Abstract
Fluoxetine (Prozac), a widely used antidepressant, is said to exert its medicinal effects almost exclusively by blocking the serotonin uptake systems. The present study shows that both muscle and neuronal nicotinic acetylcholine receptors are blocked, in a noncompetitive and voltage-dependent way, by fluoxetine, which also increases the rate of desensitization of the nicotinic receptors. Because these receptors are very widely distributed in the both central and peripheral nervous systems, the blocking action of fluoxetine on nicotinic receptors may play an important role in its antidepressant and other therapeutical effects. Our findings will help to understand the mode of action of fluoxetine, and they may also help to develop more specific medicinal drugs.
Keywords: antidepressants, Xenopus oocytes, cholinergic-serotonergic interaction, acetylcholine receptor modulation
Nicotinic acetylcholine receptors (nAcChoRs) mediate the transmission of signals across the vertebrate neuromuscular junction as well as across central and peripheral synapses (1, 2). Another widely distributed system is that in which serotonin (5-hydroxytryptamine, 5HT) is the neurotransmitter (3, 4), and it is now evident that the two systems are not completely specific. For example, dopamine activates 5HT receptors (5), and there are marked cross-interactions between serotonergic and cholinergic systems. For instance, atropine, a muscarinic antagonist, blocks both acetylcholine (AcCho) receptors and 5HT receptors in snail neurons (6); dihydro-β-erythroidine and tubocurarine, nicotinic antagonists, block 5HT3 receptors (7) and serotonin, as well as various serotonergic agonists and antagonists, block in a noncompetitive manner both muscle and neuronal nAcChoRs (8–10). Finally, 5HT acts as an antagonist on wild-type neuronal α7 nAcChoR, but it acts as an agonist on the α7 nAcChoR mutated in the M2 transmembrane region (11, 12). Because fluoxetine (Prozac), a highly efficient inhibitor of 5HT uptake, is extensively used in the treatment of depression, eating disorders, and other diseases of the brain (13), we decided to see if, like other serotonergic agents, fluoxetine would alter the function of nAcChoRs.
MATERIALS AND METHODS
The experiments were performed on voltage-clamped Xenopus oocytes expressing muscle α1β1γδ or neuronal (α2β4 or α3β4) nAcChoRs. The complementary RNA preparation, oocyte injection, and electrophysiological recordings have been described elsewhere (8, 9). Briefly, RNAs encoding mouse muscle (α1, β1, γ, and δ) or rat neuronal (α2, α3, and β4) nAcChoR subunits were transcribed in vitro. Equal quantities of cRNA subunits were combined to obtain α1β1γδ, α2β4, and α3β4 nAcChoR subtypes. Xenopus laevis oocytes were dissected from the ovary and maintained at 16°C in Barth’s solution containing 88 mM NaCl, 1 mM KCl, 0.33 mM Ca(NO3)2, 0.41 mM CaCl2, 0.82 mM MgSO4, 2.4 mM NaHCO3, 5 mM Hepes (pH 7.4; NaOH), and 0.1 mg/ml gentamicin sulfate. The next day each oocyte was injected with 0.5–50 ng of the corresponding cRNA mixture in a volume of 50 nl water, and about 2 days later the follicullar cells enveloping the oocytes were removed by collagenase treatment (Sigma type I, 140 units/ml) for 0.5–1 h (14). AcCho currents were recorded using two microelectrode voltage-clamp and single-channel recordings (15–17), 3–9 days after the cRNA injection. Oocytes were placed in a 0.1 ml volume chamber and continuously superfused at room temperature (20–23°C) with frog Ringer’s solution containing 115 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, and 5 mM Hepes (pH 7.0; NaOH). In all the experiments where currents were recorded by two-microelectrode voltage clamp, the oocytes membrane potential was maintained at −60 mV. AcCho and fluoxetine were diluted in the Ringer’s solution and applied onto the oocytes by superfusion at a rate of 7–10 ml/min. Before each test with fluoxetine AcCho was applied repeatedly until the control AcCho-current amplitude was stable.
RESULTS
Fluoxetine Action on Muscle and Nuronal AcCho Receptors.
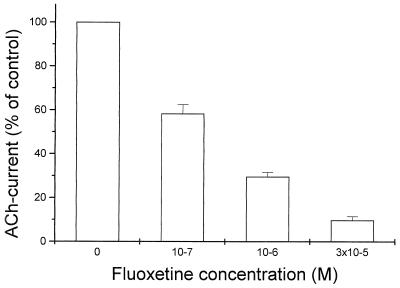
Fluoxetine reduces rapidly the amplitude of the membrane currents elicited by AcCho (AcCho current) in oocytes expressing mouse muscle α1β1γδ receptors. This action is dose-dependent and reversible and can be seen clearly even when relatively low concentrations of fluoxetine are coapplied with AcCho, as in Fig. 1.
Figure 1.
Blockage of mouse muscle nAcChoRs by fluoxetine. Xenopus oocytes were injected with a mixture of α1β1γδ cRNAs and simultaneously exposed to AcCho (4 × 10−6 M) and the indicated fluoxetine concentrations. Normalized AcCho current, mean ± SE of three oocytes.
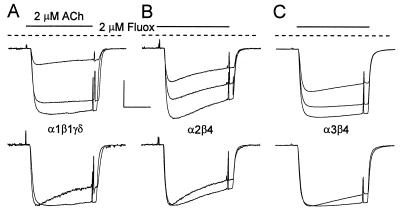
In most experiments, the membrane currents were induced by relatively low concentrations of AcCho to reduce receptor desensitization. At a concentration of 2 μM, fluoxetine had a clear blocking effect on the AcCho currents mediated by either muscle or neuronal nAcChoRs. Moreover, fluoxetine blocked with different strengths the three different types of nAcChoR studied. The order of potency for blocking of nAcChoRs was α1β1γδ > α2β4 > α3β4 (Fig. 2). Furthermore, we consistently observed that the onset of the AcCho current block by fluoxetine, as well as its recovery, was fastest for α3β4 receptors and slowest for α1β1γδ receptors. All together, these facts may reflect different affinities of fluoxetine for its binding site(s) on each receptor subtype.
Figure 2.
Blockage of muscle and neuronal nAcChoRs by fluoxetine. The columns represent the fraction of unblocked AcCho current in the presence of 2 μM fluoxetine (Fluox) with respect to the control current (mean ± SE). Data were from two donors (n = 6–8). (Inset) Typical AcCho currents recorded from oocytes expressing muscle or neuronal nAcChoRs. In this and subsequent figures, the timing of drug application is indicated by bars above the records and by depolarizing pulses (20 mV) that were also used to monitor membrane conductance; inward currents are represented by downward deflections.
At the concentrations used, fluoxetine alone had no direct gating action on either muscle or neuronal nAcChoRs, because no membrane currents were detected when the oocytes were exposed to 2 μM fluoxetine for up to 15 min, or to 10 μM for 2 min. Moreover, the extent of blockage was similar if oocytes were preincubated with fluoxetine and then the current was elicited by AcCho, still in the presence of fluoxetine as in Fig. 3; or if the drugs were applied in the opposite order—i.e., AcCho and then AcCho plus fluoxetine as in Fig. 2. For example, when the oocytes were preincubated with 2 μM fluoxetine for a few minutes and then exposed to fluoxetine plus AcCho, the amplitudes of the AcCho currents relative to controls were 0.21 ± 0.02, 0.45 ± 0.03, and 0.64 ± 0.03 for α1β1γδ, α2β4, and α3β4 respectively (n = 4–6 for all). These values are not very different from those shown in Fig. 2.
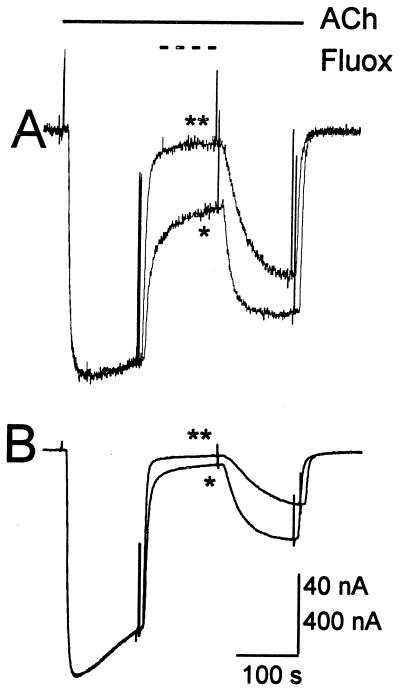
Figure 3.
Fluoxetine blockage of AcCho currents mediated by muscle or neuronal nAcChoRs. (A Upper) Superimposed records of AcCho currents from an oocyte expressing muscle α1β1γδ nAcChoRs. The largest trace shows the control current elicited by AcCho alone. After 5–8 min the oocyte was preincubated for 2 min with 2 μM fluoxetine that was maintained during the next AcCho application, which resulted in an inhibited current (record with the lowest amplitude). The middle records show partial recovery after 7–10 min. (A Lower) Normalized and superimposed records corresponding to the same control and blocked AcCho currents. (B and C) Block of AcCho currents by fluoxetine in oocytes expressing neuronal α2β4 and α3β4 nAcChoRs, respectively, following the same protocol as for A. [Horizontal calibration bar = 50 s; vertical calibration bar = 66 nA (A), 800 nA (B), and 400 nA (C).]
Fluoxetine and nAcChoR Desensitization.
Fluoxetine accelerated the time course of desensitization of the AcCho currents elicited by activation of either muscle or neuronal nAcChoRs (Fig. 3, lower sets of traces). This feature is consistent with the monotonic decrease of the AcCho current blocked by fluoxetine, even when the current did not desensitize much before fluoxetine application (e.g., Fig. 2, α3β4 nAcChoR). It is possible that fluoxetine acts on a desensitized state of the receptor-channel complex, as it has been proposed that agonists exhibit a higher affinity for the desensitized state (18), or it may be that fluoxetine blocks the open channel or that it does both (19–22). Although the rate of desensitization of the AcCho-current recovered after about 10 min of washing out the fluoxetine, the current amplitude did not recover completely for any of the three types of nAcChoRs after more than 1 h of washing. At the low AcCho concentrations used, the AcCho current amplitude repeated well and there was no run-down during observation periods of about 3 h, even when other nAcChoR blockers [5HT, 8-hydroxy-2-(di-n-propylamino)tetralin, d-tubocurarine; refs. 9 and 10] were applied. It may be that the slow recovery is due to a long-lasting blocking effect of fluoxetine on a small fraction of nicotinic receptors.
Fluoxetine seems to interact with both muscle and neuronal nAcChoRs in a noncompetitive fashion. Thus, when the concentration of AcCho was increased, maintaining the same concentration of fluoxetine, the fraction of AcCho current blocked was larger (Fig. 4), opposite to what would be expected by simple competition for the agonist binding sites. With both, nondesensitizing (elicited by low doses) or desensitizing AcCho-currents, we consistently observed that the block was faster and more pronounced, and recovery was slower and less complete with high concentrations of fluoxetine.
Figure 4.
Blockage of nAcChoRs by different concentrations of fluoxetine. (A) AcCho current recorded from an oocyte expressing muscle α1β1γδ nAcChoRs. The current was elicited by 2 μM AcCho and blocked by fluoxetine 2 μM (∗) or 10 μM (∗∗). Records are superimposed and normalized and after 5 min the recovery was (94%, ∗∗). In B, the AcCho current was elicited by 10 μM AcCho, to increase AcCho current desensitization, and fluoxetine concentrations were as in A. Records are again superimposed and normalized, the recovery was 92% (∗∗).
Voltage Dependence of Fluoxetine Block and Single-Channel Currents.
The blockage of α1β1γδ, α2β4, and α3β4 nAcChoRs by fluoxetine was stronger at hyperpolarized membrane potentials. Using a one-site blockade model (23), this voltage dependence can be used to estimate the fraction of membrane potential sensed at the site of binding of fluoxetine within the channel gated by AcCho. Judging from the values obtained, the site of interaction between fluoxetine and the three types of receptors is within the ionic channel, being 0.25 ± 0.02 (n = 4) and 0.23 ± 0.03 (n = 3) for α1β1γδ and α2β4 receptors, respectively, whereas for α3β4 it was 0.42 ± 0.05 (n = 3).
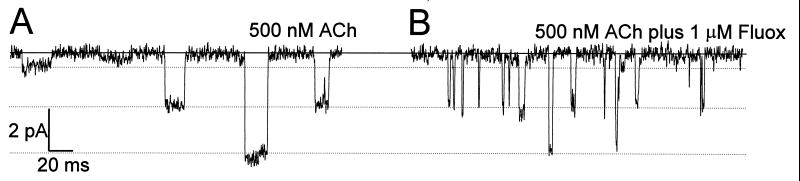
Preliminary experiments with single-channel recordings showed that the opening of muscle nAcChoR channels was clearly affected by fluoxetine (Fig. 5). The single-channel activity elicited by AcCho alone showed three different amplitudes, and when fluoxetine was included in the patch-pipette the amplitude of the events was not greatly altered. In contrast, the mean channel open time was clearly reduced for the three populations of channels, from 10.9 ms to 2.1 ms. This pattern resembles the effects of local anesthetics and curare acting as open-channel blockers on nAcChoRs (19–22); a similar effect of fluoxetine has been reported for potassium and sodium currents of rabbit corneal epithelial cells and human lens epithelium (24).
Figure 5.
Single-channel currents, elicited by AcCho in an oocyte expressing muscle α1β1γδ nAcChoRs, recorded in the cell-attached configuration. Patch pipettes (≈10 MΩ) were filled with Ringer’s solution containing 500 nM AcCho alone (A), or AcCho plus 1 μM fluoxetine (B). The resting membrane potential of the oocyte was recorded with intracellular microelectrodes just before the single-channel recording (approximately −60 mV in this oocyte). The patch-pipette potential was +120 mV. Records were filtered at 2 kHz with a low-pass Bessel filter and digitized at 20 kHz.
DISCUSSION
Fluoxetine blocks different types of nAcChoRs with different strengths, and these effects show some similarities with the action of fluoxetine on 5HT3 receptors (25–27). Thus, fluoxetine inhibits the membrane currents elicited by activation of both muscle (α1β1γδ) and neuronal (α2β4 or α3β4) nAcChoRs, either by increasing the rate of receptor desensitization and/or by inducing channel blockage, which may indirectly facilitate the desensitizing state of the receptor. Moreover, fluoxetine inhibits nAcChoRs in a dose-dependent and noncompetitive manner; the recovery from the blockage is slowly reversible or incomplete like its action on 5HT3 receptors. The site of action of fluoxetine on α1β1γδ and α2β4 nAcChoRs appears to be within the ionic channel and seems to be the same site as that for serotonergic agonists and antagonists on muscle nAcChoRs (10), whereas our preliminary results suggest that the site of action on α3β4 receptors is slightly more internal, because its estimated “electrical distance” is larger (0.42 vs. 0.25).
It is commonly thought that the therapeutic effects of fluoxetine are essentially due to its blocking action on 5HT transporters (13). However, our results show clearly that even at relatively low concentrations fluoxetine inhibits the membrane currents mediated by activation of various types of nAcChoRs. Moreover, we have also shown recently that fluoxetine inhibits the binding as well as the function of 5HT2C receptors expressed in either oocytes or HeLa cells (28). All these effects can be seen at fluoxetine concentrations that are like those reached in plasma during clinically effective treatments: 0.29–0.97 μM and in some patients up to 1.6 μM after administration of 40 mg/day during a 30-day treatment (29). It is likely that at these levels the effect of fluoxetine on the 5HT uptake system has reached saturation (see ref. 13).
Moreover, Séguéla et al. (30) have shown that 5HT innervation in the cerebral cortex is predominantly nonjunctional. Therefore, by the process of volume transmission (see ref. 31 and papers therein), 5HT and fluoxetine may be exerting important effects, not only on AcCho receptors located near the cholinergic and serotonergic terminals but also on extra-junctional receptors (32) situated away from the synaptic regions.
Therefore, it is not easy to think that the antidepressant effects, and the alleviation of other disorders, attributed to fluoxetine are entirely the consequence of blocking the 5HT transporters. On the contrary, it is becoming increasingly apparent that fluoxetine has quite a complex mechanism of action and affects a variety of membrane proteins, some of them, like the 5HT and nAcChoRs widely distributed in the organism. Furthermore, if one considers that, as a result of 5HT uptake inhibition, fluoxetine increases the extracellular levels of 5HT up to 7-fold in many brain areas, including the nucleus accumbens, striatum, thalamus, and hypothalamus (33–36); that nAcChoRs are distributed in these areas (37, 38), and that 5HT also modulates the release of AcCho (39), then all these facts point clearly to many possible interactions between the serotonergic and cholinergic systems in the central nervous system. Therefore, further studies are required to clarify how fluoxetine (Prozac) and similar drugs exert their beneficial actions. In the meantime, our findings may help in the design of better and more selective medicinal drugs.
Acknowledgments
We thank Drs. J. Boulter and S. Heinemann (The Salk Institute) for providing the nAcChoR clones, and Eli Lilly Co. for the fluoxetine. We are also grateful to Rico Miledi for computer programming and to Drs. Q.T. Nguyen and R. Arellano for reviewing the manuscript. This work was supported by a Grant NS23284 from the National Institute of Neurological Disorders and Stroke.
ABBREVIATIONS
- 5HT
5-hydroxytryptamine (serotonin)
- AcCho
acetylcholine
- nAcChoR
nicotinic AcCho receptor
References
- 1.Sargent P B. Annu Rev Neurosci. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- 2.McGehee D S, Role L W. Annu Rev Physiol. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs B L, Azmitia E C. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 4.Peroutka S J. J Neurochem. 1993;60:408–416. doi: 10.1111/j.1471-4159.1993.tb03166.x. [DOI] [PubMed] [Google Scholar]
- 5.Woodward R W, Panicker M M, Miledi R. Proc Natl Acad Sci USA. 1992;89:4708–4712. doi: 10.1073/pnas.89.10.4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerschenfeld N M, Stefani E. J Physiol (London) 1966;185:684–700. doi: 10.1113/jphysiol.1966.sp008010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elsele J L, Bertrand S, Galzi J L, Devillers-Thiery A, Changeux J P, Bertrand D. Nature (London) 1993;366:479–483. doi: 10.1038/366479a0. [DOI] [PubMed] [Google Scholar]
- 8.Grassi F, Polenzani L, Mileo A M, Caratsch C G, Eusebi F, Miledi R. J Neurosci Res. 1993;34:562–570. doi: 10.1002/jnr.490340508. [DOI] [PubMed] [Google Scholar]
- 9.García-Colunga J, Miledi R. Proc Natl Acad Sci USA. 1995;92:2919–2923. doi: 10.1073/pnas.92.7.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.García-Colunga J, Miledi R. Proc Natl Acad Sci USA. 1996;93:3990–3994. doi: 10.1073/pnas.93.9.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palma E, Mileo A M, Eusebi F, Miledi R. Proc Natl Acad Sci USA. 1996;93:11231–11235. doi: 10.1073/pnas.93.20.11231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palma E, Eusebi F, Miledi R. Proc Natl Acad Sci USA. 1996;93:11231–11235. doi: 10.1073/pnas.93.20.11231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong D T, Bymaster F P, Engleman E A. Life Sci. 1995;57:411–441. doi: 10.1016/0024-3205(95)00209-o. [DOI] [PubMed] [Google Scholar]
- 14.Miledi R, Woodward R M. J Physiol (London) 1989;416:601–621. doi: 10.1113/jphysiol.1989.sp017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miledi R. Proc R Soc London B. 1982;215:491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- 16.Hamill O P, Marty E, Neher E, Sakmann B, Sigworth F J. Pflügers Arch. 1981;392:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 17.Miledi R, Parker I, Sumikawa K. Proc R Soc London B. 1983;218:481–484. doi: 10.1098/rspb.1983.0053. [DOI] [PubMed] [Google Scholar]
- 18.Katz B, Thesleff S. J Physiol (London) 1957;138:63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz B, Miledi R. Proc R Soc London B. 1978;203:119–123. doi: 10.1098/rspb.1978.0096. [DOI] [PubMed] [Google Scholar]
- 20.Neher E, Steinbach J H. J Physiol (London) 1978;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trautmann A. Nature (London) 1982;298:272–275. doi: 10.1038/298272a0. [DOI] [PubMed] [Google Scholar]
- 22.Clapham D E, Neher E. J Physiol (London) 1984;347:255–277. doi: 10.1113/jphysiol.1984.sp015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodhull A M. J Gen Physiol. 1973;61:687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rae J L, Rich A, Zamudio A C, Candia O A. Am J Physiol. 1995;269:C250–C256. doi: 10.1152/ajpcell.1995.269.1.C250. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt A W, Peroutka S J. Eur J Pharmacol. 1989;163:397–398. doi: 10.1016/0014-2999(89)90215-x. [DOI] [PubMed] [Google Scholar]
- 26.Fan P. Br J Pharmacol. 1994;112:741–744. doi: 10.1111/j.1476-5381.1994.tb13140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucchelli A, Santagostino-Barbone M G, Barbieri A, Candura S M, Tonini M. Br J Pharmacol. 1995;114:1017–1025. doi: 10.1111/j.1476-5381.1995.tb13307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ni Y, Miledi R. Proc Natl Acad Sci USA. 1997;94:2036–2040. doi: 10.1073/pnas.94.5.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodnick P J. Psychopharmacol Bull. 1991;27:503–512. [PubMed] [Google Scholar]
- 30.Séguéla P, Watkins K C, Descarries L. J Comp Neurol. 1989;289:129–142. doi: 10.1002/cne.902890111. [DOI] [PubMed] [Google Scholar]
- 31.Fuxe K, Aquati L F, editors. Volume Transmission in the Brain: Novel Mechanisms for Neural Transmission. New York: Raven; 1991. [Google Scholar]
- 32.Miledi R. J Physiol (London) 1960;151:24–30. [PMC free article] [PubMed] [Google Scholar]
- 33.Guan X M, McBride W J. Brain Res Bull. 1988;21:43–46. doi: 10.1016/0361-9230(88)90118-9. [DOI] [PubMed] [Google Scholar]
- 34.Perry K W, Fuller R W. Life Sci. 1992;50:1683–1690. doi: 10.1016/0024-3205(92)90423-m. [DOI] [PubMed] [Google Scholar]
- 35.Dailey J W, Yan Q S, Mishra P K, Burger R L, Jobe P C. J Pharmacol Exp Ther. 1992;260:533–540. [PubMed] [Google Scholar]
- 36.Perry K W, Fuller R W. J Pharm Pharmacol. 1993;45:759–761. doi: 10.1111/j.2042-7158.1993.tb07105.x. [DOI] [PubMed] [Google Scholar]
- 37.Meredith G E, Blanf B, Groenewegen H J. Neuroscience. 1989;31:327–345. doi: 10.1016/0306-4522(89)90377-1. [DOI] [PubMed] [Google Scholar]
- 38.Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson W. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- 39.Rada P V, Mark G P, Hoebel B G. Brain Res. 1993;629:98–104. doi: 10.1016/0006-8993(93)91600-w. [DOI] [PubMed] [Google Scholar]