Abstract
Mole rats (Spalax ehrenbergi superspecies) perform the heavy work of digging their subterranean burrows in Israel under highly hypoxic/hypercapnic conditions. Unlike most other mammals, they can achieve high levels of metabolic rate under these conditions, while their metabolic rate at low work rates is depressed. We explored, by comparing mole rats with white rats, whether and how this is related to adaptations in the design of the respiratory system, which determines the transfer of O2 from the lung to muscle mitochondria. At the same body mass, mole rats were found to have a significantly smaller total skeletal muscle mass than ordinary white rats (−22%). In contrast, the fractional volume of muscle mitochondria was larger by 46%. As a consequence, both species had the same total amount of mitochondria and achieved, under normoxia, the same V̇O2max. Whereas the O2 transport capacity of the blood was not different, we found a larger capillary density (+31%) in the mole rat muscle, resulting in a reduced diffusion distance to mitochondria. The structural pulmonary diffusing capacity for O2 was greater in the mole rat (+44%), thus facilitating O2 uptake in hypoxia. We conclude that structural adaptations in lung and muscle tissue improve O2 diffusion conditions and serve to maintain high metabolic rates in hypoxia but have no consequences for achieving V̇O2max under normoxic conditions.
The purpose of the present investigation was to estimate determinants of oxygen flow from lungs to skeletal muscle mitochondria in mole rats and to compare them to white rats of the same body mass. The blind mole rats of the Spalax ehrenbergi superspecies (1) live underground in Israel and are known to have many adaptations to the subterranean hypoxic environment, in particular to low O2 concentrations in the burrow (1–11). The resting normoxic mole rat has a low, arrhythmic heart frequency with 142 beats per min, compared with 314 beats per min in white rats (8). In hypoxia, the heart rate of mole rats increases by 230%, almost twice that of white rats under similar condition (12). Efficient O2 unloading is achieved when tissue PO2 is very low, indicating that a large O2 saturation difference can still exist in hypoxia (13). Adaptation of mole rats to hypoxia is further supported by the finding that general metabolism seems not affected by chronic exposure to hypoxia, in contrast to white rats (11).
Under hypoxic conditions, O2 uptake in the lung is a critical step, and the question arises whether the pulmonary gas exchanger shows adaptive features resulting in a higher diffusing capacity.
We advance the hypothesis that the capacity to do aerobic work under low O2 pressures is due to adaptations in the design of the respiratory system, such that structures determining oxygen uptake in the lung and oxygen supply to muscle cells are adjusted to ensure adequate O2 supply at very low ambient O2 concentrations. This hypothesis is tested by comparing mole rats to white rats. The physiology of the white rat conforms in many aspects to mammalian allometric rules. In addition, the white rat belongs to the same suborder as the mole rat (Myomorpha) and is relatively tolerant to hypoxia/hypercapnia (11), which makes it a suitable animal for comparison with the mole rat (12). Remarkably, the critical PO2 values (i.e., the lowest oxygen pressure animals can sustain for prolonged periods of time) were found to be lower for the mole rats than for any other rodent studied so far (2, 11). In this ability to withstand hypoxic conditions, they even exceed many high altitude mammals (2, 11). We observed that various parameters in mole rat lung and skeletal muscle facilitate oxygen transfer without affecting maximal oxygen consumption in normoxia. Mole rats show several congruent structural adaptations, representing a shared adaptive effort, allowing for survival in a hypoxic environment.
MATERIALS AND METHODS
Animals.
The study was carried out on four white rats (CD strain, Charles River Breeding Laboratories) and four mole rats (S. ehrenbergi, supplied by Eviatar Nevo, Haifa University, Israel). The chromosomal species characteristics of the mole rats in this study were mixed. All lung data were obtained on animals with 2n = 52; two of the mole rats used for muscle studies were 2n = 52 and one was 2n = 60. Animals were housed in individual cages and kept under controlled conditions. Mole rats were given food and water ad libitum, while white rats were given a food-restricted diet to maintain a matched body weight. Due to difficulties in working with mole rats, some parts of the analysis had to be done on different sets of animals; V̇O2max measurements and hypoxia tolerance tests were carried out on four animals, whereas muscle sampling was done on a subset of the animals that had undergone functional analysis (n = 3). Due to respiratory tract disease at the time of sacrifice, lung data had to be obtained in part from different animals (n = 4).
Mass-specific oxygen consumption was measured in an open-flow system on a treadmill (14). Speed was increased until oxygen consumption no longer increased with increasing speed; this value was retained as V̇O2max. During hypoxic runs a premixed gas of compressed air diluted by various concentrations of N2 was used (14). CO2 content was continuously monitored for all experiments. Critical oxygen concentration was defined as the lowest oxygen tension at which the animals could just maintain their resting metabolism and survive for significant periods of time.
Surgery and Tissue Sampling.
Terminal experiments were started with a light halothane anesthesia to minimize stress to the animals followed by intraperitoneal injection of a solution containing a combination of ketamine, xylazine, and acepromazine (70, 7, and 0.7 mg per kg of body weight, respectively). Venous blood was taken by puncture of the vena jugularis for determination of physiological hematocrit. The deeply anesthetized animals were tracheotomized and a cannula was inserted. After laparotomy, a pneumothorax was set by incision of the diaphragm and a snare was slung around the atrioventricular sulcus of the heart. During this procedure, the lungs were oxygenated regularly to keep a normal blood oxygen level and to prevent a collapse of the lung tissue. Instillation of the lungs with a glutaraldehyde solution was done according to the fixation methods described in detail earlier (15). Following muscle sampling (see below), the lungs were removed from the chest. Lung volume was assessed using the method of water displacement (15). After removal of the lungs, the animals were totally dissected to determine the mass of individual muscles and muscle strata. All samples for the morphometric analysis were obtained within 1 hr after death.
Analysis of the Muscle Tissue.
Random muscle samples from 15 locations were taken on one half of the carcass, according to a systematic and volume weighted sampling procedure (16). M. deltoideus and M. rectus femoris were collected for the stereological analysis of single muscles. These muscles were chosen to represent the chief motor muscles in each species. In addition, the heart was collected, weighed, and sampled for electron microscopy. For the stereological analysis, one block was sectioned from each of the random samples from the whole body muscle tissue (10 micrographs per block analyzed) whereas for the single muscles four randomly selected blocks were cut and analyzed (40 micrographs per animal). The blocks were cut to obtain IUR (isotropic uniform random) sections on which the volume density of mitochondria in muscle fibers was estimated by point counting, and capillary length density was estimated from a count of capillary profiles (17). From these density data, the absolute volume of muscle mitochondria and the total capillary length were obtained by multiplying with the muscle volume.
Analysis of Myoglobin in Muscle Tissue.
Determination of myoglobin content was done according to the method of Reynafarje (18) with some modifications. In brief, frozen muscle tissue was homogenized on ice in a buffer solution (containing 0.12 M KCl, 2.0 mM Hepes-KO/H, and 5.0 mM MgCl2, pH 7.5). After a brief centrifugation step, the supernatant was gassed with 10% CO/90% N2 for ≈5 min. A few crystals of Na-dithionit were added to the sample, and the solution was further gassed for another 3 min. Spectrophotometrical analysis was done immediately after that step at 568 nm and 538 nm.
To verify the outcome of these experiments, Western blotting was performed on parallel samples. Proteins were analyzed by SDS/mini-PAGE essentially according to the method of Laemmli (19) with some modifications (see ref. 20). Lysates were subjected to a 15% SDS/polyacrylamide separating gel, and proteins were blotted onto a polyvinylidene difluoride (PVDF) membrane (Bio-Rad). Electrophoretic transfer was performed at a constant current of 0.2 A for 2.5 hr in an ice-cold water bath. Blots were decorated with myoglobin antibodies (Dako) at a dilution of 1:2000. Proteins were visualized with peroxidase-linked secondary antibodies and the enhanced chemiluminescence (ECL) method (Amersham). Experiments were done in triplicate. The outcome of the Western blotting was similar to that obtained for the spectrophotometric analysis. Mole rat and rat myoglobin migrated in a similar way as it was observed in control experiments with human and horse myoglobin (data not shown).
Analysis of the Lung.
The lungs were carefully dissected and embedded in agar and serial slices were sectioned for light and electronmicroscopical analysis. Slices for light microscopical studies were embedded in methyl methacrylate, whereas for ultrastructural analysis, small pieces were cut, postfixed, and embedded in Epon (15). Morphometric analysis of lung tissue was done as previously described (15). Eleven randomly chosen pictures were taken from each of the nine blocks per animal.
The pulmonary diffusing capacity DlO2 was estimated from morphometric data using the equations described in detail elsewhere (15), with the modifications recently introduced for estimating the membrane diffusing capacity, which amounts to considering the tissue and plasma layers as a single diffusion barrier (21).
Statistics.
Gaussian distribution of the residuals and homogeneity of the variances could not be confirmed for all variables, so statistical analysis was done using an improved bootstrap method with bias-corrected confidence limit intervals (22). Data are expressed as mean ± SEM. Differences were considered statistically significant at P < 0.05.
RESULTS
Physiological Study.
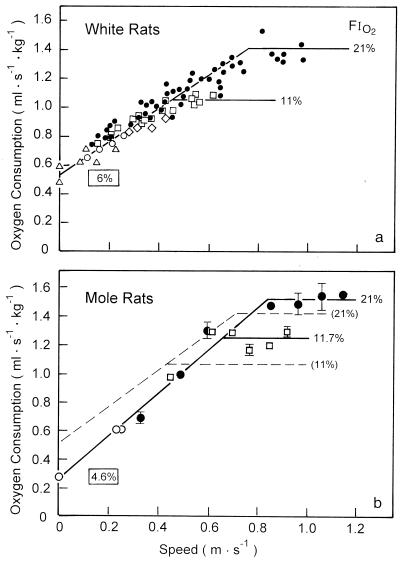
To compare mole rats to white rats, the latter were diet-controlled to ensure that all animals were of similar body mass during the study, up to the time of sacrifice (Table 1). When the animals were running on a treadmill, it was found that O2 consumption, V̇O2, increased linearly with running speed up to maximal O2 consumption, V̇O2max, when V̇O2 leveled off (Fig. 1). Under normoxic conditions (FiO2 = 21%), both species achieved the same V̇O2max. Under hypoxic conditions (FiO2 = 11%) the increase of V̇O2 was superimposed on the normoxic slope in both species, but the mole rats reached the maximum at a significantly higher level. The major difference between the two species was that the mole rats have a significantly lower resting V̇O2 (y-intercept of V̇O2 increase with running speed) so that the slope of V̇O2 increase was steeper (Fig. 1). When FiO2 was further reduced to estimate critical PO2, we found that rats could maintain their resting V̇O2 down to 6% [critical PO2, ≈40 torr (1 torr = 133 Pa)], whereas mole rats could well support a critical FiO2 of 4.6% (critical PO2, ≈30 torr). This difference was statistically significant. The venous hematocrit and the capillary hematocrit estimated by morphometry did not differ between mole rats and white rats (data not shown).
Table 1.
Morphometric muscle data
| Tissue | Mb, g | Mm/Mb, g/kg | VV(mt,f), % | JV(c,f), mm·mm−3 | V(mt), cm3 | J(c), m |
|---|---|---|---|---|---|---|
| Mole rats | 136.8 ± 10.0 | |||||
| (108–151) | ||||||
| Skeletal muscle | 272.2 ± 27.8* | 8.83 ± 0.17* | 1757 ± 167* | 3.14 ± 0.60 | 60,241 ± 7138 | |
| (206–295) | (8.5–9.1) | (1498–2068) | (1.98–4.00) | (46,081–68,900) | ||
| Heart | 3.89 ± 0.42 | 31.76 ± 0.95 | 4462 ± 491 | 0.164 ± 0.010 | 2340 ± 393 | |
| (3.10–4.50) | (29.87–32.87) | (3480–4692) | (0.152–0.183) | (1691–3047) | ||
| White rats | 139.5 ± 3.66 | |||||
| (130–147) | ||||||
| Skeletal muscle | 346.7 ± 10.6 | 6.04 ± 0.59 | 1346 ± 70 | 2.77 ± 0.31 | 61,282 ± 3519 | |
| (315–361) | (5.2–7.7) | (1210–1482) | (2.31–3.69) | (55,980–70,884) | ||
| Heart | 3.23 ± 0.05 | 34.54 ± 1.41 | 5280 ± 430 | 0.156 ± 0.005 | 2384 ± 197 | |
| (3.11–3.32) | (30.90–37.01) | (4046–5902) | (0.141–0.165) | (1802–2672) |
Values are given as mean ± SEM (n = 3 for mole rats and n = 4 for white rats). Numbers in parentheses indicate range of values. Mb, body mass; Mm/Mb, relative whole body muscle mass; VV(mt,f), volume density of total mitochondria; JV(c,f), capillary length density; V(mt), total mitochondrial volume; and J(c), total muscle capillary length.
Significantly different from white rat (P < 0.05).
Figure 1.
Rate of O2 consumption as function of running speed in white rats (a) and mole rats (b). Solid symbols represent normoxic measurements (FiO2 = 21%), and the open symbols measurements represent hypoxia. Framed values of FiO2 represent the lowest sustainable O2 concentration (critical PO2). The regressions for the white rats are reported in the mole rat graph as a dashed line.
Morphology of Skeletal Muscle Tissue.
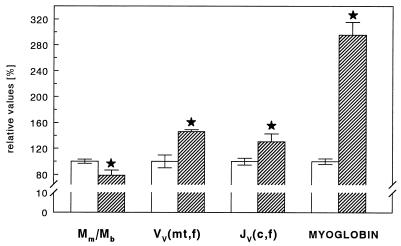
Mole rats show a significantly smaller muscle mass (by 22%) compared with white rats, hence the ratio of muscle mass to body mass was significantly smaller in mole rats (Table 1 and Fig. 2). Comparing the two species, we observed that the muscle mass is not distributed similarly over the whole body. Like other subterranean rodents, mole rats show a preference to locate more of their muscle mass in upper trunk and forelimb areas. This is also reflected in individual muscle weights. We found that in the mole rat the muscle mass of the front limb muscle M. deltoideus was larger by 1.5-fold, while the mass of the hindlimb muscle M. rectus femoris was smaller by 0.6-fold, compared with white rats.
Figure 2.
Morphometric properties of skeletal musculature for mole rats (hatched bars) and white rats (open bars). Mm/Mb, relative whole body skeletal muscle mass; VV(mt,f), mitochondrial volume density; JV(c,f), capillary length density; MYOGLOBIN, myoglobin concentrations for mole rat and white rat tissue; mt, mitochondrial; c, capillary; and f, muscle fibers. Data are reported as mean ± SEM. ∗, Significantly different vs. white rat, P < 0.05.
Analysis of key structures in muscle tissue involved in transport and consumption of oxygen showed distinct differences between the two species. Total volume density of mitochondria was found to be significantly higher in mole rats (Table 1 and Fig. 2). Similar observations could be made for single muscles so that mole rats had more mitochondria than white rats in M. deltoideus (11.53 ± 0.72 vs. 8.12 ± 1.02) and M. rectus femoris (10.22 ± 0.83 vs. 6.13 ± 0.95). In both species, close to 25% of the mitochondria were found in subsarcolemmal locations in all locomotor muscles analyzed.
Mass-specific total mitochondrial volume was not found to be different between mole rats and white rats (Table 1). Due to the larger muscle mass of M. deltoideus, total mitochondrial volume was significantly greater (0.029 ± 0.002 vs. 0.013 ± 001) in mole rats. The opposite was found in M. rectus femoris, which in absolute terms showed a smaller mitochondrial volume (0.021 ± 0.004 vs. 0.028 ± 0.006) in mole rats.
Capillary length density as assessed by the analysis of whole body random samples showed significantly larger values for mole rats (Table 1 and Fig. 2), thus resulting in shorter diffusion distances to the mitochondria. Total capillary length, however, did not differ between groups (Table 1). The myoglobin content of mole rat muscles was found to be 3-fold higher than that of white rats (Fig. 2). This holds as well for whole body random samples as for single muscles.
Morphology of Heart Muscle Tissue.
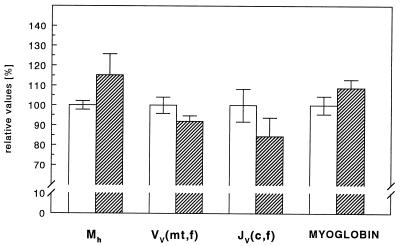
The heart mass relative to the body mass of the mole rat did not differ significantly from that of white rats (Table 1 and Fig. 3) and was in the range of previously reported values [0.34% ± 0.06 (23)]. Mitochondrial density and capillary length density did not differ between the two groups. Hence, myocardial total mitochondrial volume and total capillary length are the same for both species (Table 1 and Fig. 3). Also heart myoglobin concentration of mole rat and white rat did not differ (Fig. 3).
Figure 3.
Morphometric properties relevant to oxygen supply in the heart for mole rats (hatched bars) and white rats (open bars). Mh, mass of the heart; VV(mt,f), mitochondrial volume density; JV(c,f), capillary length density; MYOGLOBIN, myoglobin content in heart muscle; mt, mitochondrial; c, capillary; and f, muscle fibers. Data are reported as mean ± SEM.
Morphology of the Lung.
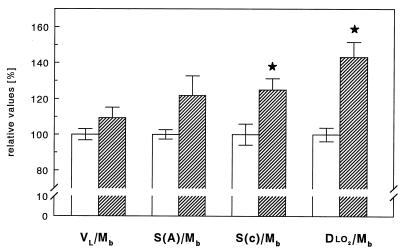
The lung volume was marginally larger in the mole rat (Table 2), amounting to ≈110% of that of the white rat when expressed per unit body mass (Fig. 4). The morphometric study revealed that the volume density of alveolar septa was also ≈10% larger in the mole rat, but this small difference did not reach statistical significance. As a combined result of these small differences, the alveolar surface area V(c) and the capillary volume S(a) were found to be higher by ≈20% in the mole rats, but these differences were significant only at the level of P < 0.1, largely due to a wide range of individual data in the mole rats. The difference in capillary surface area per unit body weight reached statistical significance (Fig. 4). The capillary loading of the gas exchange surface V(c)/S(a) was found to be the same in white and mole rats (Table 2). The arithmetic mean barrier thickness as well as the harmonic mean barrier thickness between alveolar surface and erythrocytes (a measure for the distance oxygen must travel from alveolar space to erythrocyte surface) only differed marginally with a tendency to smaller values in the mole rat lung (Table 2).
Table 2.
Lung structural data
| Animal | Vl, ml | VV(s,L), ml·ml−1 | V(c), ml | S(a), cm2 | V(c)/S(a), ml·m−2 | τt, μm | τhb, μm | DlO2* |
|---|---|---|---|---|---|---|---|---|
| Mole rats | 7.50 ± 0.33 | 0.210 ± 0.0074 | 0.901 ± 143 | 6406 ± 677 | 1.483 ± 0.242 | 1.040 ± 0.083 | 0.630 ± 0.042 | 0.026 ± 0.002† |
| (7.16–8.43) | (0.194–0.223) | (0.654–1.287) | (4932–8099) | (0.829–1.979) | (0.875–1.244) | (0.548–0.738) | (0.021–0.032) | |
| White rats | 7.01 ± 0.18 | 0.194 ± 0.0124 | 0.719 ± 0.068 | 5378 ± 165 | 1.371 ± 0.125 | 1.228 ± 0.079 | 0.770 ± 0.068 | 0.018 ± 0.001 |
| (6.72–7.46) | (0.162–0.221) | (0.569–0.857) | (5044–5835) | (1.068–1.666) | (1.077–1.454) | (0.666–0.96) | (0.016–0.021) |
Values indicate means ± SEM (n = 4 animals per group). Numbers in parentheses indicate range of data points. Data were collected as given in Materials and Methods. VL, lung volume; VV(s,L), septum volume per lung volume; Vc, volume of capillaries per lung; Sa, alveolar surface area per lung; Vc/Sa, capillary loading; τt, arithmetic mean tissue thickness; τhb, harmonic mean tissue thickness between alveolar surface and erythrocytes; and DlO2, pulmonary oxygen diffusing capacity.
Expressed in ml of O2 per sec per mmHg.
Significantly different from white rat (P < 0.05).
Figure 4.
Summary of key parameters determining oxygen uptake in the lungs in mole rats (hatched bars) compared with white rats (open bars). VL/Mb, lung volume related to body mass; S(a)/Mb, body mass-specific alveolar surface area; S(c)/Mb, capillary surface area relative to body mass; and DlO2/Mb, body mass-specific pulmonary diffusing capacity for oxygen. Data are reported as mean ± SEM. ∗, Significantly different vs. white rat, P < 0.05.
The combination of all the structural parameters resulted in a pulmonary diffusing capacity, DlO2, which was significantly higher by 43% in the mole rat lung (Table 2 and Fig. 4).
DISCUSSION
In considering the physiological data, it is first noted that the estimates of V̇O2 in white rats agree with previous estimates obtained with the same method (24). This applies to both the estimates of V̇O2max as for the y-intercept of the slope of V̇O2 increase with running speed. The mole rats achieved a similar V̇O2max at about the same running speed as the white rats (Fig. 1), which is probably related to the fact that the total muscle mitochondrial volume is about the same in both species (Table 1).
The main physiological difference between the two species is found in their tolerance of hypoxia. Under hypoxic conditions mole rats achieve a higher V̇O2max than white rats, and they tolerate a lower critical inspired PO2. This appears related to the fact that mole rats have a resting V̇O2 that is about half that of the white rats. The y-intercept of the V̇O2 increase with running speed is indeed much lower than that found in other mammals of the same body size. It seems that the physiological adaptation of mole rats to their hypoxic environment enables them to survive at a lower PO2 as compared with white rats (7). The depression of metabolic rate at rest is further supported by our observation that the resting heart rate of the mole rat is only 160 beats per min, as compared with 350 beats per min in the white rat, as shown previously (8). This may explain why the mole rats can sustain a critical PO2 that is 50% lower than that in the white rat. We therefore conclude that one of the fundamental adaptive strategies for survival under chronically hypoxic conditions in the mole rat is a depression of metabolic rate at low work rates. As a consequence, O2 consumption increases more steeply than in white rats when work rate increases to finally achieve a similar maximal rate in normoxia. That mole rats achieve a higher V̇O2max under hypoxic conditions than white rats is likely related to other physiological adaptations, mainly in the blood and circulation (9), and possibly in the design of the lung and the muscle microvasculature (see below).
Despite a similar body mass, mole rats have a significantly smaller whole body skeletal muscle mass than white rats, and it is differently distributed. Mole rats, which are teeth and head-lift diggers, have evolutionarily adjusted to this chief locomotor pattern by moving muscle mass to the front limb, shoulder, and neck (1). The mass of the front limb muscle M. deltoideus is larger in mole rats than in white rats. In contrast, the mass of their M. rectus femoris (located on the rear limbs) is less than that in white rats. Within the reduced muscle mass, both mitochondrial and capillary densities were increased significantly so that the total mitochondrial volume and the total capillary length were nearly the same in both species. When V̇O2max is related to total mitochondrial volume, we find that 1 ml of mitochondria consumes ≈5 ml of O2 per min at V̇O2max in both species, a value close to that found in other mammals (25, 26). Hence, there is no difference in the total mitochondrial capacity for oxidative phosphorylation.
The evidence concerning muscle structural adaptations to chronic hypoxia exposure, with regard to both muscle capillarity and muscle oxidative potential, is controversial. Hochachka et al. (27) reported that adaptations to chronic hypoxia are characterized by an increase in capillarity and muscle oxidative capacity, allowing organisms in hypoxia to maintain an acceptable level of aerobic metabolism in the face of a decreased oxygen supply. This view is not supported by experiments analyzing skeletal muscle tissue from human highland populations. Studies on both natives of the Andes (28) and Tibetans (29) report evidence of lower muscle oxidative capacities and a low to normal capillarity, as judged by lowland reference data. In the case of the Tibetans, these features seem to be genetically fixed, because they can also be demonstrated in Tibetans never exposed to high altitude (30). In the case of the mole rat, we find that both mitochondrial and capillary densities are significantly higher than those in white rats of the same body mass. Interestingly, this difference is brought about by a decrease in muscle mass at a constant absolute mitochondrial volume and capillary length. This has consequences for the O2 supply of skeletal muscle mitochondria, as discussed below. The reason for the discrepancy of findings regarding the strategies of skeletal muscle adaptations to hypoxia are not obvious and open to speculation (see also ref. 31). One specific aspect of the hypoxia adaptations of the mole rats might be related to the fact that these burrowing animals are exposed to high PCO2, not observed with high altitude hypoxia.
The local supply of O2 to the mitochondria in muscle cells is determined by the mitochondrial and capillary densities, both of which were found to be higher in the mole rat by 1.5 and 1.3 times, respectively. As a result, the cross-sectional area of a pericapillary cylinder of muscle cells (Krogh cylinder) is 0.75 times smaller in the mole rat, but it contains 10 percent more mitochondria. The combination of these two features leads to the conclusion that the mean diffusion distances from capillary to mitochondria in the mole rats is about two/thirds that in the white rat. Even with a lower capillary PO2, mole rats therefore can maintain an adequate O2 diffusion gradient to the mitochondria. This transfer is further facilitated by the 3-fold higher myoglobin concentration observed in the mole rat, a process that is particularly important at low O2 pressures (2, 32). Myoglobin facilitates diffusion and steepens the gradient from red cell to mitochondria by lowering the concentration of free oxygen near the sarcolemma. In effect, during heavy exercise, the myoglobin-facilitated flux is dominant, which is likely the case during digging and foraging underground.
No structural specializations were detected in the heart of mole rats nor were myoglobin levels different from those of white rats. The identical design of rat and mole rat hearts is not surprising, in view of the fact that the peripheral vascular bed as well as the oxidative capacity that the hearts are subserving are identical in both species; also, both species achieve the same maximal heart frequency in exercise. The absence of differences in capillarization and myoglobin concentration points to the fact that, for the heart, there seems to be no oxygen diffusion problem. Mole rats use specific modifications of heart functions when dealing with hypoxia (12, 23). It is reported that the mole rat maintains up to 2 times higher heart rates in hypoxia than any other rodents (12). It is interesting that we observed the mole rats to increase their resting heart rate as FiO2 was reduced, whereas this did not occur in white rats. Oxygen transport in mole rat at a low resting heart rate in normoxia is achieved by extremely low venous saturations (of ≈6%) and a very low tissue PO2 [of 15.1 mmHg (1 mmHg = 133 Pa; ref. 10]. Incidentally, we found that the hematocrit was not significantly different between white rats and the mole rats of this study, although it may vary significantly among the S. ehrenbergi species (5).
Oxygen uptake in the lung is driven by the O2 pressure difference between air and blood and determined by the pulmonary diffusing capacity, which depends on a combination of several structural parameters (12, 15, 21, 33).
The morphometric study revealed that the alveolar surface area and the alveolar capillary volume were each ≈20% larger in the mole rat, whereas the diffusion barrier thickness was somewhat reduced. As a result, the pulmonary diffusing capacity was estimated to be ≈40% higher in the mole rat. From previous studies (34), we would predict that the higher pulmonary diffusing capacity should allow the mole rats to maintain V̇O2max, even if inspired PO2 is reduced. For the same reason, the observation that mole rats achieve a slightly higher V̇O2max than white rats at 11% FiO2 may also be related to their higher pulmonary diffusing capacity, which would facilitate loading O2 onto the blood if the inspired PO2 as driving force is reduced.
In conclusion, the mole rat has evolved a number of structural and functional modifications in its respiratory system that appear as adaptations to an active life in a hypoxic environment in their burrows. Their metabolic rate at low work rates is depressed, which allows them to survive when O2 is very scarce. Their muscles are provided with the same amount of mitochondria as white rats, which allows them to achieve the same maximal rate of O2 consumption at similar work rates under normoxic conditions. Furthermore, the diffusing capacity of their lungs is increased to a sufficient extent to allow them to achieve higher maximal rates of oxidative metabolism, even if the inspired PO2 is reduced to half of normal. Survival underground evidently requires several different adaptations in the pathway for oxygen, both physiological and morphometric, from the muscle and heart up to the lung, and this appears fulfilled in the mole rat.
Acknowledgments
Some of the physiological results were obtained by Evan S. Ong and Larry K. Fan as part of their masters theses. We are grateful to Dr. E. Altpeter, H. Claassen, Dr. T. Roberts, L. Tüscher, and E. Wagner for their contributions. E.N. thanks the Israeli Discount Bank Chair of Evolutionary Biology and the Ancell–Teicher Research Foundation for Genetics and Molecular Biology for financial support. The study was supported by grants from the Swiss National Science Foundation (to H.H. and E.R.W.), the National Institutes of Health (to C.R.T.), and the Maurice E. Müller Foundation.
References
- 1.Nevo E. In: Evolutionary Biology. Hecht H K, Wallace B, MacIntyre R J, editors. Vol. 25. New York: Plenum; 1991. pp. 1–125. [Google Scholar]
- 2.Arieli R. In: Evolution of Subterranean Mammals at the Organismal and Molecular Levels. Nevo E, Reig O A, editors. New York: Wiley–Liss; 1990. pp. 251–268. [Google Scholar]
- 3.Arieli R, Ar A. J Appl Physiol. 1979;47:1011–1017. doi: 10.1152/jappl.1979.47.5.1011. [DOI] [PubMed] [Google Scholar]
- 4.Yahav S, Simson S, Nevo E. Oecologia. 1988;77:533–536. doi: 10.1007/BF00377270. [DOI] [PubMed] [Google Scholar]
- 5.Arieli R, Heth G, Nevo E, Hoch D. Experientia. 1986;42:441–443. doi: 10.1007/BF02118650. [DOI] [PubMed] [Google Scholar]
- 6.Nevo E. Annu Rev Ecol Syst. 1979;10:269–308. [Google Scholar]
- 7.Arieli R, Kerem D. Undersea Biomed Res. 1984;11:275–285. [PubMed] [Google Scholar]
- 8.Storier D, Wollberg Z, Ar A. J Comp Physiol B. 1981;142:533–538. [Google Scholar]
- 9.Arieli R, Nevo E. Comp Biochem Physiol. 1991;100:543–545. doi: 10.1016/0300-9629(91)90367-l. [DOI] [PubMed] [Google Scholar]
- 10.Ar A, Arieli R, Shkolnik A. Respir Physiol. 1977;30:201–218. doi: 10.1016/0034-5687(77)90031-7. [DOI] [PubMed] [Google Scholar]
- 11.Arieli R, Ar A, Shkolnik A. Physiol Zool. 1977;50:61–75. doi: 10.1016/0034-5687(77)90031-7. [DOI] [PubMed] [Google Scholar]
- 12.Arieli R, Ar A. Physiol Zool. 1981;54:14–21. [Google Scholar]
- 13.Ar, A. (1987) in Comparative Physiology of Environmental Adaptations, Eighth European Society for Comparative Physiology Conference, Strasbourg, France, ed. Dejours, P. (Karger, Basel), pp. 208–221.
- 14.Fedak M A, Rome L, Seeherman H J. J Appl Physiol Respir Environ Exercise Physiol. 1981;51:772–776. doi: 10.1152/jappl.1981.51.3.772. [DOI] [PubMed] [Google Scholar]
- 15.Vock R, Weibel E R. Exp Lung Res. 1993;19:559–557. doi: 10.3109/01902149309031728. [DOI] [PubMed] [Google Scholar]
- 16.Hoppeler H, Lindstedt S L, Uhlmann E, Niesel A, Cruz-Orive L M, Weibel E R. J Comp Physiol B. 1984;155:51–61. [Google Scholar]
- 17.Vock R, Weibel E R, Hoppeler H, Ordway G, Weber J M, Taylor C R. J Exp Biol. 1996;199:1675–1688. doi: 10.1242/jeb.199.8.1675. [DOI] [PubMed] [Google Scholar]
- 18.Reynafarje B. J Lab Clin Med. 1963;61:138–165. [PubMed] [Google Scholar]
- 19.Laemmli U K. Nature (London) 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Widmer H R, Hefti F. Eur J Neurosci. 1994;6:1669–1679. doi: 10.1111/j.1460-9568.1994.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 21.Weibel E R, Federspiel W J, Fryder-Doffey F, Hsia C C W, König M, Stalder-Navarro V, Vock R. Respir Physiol. 1993;93:125–149. doi: 10.1016/0034-5687(93)90001-q. [DOI] [PubMed] [Google Scholar]
- 22.Efron B, Tibshirani R J. An Introduction to the Bootstrap. London: Chapman & Hall; 1993. [Google Scholar]
- 23.Edoute Y, Arieli R, Nevo E. J Comp Physiol B. 1988;158:575–582. doi: 10.1007/BF00692566. [DOI] [PubMed] [Google Scholar]
- 24.Seeherman H J, Taylor C R, Maloiy G M O, Armstrong R B. Respir Physiol. 1981;44:11–17. doi: 10.1016/0034-5687(81)90074-8. [DOI] [PubMed] [Google Scholar]
- 25.Weibel E R, Taylor C R, Hoppeler H. Respir Physiol. 1992;87:325–348. doi: 10.1016/0034-5687(92)90015-o. [DOI] [PubMed] [Google Scholar]
- 26.Weibel E R, Taylor C R, Hoppeler H. Proc Natl Acad Sci USA. 1991;88:10357–10361. doi: 10.1073/pnas.88.22.10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hochachka P W, Stanley C, Merkt J, Sumar-Kalinowski J. Respir Physiol. 1983;52:303–313. doi: 10.1016/0034-5687(83)90087-7. [DOI] [PubMed] [Google Scholar]
- 28.Desplanches D, Hoppeler H, Tüscher L, Mayhet M H, Spielvogel H, Ferretti G, Kayser B. J Appl Physiol. 1996;81:1946–1951. doi: 10.1152/jappl.1996.81.5.1946. [DOI] [PubMed] [Google Scholar]
- 29.Kayser B, Hoppeler H, Claassen H, Cerretelli P. J Appl Physiol. 1991;70:938–1942. doi: 10.1152/jappl.1991.70.5.1938. [DOI] [PubMed] [Google Scholar]
- 30.Kayser B, Hoppeler H, Desplanches D, Marconi C, Broers B, Cerretelli P. J Appl Physiol. 1996;81:419–425. doi: 10.1152/jappl.1996.81.1.419. [DOI] [PubMed] [Google Scholar]
- 31.Banchero E. Annu Rev Physiol. 1987;49:465–474. doi: 10.1146/annurev.ph.49.030187.002341. [DOI] [PubMed] [Google Scholar]
- 32.Honig C R, Connett R J, Gayeski E J. Med Sci Sports Exercise. 1992;24:47–53. [PubMed] [Google Scholar]
- 33.Weibel E R, Marques L B, Constantinopol M, Doffey F, Gehr P, Taylor C R. Respir Physiol. 1987;69:81–100. [Google Scholar]
- 34.Karas R H, Taylor C R, Rösler K, Hoppeler H. Respir Physiol. 1987;69:65–81. doi: 10.1016/0034-5687(87)90097-1. [DOI] [PubMed] [Google Scholar]