C. Proc Natl Acad Sci USA. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cordon-Cardo C, O’Brien J P, Casals D, Rittman-Grauer L, Biedler J L, Melamed M R, Bertino J R. Proc Natl Acad Sci USA. 1989;86:695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fojo A T, Ueda K, Slamon D J, Poplack D G, Gottesman M M, Pastan I. Proc Natl Acad Sci USA. 1987;84:265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Croop J, Raymond M, Haber D, Devault A, Arceci R J, Gros P, Housman D. Mol Cell Biol. 1989;9:1346–1350. doi: 10.1128/mcb.9.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugawara I, Kataoka I, Morishita Y, Hamada H, Tsuruo T, Itoyama S, Mori S. Cancer Res. 1988;48:1926–1929. [PubMed] [Google Scholar]
- 9.Cordon-Cardo C, O’Brien J P, Boccia J, Casals D, Bertino J R, Melamed M R. J Histochem Cytochem. 1990;38:1277–1287. doi: 10.1177/38.9.1974900. [DOI] [PubMed] [Google Scholar]
- 10.Schinkel A H, Smit J J, Van Tellingen O, Beijnen J H, Wagenaar E, Van Deemter L, Mol C A, Van der Valk M A, Robanus-Maandag E C, Te Riele H P, Berns A J M, Borst P. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 11.Arceci R J, Croop J M, Horwitz S B, Housman D. Proc Natl Acad Sci USA. 1988;85:4350–4354. doi: 10.1073/pnas.85.12.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teeter L D, Becker F F, Chisari F V, Li D J, Kuo M T. Mol Cell Biol. 1990;10:5728–5735. doi: 10.1128/mcb.10.11.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiebaut F, Tsuruo T, Hamada H, Gottesman M M, Pastan I, Willingham M C. J Histochem Cytochem. 1989;37:159–164. doi: 10.1177/37.2.2463300. [DOI] [PubMed] [Google Scholar]
- 14.Ames B N, Profet M, Gold L S. Proc Natl Acad Sci USA. 1990;87:7782–7786. doi: 10.1073/pnas.87.19.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunter J, Hirst B H, Simmons N L. Pharm Res. 1993;10:743–749. doi: 10.1023/a:1018972102702. [DOI] [PubMed] [Google Scholar]
- 16.Leu B L, Huang J D. Cancer Chemother Pharmacol. 1995;35:432–436. doi: 10.1007/s002800050258. [DOI] [PubMed] [Google Scholar]
- 17.Hsing S, Gatmaitan Z, Arias I M. Gastroenterology. 1992;102:879–885. doi: 10.1016/0016-5085(92)90173-v. [DOI] [PubMed] [Google Scholar]
- 18.Hunter J, Hirst B H, Simmons N L. Br J Cancer. 1991;64:437–444. doi: 10.1038/bjc.1991.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Asperen J, Schinkel A H, Beijnen J H, Nooijen W J, Borst P, van Tellingen O. J Natl Cancer Inst. 1996;88:994–999. doi: 10.1093/jnci/88.14.994. [DOI] [PubMed] [Google Scholar]
- 20.McGuire W P, Hoskins W J, Brady M F, Kucera P R, Partridge E E, Look K Y, Clarke-Pearson D L, Davidson M. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 21.Huizing M T, Sewberath Misser V H, Pieters R C, Ten Bokkel Huinink W W, Veenhof C H N, Vermorken J B, Pinedo H M, Beijnen J H. Cancer Invest. 1995;13:381–404. doi: 10.3109/07357909509031919. [DOI] [PubMed] [Google Scholar]
- 22.Eiseman J L, Eddington N D, Leslie J, MacAuley C, Sentz D L, Zuhowski M, Kujawa J M, Young D, Egorin M J. Cancer Chemother Pharmacol. 1994;34:465–471. doi: 10.1007/BF00685656. [DOI] [PubMed] [Google Scholar]
- 23.Sparreboom A, Huizing M T, Boesen J J B, Nooijen W J, Van Tellingen O, Beijnen J H. Cancer Chemother Pharmacol. 1995;36:299–304. doi: 10.1007/BF00689047. [DOI] [PubMed] [Google Scholar]
- 24.Sparreboom A, van Tellingen O, Nooijen W J, Beijnen J H. J Chromatogr. 1995;664:383–391. doi: 10.1016/0378-4347(94)00495-q. [DOI] [PubMed] [Google Scholar]
- 25.Sparreboom A, van Tellingen O, Huizing M T, Nooijen W J, Beijnen J H. J Chromatogr. 1996;681:355–362. doi: 10.1016/0378-4347(95)00544-7. [DOI] [PubMed] [Google Scholar]
- 26.Sparreboom A, van Tellingen O, Nooijen W J, Beijnen J H. Anti-Cancer Drugs. 1996;7:78–86. doi: 10.1097/00001813-199601000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Lum B L, Kaubisch S, Yahanda A M, Adler K M, Jew L, Ehsan M N, Brophy N A, Halsey J, Gosland M P, Sikic B I. J Clin Oncol. 1992;10:1635–1642. doi: 10.1200/JCO.1992.10.10.1635. [DOI] [PubMed] [Google Scholar]
- 28.Bartlett N L, Lum B L, Fischer G A, Brophy N A, Ehsan M N, Halsey J, Sikic B I. J Clin Oncol. 1994;12:835–842. doi: 10.1200/JCO.1994.12.4.835. [DOI] [PubMed] [Google Scholar]
- 29.Berg S L, Tolcher A, O’Shaughnessy J A, Denicoff A M, Noone M, Ognibene F P, Cowan K H, Balis F M. J Clin Oncol. 1995;13:2039–2042. doi: 10.1200/JCO.1995.13.8.2039. [DOI] [PubMed] [Google Scholar]
- 30.Sparreboom A, van Tellingen O, Nooijen W J, Beijnen J H. Cancer Res. 1996;56:2112–2115. [PubMed] [Google Scholar]
- 31.Harris J W, Rahman A, Kim B-R, Guengerich F P, Collins J M. Cancer Res. 1994;54:4026–4035. [PubMed] [Google Scholar]
- 32.Rahman A, Korzekwa K R, Grogan J, Gonzalez F J, Harris J W. Cancer Res. 1994;54:5543–5546. [PubMed] [Google Scholar]
- 33.Bezek S, Scasnar V, Kukan M, Trnovec T. Biopharm Drug Dispos. 1990;11:403–409. doi: 10.1002/bdd.2510110505. [DOI] [PubMed] [Google Scholar]
- 34.Georg C F, Gruchy B S. J Pharm Pharmacol. 1979;31:643–651. doi: 10.1111/j.2042-7158.1979.tb13613.x. [DOI] [PubMed] [Google Scholar]
- 35.Fabre J, Rudhardt M, Dayer P, Trimble E R, Male P J. Arzneim-forsch. 1984;34:789–791. [PubMed] [Google Scholar]
- 36.Mayer U, Wagenaar E, Beijnen J H, Smit J W, Meijer D K F, van Asperen J, Borst P, Schinkel A H. Br J Pharmacol. 1996;119:1038–1044. doi: 10.1111/j.1476-5381.1996.tb15775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wacher V J, Salphati L, Benet L Z. Adv Drug Deliv Rev. 1996;20:99–112. doi: 10.1016/s0169-409x(00)00126-5. [DOI] [PubMed] [Google Scholar]