Abstract
It is increasingly clear that growth hormone (GH) has growth-promoting effects in fishes, which are mediated in part by the insulin-like growth factor (IGF)-I. Growth-promoting actions of prolactin (PRL) have been reported in higher vertebrates, but are less well established in teleosts. We examined the effects of injecting homologous GH or the two homologous tilapia PRLs (tPRL177 and tPRL188) on the in vitro incorporation of [35S]sulfate (extracellular matrix synthesis) and [3H]thymidine (DNA synthesis) by ceratobranchial cartilage explants and on IGF-I mRNA levels in tilapia liver. Tilapia GH (tGH) and tPRL177 stimulated sulfate uptake at the highest doses examined. Thymidine incorporation was stimulated by tPRL177. tPRL188 was without these effects. Consistent with its somatotropic actions, tGH elevated IGF-I mRNA levels in the liver. tPRL177 also elevated liver IGF-I levels. Consistent with the previously described osmoregulatory actions of GH and PRL in teleosts, we observed that tGH elevated and tPRL177 and tPRL188 lowered levels of gill Na+,K+-ATPase activity. High-affinity, low-capacity binding sites for tGH in the tilapia liver were identified. tPRL177 binds with lower affinity than tGH to these sites but can displace 125I-labeled tGH from its receptor. The ability of tPRL177 to displace tGH was similar to that of ovine GH. tPRL188 did not displace 125I-labeled tGH binding. Collectively, this work suggests that tPRL177 may possess somatotropic actions similar to tGH, but only in freshwater tilapia where tPRL177 levels are sufficiently high for it to act as a competitive ligand for GH receptors.
Keywords: insulin-like growth factor, growth promotion
Growth hormone (GH), prolactin (PRL), and somatolactin belong to a family of hormones that share similarities in structure and function. Based on the similarities in structure, function, and gene sequences, it has been proposed that these hormones evolved from a common ancestral gene through duplication and subsequent divergence (1, 2, 53).
GH regulates growth in all vertebrates, including fish (2, 3). Recently, GH has been implicated in seawater osmoregulation of salmonid and cichlid teleosts (4–6). The actions of GH are mediated to an important degree through insulin-like growth factor (IGF)-I (7, 8), and the primary source of circulating IGF-I is the liver (8).
PRL is the most versatile of the pituitary hormones. It shows lactogenic, luteotropic, mitogenic, somatotropic, metamorphic, antimetamorphic, and osmoregulatory activities (9–14). Among teleosts, the most prominent action of PRL is its osmoregulatory role in freshwater adaptation (10). Since it may be argued that the somatotropic activities ascribed to PRL result from the binding of heterologous PRL to GH receptors (15, 16), more interest has been directed toward examining the growth-promoting and osmoregulatory actions of homologous hormones in teleosts.
Two forms of PRL have been identified in the tilapia, one 177 aa (tPRL177) and the other 188 aa (tPRL188; refs. 17 and 18). Each is encoded by a separate gene, suggesting unique roles for these two PRLs in tilapia (19). Studies aimed at identifying unique actions of each tPRL have not revealed consistent differences, but it is clear that both hormones facilitate osmoregulatory adaptation to freshwater (17, 20, 21).
Until now, evidence for the somatotropic actions of homologous PRL in teleosts is largely lacking. We examined the effects of homologous tGH, tPRL177, and tPRL188 on growth in hypophysectomized (Hx) tilapia, as determined by ceratobranchial cartilage [35S]sulfate and [3H]thymidine incorporation in vitro and measurements of liver IGF-I mRNA levels. Since the osmoregulatory and growth-promoting actions of GH and PRL are specific and depend upon circulating levels and the availability and specificity of tissue receptors, we evaluated the affinity of hepatic GH receptors for 125I-labeled tGH. We also evaluated the actions of tGH and tPRLs on gill Na+,K+-ATPase activity.
MATERIALS AND METHODS
Animals and Hypophysectomy.
Animal husbandry is described elsewhere (4, 22). Mature tilapia (Oreochromis mossambicus) of both sexes (35–90 g) were Hx as described elsewhere (23). Postoperative procedures are also described elsewhere (4).
Hormones.
Tilapia pituitary tPRL177, tPRL188, and tGH were administered by intraperitoneal injection every 2 days for 8 days as described elsewhere (4). In this study, we employed hormone doses of 15 (low), 150 (medium), and 500 (high) ng per g of body weight.
Experimental Procedure.
Experimental procedures for the injection studies are described elsewhere (4), with the following modifications. Twenty-four hours after the last injection, gill arches were removed and ceratobranchial cartilages were prepared for incubation. Liver tissue and gill filaments were removed, flash-frozen, and stored at −80°C for subsequent IGF-I mRNA analysis and Na+,K+-ATPase analysis, respectively.
Ceratobranchial Cartilage Bioassay.
The methods employed are those of Duan (24) as modified by Shepherd et al. (25) with the following exceptions: (i) no tilapia plasma was used in the incubation medium, and (ii) 2.5 μCi/ml (1 Ci = 37 GBq) of [3H]thymidine and 5.0 μCi/ml [35S]sulfate (DuPont/NEN) were used in the incubation medium.
Hepatic GH Radioreceptor Assay.
Our methods were identical to those of Hirano (26), which have been used successfully to study liver hormone receptors in different teleost species. Using these procedures, we found that tilapia liver membranes demonstrated high specific binding of 125I-labeled tGH, which was linear up to 100 mg protein and saturable with increasing amounts of membrane protein. The optimal pH for specific binding was 7.4, and equilibrium was achieved after 15 hr at 15°C.
RNase Protection Assay.
Total RNA was isolated from frozen tissues by the method of Chomczynski and Sacchi (27) and subsequently quantified by UV spectrophotometry. cDNAs encoding mature IGF-I (B–D domains) derived from teleost species are highly conserved; however, the identity between rainbow trout IGF-I and IGF-II is only 46.1% (2, 28). Total IGF-I mRNA transcripts were measured using a riboprobe complementary to a 213-bp region coding for all rainbow trout IGF-I mRNA forms (29). Crossreactivity of the antisense IGF-I riboprobe with all IGF-I mRNA forms, but not of IGF-II sense cRNA, has been described elsewhere (29). Sense IGF-I cRNA for use in standard curves was generated by transcription of an XhoI-linearized construct with T3 polymerase. The antisense IGF-I cRNA probe was generated using a NotI-linearized construct with T7 polymerase. The IGF-I riboprobe protects an ≈100-bp region of tilapia IGF-I. The protected base component represents all transcripts coding for tilapia IGF-I forms (29).
The RNase protection assay was performed as described elsewhere (29, 30), with the following modifications. Briefly, RNA (50 μg) was hybridized with probe overnight at 50°C in 30 μl of hybridization buffer. Digestion was carried out under stringent conditions with the addition of 250 μl of RNase-T2 (GIBCO/BRL) solution (30 units/ml) for 1 hr at 37°C. For validation, protected fragments were isolated, precipitated, and separated on 6% polyacrylamide/8 M urea gels and exposed to x-ray film. For quantification, the digested RNA was precipitated by the addition of 300 μg of yeast tRNA and 300 μl of ice-cold 10% trichloroacetic acid and kept on ice for 10 min. Precipitates were collected on GF/C glass fiber filters (Whatman) using a vacuum-manifold harvester (Brandel, Bethesda, Maryland). Filters were washed three times with ice-cold 20 mM sodium pyrophosphate in 5% trichloroacetic acid, washed once with 70% ethanol, air-dried, and processed for scintillation counting on a Beckman LS-3801 counter. The amount of IGF-I mRNA in a sample was calculated from standard curves. Results are expressed as pg of mRNA per μg of RNA. To determine whether equal amounts of total RNA were used in our assays, levels of 18S rRNA were assessed by Northern blot procedures as described elsewhere (30).
Gill Na+,K+-ATPase Activity.
Gill Na+, K+-ATPase activity was analyzed at 25°C according to the method of McCormick and Bern (31), and values were expressed as μmol of ADP per mg of protein per hr.
Statistical Analyses.
Significant differences among group means were evaluated using one-way analysis of variance (minitab, State College, PA) coupled with Fisher’s protected least significant difference (FPLSD) test for predetermined pairwise comparisons (32).
RESULTS
Cartilage [35S]Sulfate and [3H]Thymidine Incorporation.
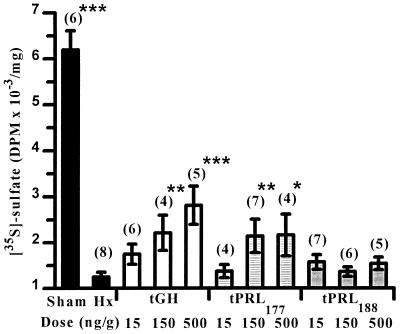
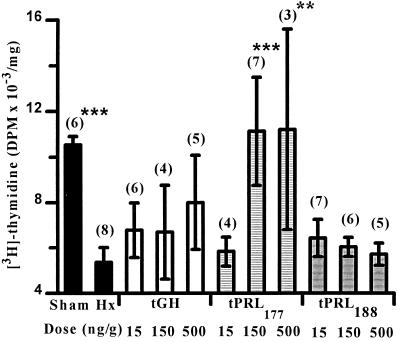
Hypophysectomy lowered levels of sulfate incorporation into cartilage tissues compared with sham-operated control values (Fig. 1). tGH increased sulfate uptake in tissues in a dose-related manner. tPRL177 treatment increased sulfate uptake in tissues at the two highest doses. Tissues from Hx controls showed lower levels of thymidine incorporation than sham-operated animals (Fig. 2). Thymidine incorporation in tissues from tPRL177-injected animals was stimulated at the two highest doses. tPRL188 had no effect on sulfate or thymidine incorporation. This study was repeated and we observed some differences: (i) hypophysectomy did not measurably alter thymidine incorporation in cartilage tissues (data not shown); (ii) tPRL177 injection at the lowest dose significantly (P < 0.01) stimulated sulfate uptake; and (iii) tGH at the highest dose significantly (P < 0.001) stimulated thymidine incorporation.
Figure 1.
Effects of hypophysectomy and homologous tGH, tPRL177, and tPRL188 replacement at different doses on the in vitro uptake of [35S]sulfate by ceratobranchial cartilage explants. Sham-operated and Hx controls were injected with vehicle only. ∗, P < 0.05, ∗∗, P < 0.01, and ∗∗∗, P < 0.001 compared with Hx controls (FPLSD). Values represent the mean ± SEM and are expressed as decays per minute (DPM) per mg of dry weight of cartilage. Numbers in parentheses denote sample size.
Figure 2.
Effects of hypophysectomy and homologous tGH, tPRL177, and tPRL188 replacement at different doses on the in vitro uptake of [3H]thymidine by ceratobranchial cartilage explants. Sham-operated and Hx controls were injected with vehicle only. ∗∗, P < 0.01, and ∗∗∗, P < 0.001 compared with Hx controls (FPLSD). Values represent the mean ± SEM and are expressed as decays per minute (DPM) per mg of dry weight of cartilage. Numbers in parentheses denote sample size.
Presence of Hepatic tGH- and tPRL177-Dependent IGF-I mRNA Expression.
The effects of tGH and tPRL(s) replacement on IGF-I mRNA levels in liver tissue were examined by RNase protection assay. Compared with sham-operated values, hypophysectomy had no effect on IGF-I mRNA levels (Table 1). tGH treatment significantly increased IGF-I mRNA levels at the highest dose. tPRL177 stimulated IGF-I mRNA levels at the two highest doses. tPRL188 had no effect on IGF-I mRNA levels (Table 1). Northern blot procedures for 18S rRNA revealed that equal amounts of RNA were used in our RNase protection assay; therefore, our IGF-I mRNA values were normalized to total RNA and values are expressed as pg of IGF-I mRNA per μg of total RNA.
Table 1.
Liver IGF-I mRNA levels
| Treatment | Dose
|
||
|---|---|---|---|
| 15 ng/g | 150 ng/g | 500 ng/g | |
| tGH | 1.8 ± 0.7 | 2.5 ± 0.9 | 15.0 ± 5.0* |
| tPRL177 | 0.2 ± 0.0 | 2.6 ± 0.6* | 2.9 ± 0.5* |
| tPRL188 | 1.6 ± 1.2 | 1.2 ± 0.8 | 0.8 ± 0.3 |
Effects of hypophysectomy and hormone replacement on liver IGF-I mRNA levels. IGF-I values for vehicle-injected Hx and sham-operated control groups are 0.2 ± 0.0 and 0.8 ± 0.3 pg per mg of total RNA, respectively. Values represent the mean ± SEM (n = 3) of duplicate determinations and are in pg per mg of total RNA.
P < 0.001 compared with Hx controls (FPLSD for those groups being compared).
Hepatic Radioreceptor Assay: Conditions and Scatchard Analysis.
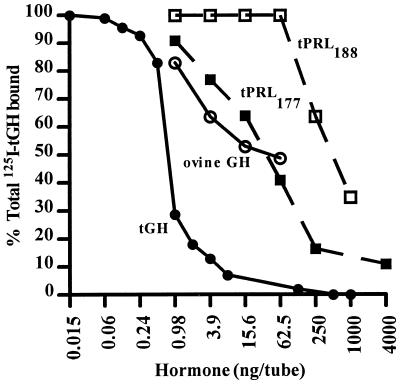
Scatchard analysis indicated a single class of binding sites with a capacity of 241 ± 99 fmol per mg of membrane protein and a binding affinity of 0.75 ± 0.11 nM−1. Significant specific binding was also found in membrane preparations from gill, heart, kidney, muscle, and testis, but levels were <30% of that observed from liver (data not shown). tGH competed equally well as eel GH with eel liver membrane preparations (data not shown). Eel GH competed as well as tGH with tilapia liver membrane preparations (data not shown). In tilapia liver membrane preparations, ovine GH was almost 20-fold less potent than tGH (Fig. 3 and Table 2). We also observed that 125I-labeled tGH can be displaced equally well by tPRL177 and ovine GH, but not to any appreciable degree by tPRL188 (Fig. 3 and Table 2) or ovine PRL (data not shown). tPRL177 was 50-fold less potent than tGH at displacing 125I-tGH from tilapia liver binding sites (Table 2). In eel liver membrane preparations, tPRL177 did not compete for eel GH binding sites (data not shown).
Figure 3.
Displacement of 125I-labeled tGH from high-affinity, low-capacity hepatic binding sites by tGH (•), ovine GH (○), tPRL177 (▪), and tPRL188 (□). Specific binding is shown as a percentage of total radioactivity in each tube. Each point represents the mean of duplicate determinations.
Table 2.
Potencies of tGH and tPRLs in radioreceptor assays
| Membrane | tGH ED50, ng per tube | Potency relative to tGH*
|
||
|---|---|---|---|---|
| tGH | tPRL177 | tPRL188 | ||
| Tilapia liver | 0.68 | 100 | 2.0 | 0.15 |
| Eel liver | 0.70 | 100 | <0.0006 | <0.0006 |
Potency of the tGH standard was designated as 100%.
Gill Na+,K+-ATPase Activity.
As seen in Table 3, hypophysectomy lowered levels of gill Na+,K+-ATPase compared with sham-operated values. tGH elevated Na+,K+-ATPase at all doses compared with Hx control values. tPRL177 at the low and high doses had no effect on gill Na+,K+-ATPase, but at the middle dose it was effective at depressing Na+,K+-ATPase levels below Hx values. tPRL188 at the two lowest doses had no effect, but the high dose showed a mean Na+,K+-ATPase level that was lower than levels observed at the other two doses and in the Hx group.
Table 3.
Gill Na+,K+-ATPase activity
| Treatment | Dose
|
||
|---|---|---|---|
| 15 ng/g | 150 ng/g | 500 ng/g | |
| tGH | 1.4 ± 0.2* | 1.3 ± 0.1† | 1.2 ± 0.1‡ |
| tPRL177 | 1.0 ± 0.1 | 0.7 ± 0.1‡ | 0.8 ± 0.1 |
| tPRL188 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.0 |
Effects of hypophysectomy and homologous tGH, tPRL177, and tPRL188 replacement at different doses on gill filament Na+,K+-ATPase activity. Na+,K+-ATPase values for vehicle-injected Hx and sham-operated control groups are 0.9 ± 0.1 and 1.9 ± 0.1 μmol of ADP per mg of protein per hr, respectively. Values represent the mean ± SEM (n = 6-20) and are in μmol of ADP per mg of protein per hr.
P < 0.001;
P < 0.005; and
P < 0.05, compared with Hx controls (FPLSD).
DISCUSSION
Results of the ceratobranchial sulfate/thymidine assay and analyses of liver IGF-I mRNA levels from the present study offer evidence, in a teleost fish, that the homologous pituitary hormones tPRL177 and tGH promote growth and alter levels of liver IGF-I in Hx adult animals.
In teleosts, liver IGF-I and IGF-II production is GH-dependent, and the incorporation of [35S]sulfate into cartilage has been shown to be stimulated by IGF-I (24, 29). We have found that tGH and tPRL177 treatment increase sulfate uptake in vitro in cartilage. Studies on other vertebrates have also shown a stimulatory effect of both heterologous or homologous PRLs on cartilage sulfation and on liver IGF-I activity (for review see refs. 3 and 11). In contrast, Duan et al. (33, 34) found that GH and somatolactin stimulated liver IGF-I mRNA expression in sockeye and coho salmon, but PRL did not. The differences observed between our work and that of Duan et al. (33, 34) may derive from the use the use of PRL in animals in physiological states wherein PRL is known to not be active (as in salmonid smolts) or antagonistic to seawater survival (4, 6, 35, 36). We observed that hypophysectomy did not affect liver IGF-I mRNA levels. This may be due to the extended period of fasting (14–16 days during our experiment), which reduces circulating IGF-I levels and liver IGF-I gene expression in other vertebrates (37, 38).
Some work on tilapia has focused on identifying the possible differences between the two PRLs (17, 20, 39, 40), but results have been inconsistent (41, 42). Recent work, however, suggests that tPRL188 possesses greater sodium-retaining and calciotropic activity than does tPRL177 (4, 20, 21).
GH and PRL have osmoregulatory actions in tilapia and other teleosts (4, 5, 10, 43). GH treatment results in increased levels of gill Na+,K+-ATPase that are consistent with the seawater-adapting role of this hormone in salmonids and cichlids (4, 6). Conversely, PRL treatment reduces gill Na+,K+-ATPase levels, consistent with its role as the freshwater osmoregulatory hormone (6, 35). More important to this study is that the actions of our GH and PRL preparations are consistent with their osmoregulatory roles in this teleost (4, 22) and strongly suggest that the somatotropic activity of our tPRL177 preparation is not due to tGH contamination.
Binding sites for GH and PRL have been identified in several tissues of the tilapia (15). Few studies, however, have examined the relationships between environmental adaptation and the hormones and receptors involved (44–46). We have undertaken radioreceptor studies to understand how GH and PRL exert their biological activities in the tilapia. We have identified high-affinity, low-capacity binding sites for GH in tilapia liver. tPRL177 binds to these sites, albeit with lower affinity than tGH, but equally as well as ovine GH, the actions of which on growth in teleosts have been described (12, 47, 48). tPRL188 does not compete for tGH binding sites. In eel liver membrane preparations, tPRL177 did not compete with eel GH or tGH for binding sites, which suggests that the ability of tPRL177 to displace tGH may not be general in teleosts. It is interesting that tPRL177, but not tPRL188, can displace 125I-labeled tGH from its receptor given the structural similarities of these hormones and their receptors (49, 50).
tPRL177 may also have a novel cartilage or liver receptor to which tPRL188 does not bind. Such a possibility is suggested by the stimulatory effect of tPRL177 on cartilage [3H]thymidine incorporation observed in our first study. Recent work on Oreochromis niloticus has demonstrated the presence of a single class of PRL receptors and a recombinant PRL receptor, which have higher affinity for tPRL188 than for tPRL177 (45, 50). This suggests that the actions of tPRL177 may overlap with those of tPRL188 and tGH through its ability to bind to their receptors. This may be analogous to the phenomenon of primate GH binding to GH and PRL receptors (9, 51).
Our findings suggest that tPRL177, in addition to its freshwater osmoregulatory role, may also be somatotropic in freshwater tilapia, where circulating levels are high (22, 25, 39). Also suggested by our work and that of others is that GH possesses both osmoregulatory and somatotropic actions in seawater tilapia (4, 25, 52). Collectively, these findings point to the existence of a GH/PRL–IGF-I axis in the tilapia.
Acknowledgments
We thank Drs. S. Hyodo, C. M. Lin, C. Raghberg, M. Shamblott, N. Vrolicjk, and G. Weber, Mrs. C. Ball, Mrs. M. Shepherd, and Mr. C. Morrey for their assistance during this study. We also thank Dr. D. S. King for purifying tGH and tPRLs. The work was supported in part by a Japan Society for the Promotion of Science fellowship to T.S. and grants-in-aid from the Ministry of Education and from the Fisheries Agency of Japan to T.H.; grants from the California State Resources Agency and University of California Sea Grant College Program [National Oceanic and Atmospheric Administration (NOAA)], NA89AA-D-SG138 R/F-145 to H.A.B.; grants from the Edwin W. Pauley Foundation, Hawaii Aquaculture Development Program Grant 35965, National Science Foundation Grant DCB 9104494, U.S. Department of Agriculture Grant 95-37206-2283, grants from the University of Hawaii Sea Grant College Program (R/AQ-37), and School of Ocean and Earth Science and Technology Institutional Grant NA 36RG0507 (Yr. 27–28) to E.G.G.; and Program Development Grants R/MR-55 PD and E/ET-23PD to B.S.S. through E.G.G. from NOAA. This paper represents University of Hawaii Sea Grant College contribution no. UNIHI-SEA GRANT-JC97-02.
ABBREVIATIONS
- GH
growth hormone
- tGH
tilapia GH
- PRL
prolactin
- tPRL177 and tPRL188
177-aa and 188-aa tilapia PRL, respectively
- IGF
insulin-like growth factor
- Hx
hypophysectomized
References
- 1.Scanes C G, Campbell R M. In: Growth Hormone. Harvey S, Scanes C G, Daughaday W H, editors. London: CRC; 1995. pp. 25–37. [Google Scholar]
- 2.Chen T T, Marsh A, Shamblott M, Chan K-M, Tang Y-L, Cheng C M, Yang B Y. In: Fish Physiology: Molecular Endocrinology of Fish. Sherwood N M, Hew C L, editors. Vol. 13. New York: Academic; 1994. pp. 179–209. [Google Scholar]
- 3.Scanes C G, Daughaday W H. In: Growth Hormone. Harvey S, Scanes C G, Daughaday W H, editors. London: CRC; 1995. pp. 351–370. [Google Scholar]
- 4.Sakamoto, T., Shepherd, B. S., Nishioka, R. S., Madsen, S. S., Siharath, K., Bern, H. A. & Grau, E. G. (1997) Gen. Comp. Endocrinol., in press. [DOI] [PubMed]
- 5.Sakamoto T, McCormick S D, Hirano T. Fish Physiol Biochem. 1993;11:155–164. doi: 10.1007/BF00004562. [DOI] [PubMed] [Google Scholar]
- 6.McCormick S D. In: Fish Physiology: Cellular and Molecular Approaches to Fish Ionic Regulation. Hoar W S, Randall D J, Farrell A P, editors. Vol. 14. San Francisco: Academic; 1995. pp. 285–315. [Google Scholar]
- 7.Siharath, K. & Bern, H. A. (1993) Proc. Zool. Soc. (Calcutta), Haldane Commem. Vol., 113–124.
- 8.Cohick W S, Clemmons D R. Annu Rev Physiol. 1993;55:131–153. doi: 10.1146/annurev.ph.55.030193.001023. [DOI] [PubMed] [Google Scholar]
- 9.Nicoll C S. In: Handbook of Physiology: Endocrinology IV. Knobil E, Sawyer W H, editors. Vol. 2. Baltimore: Williams & Wilkins; 1974. pp. 253–291. [Google Scholar]
- 10.Hirano T. In: Progress in Clinical And Biological Research: Comparative Endocrinology: Developments and Directions. Ralph C L, editor. Vol. 205. New York: Liss; 1986. pp. 53–74. [PubMed] [Google Scholar]
- 11.Nicoll C S. In: The Endocrinology of Growth, Development, and Metabolism in Vertebrates. Schreibman M P, Scanes C G, Pang P K T, editors. New York: Academic; 1993. pp. 151–182. [Google Scholar]
- 12.Clarke W C, Walker Farmer S, Hartwell K M. Gen Comp Endocrinol. 1977;33:174–178. doi: 10.1016/0016-6480(77)90241-6. [DOI] [PubMed] [Google Scholar]
- 13.Walker Farmer S, Papkoff H, Bewley T A, Hayashida T, Nishioka R S, Bern H A, Li C H. Gen Comp Endocrinol. 1977;31:60–71. doi: 10.1016/0016-6480(77)90191-5. [DOI] [PubMed] [Google Scholar]
- 14.Licht P, Hoyer H. Gen Comp Endocrinol. 1968;11:338–346. doi: 10.1016/0016-6480(68)90090-7. [DOI] [PubMed] [Google Scholar]
- 15.Prunet P, Auperin B. In: Fish Physiology: Molecular Endocrinology of Fish. Sherwood N M, Hew C L, editors. Vol. 13. New York: Academic; 1994. pp. 367–391. [Google Scholar]
- 16.Brelje T C, Allaire P, Hegre O, Sorenson R L. Endocrinology. 1989;125:2392–2399. doi: 10.1210/endo-125-5-2392. [DOI] [PubMed] [Google Scholar]
- 17.Specker J L, King D S, Nishioka R S, Shirahata K, Yamaguchi K, Bern H A. Proc Natl Acad Sci USA. 1985;82:7490–7494. doi: 10.1073/pnas.82.22.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaguchi K, Specker J L, King D S, Yokoo Y, Nishioka R S, Hirano T, Bern H A. J Biol Chem. 1988;263:9113–9121. [PubMed] [Google Scholar]
- 19.Rentier-Delrue F, Swennen D, Prunet P, Lion M, Martial J A. DNA. 1989;8:261–270. doi: 10.1089/dna.1.1989.8.261. [DOI] [PubMed] [Google Scholar]
- 20.Auperin B, Rentier-Delrue F, Martial J A, Prunet P. J Mol Endocrinol. 1994;12:13–24. doi: 10.1677/jme.0.0120013. [DOI] [PubMed] [Google Scholar]
- 21.Flik G, Rentier-Delrue F, Wendelaar-Bonga S E. Am J Physiol. 1994;266:R1302–R1308. doi: 10.1152/ajpregu.1994.266.4.R1302. [DOI] [PubMed] [Google Scholar]
- 22.Yada T, Hirano T, Grau E G. Gen Comp Endocrinol. 1994;93:214–223. doi: 10.1006/gcen.1994.1025. [DOI] [PubMed] [Google Scholar]
- 23.Nishioka R S. In: Biochemistry and Molecular Biology of Fishes: Analytical Techniques. Hochachka P W, Mommsen T P, editors. Vol. 3. New York: Elsevier; 1994. pp. 49–58. [Google Scholar]
- 24.Duan C. In: Biochemistry and Molecular Biology of Fishes: Analytical Techniques. Hochachka P W, Mommsen T P, editors. Vol. 3. New York: Elsevier; 1994. pp. 525–533. [Google Scholar]
- 25.Shepherd, B. S., Ron, B., Burch, A., Sparks, R., Richman, N. H., Shimoda, S. K., Stetson, M. H., Lim, C. & Grau, E. G. (1997) Fish Physiol. Biochem., in press.
- 26.Hirano T. Gen Comp Endocrinol. 1991;81:383–390. doi: 10.1016/0016-6480(91)90165-3. [DOI] [PubMed] [Google Scholar]
- 27.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 28.Duguay S J, Lai-Zhang J, Steiner D F, Funkenstein B, Chan S J. J Mol Endocrinol. 1996;16:123–132. doi: 10.1677/jme.0.0160123. [DOI] [PubMed] [Google Scholar]
- 29.Shamblott M J, Cheng C M, Bolt D, Chen T T. Proc Natl Acad Sci USA. 1995;92:6943–6946. doi: 10.1073/pnas.92.15.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shamblott M J, Chen T T. Mol Mar Biol Biotechnol. 1993;2:351–361. [PubMed] [Google Scholar]
- 31.McCormick S D, Bern H A. Am J Physiol. 1989;256:R707–R715. doi: 10.1152/ajpregu.1989.256.3.R707. [DOI] [PubMed] [Google Scholar]
- 32.Steele R G D, Torrie J H. Principles and Procedures of Statistics: A Biometrical Approach. New York: McGraw–Hill; 1980. [Google Scholar]
- 33.Duan C, Hanzawa N, Takeuchi Y, Hamada E, Miyachi S, Hirano T. Zool Sci. 1993;10:473–480. [Google Scholar]
- 34.Duan C, Duguay S J, Plisetskaya E M. Fish Physiol Biochem. 1993;11:371–379. doi: 10.1007/BF00004587. [DOI] [PubMed] [Google Scholar]
- 35.Madsen S S, Bern H A. Zool Sci. 1992;9:775–784. [Google Scholar]
- 36.Prunet P, Avella M, Fostier A, Björnsson B, Boeuf G, Haux C. In: Progress in Comparative Endocrinology. Epple A, Scanes C G, Stetson M H, editors. New York: Wiley–Liss; 1990. pp. 547–552. [Google Scholar]
- 37.Thissen J-P, Ketelslegers J-M, Underwood L E. Endocr Rev. 1994;15:80–101. doi: 10.1210/edrv-15-1-80. [DOI] [PubMed] [Google Scholar]
- 38.Duan C, Duguay S J, Swanson P, Dickhoff W W, Plisetskaya E M. In: Perspectives in Comparative Endocrinology. Davey K G, Peter R E, Tobe S S, editors. Ottawa: Natl. Res. Counc. of Canada; 1994. pp. 365–372. [Google Scholar]
- 39.Ayson F G, Kaneko T, Tagawa M, Hasegawa S, Grau E G, Nishioka R S, King D S, Bern H A, Hirano T. Gen Comp Endocrinol. 1993;89:138–148. doi: 10.1006/gcen.1993.1017. [DOI] [PubMed] [Google Scholar]
- 40.Rubin D A, Specker J L. Gen Comp Endocrinol. 1992;87:189–196. doi: 10.1016/0016-6480(92)90022-c. [DOI] [PubMed] [Google Scholar]
- 41.Specker J L, Brown P S, Brown S C. Fish Physiol Biochem. 1989;7:119–124. doi: 10.1007/BF00004697. [DOI] [PubMed] [Google Scholar]
- 42.Young P S, McCormick S D, Demarest J R, Lin R J, Nishioka R S, Bern H A. Gen Comp Endocrinol. 1988;71:85–92. doi: 10.1016/0016-6480(88)90267-5. [DOI] [PubMed] [Google Scholar]
- 43.Richman N H, III, Zaugg W S. Gen Comp Endocrinol. 1987;65:189–198. doi: 10.1016/0016-6480(87)90165-1. [DOI] [PubMed] [Google Scholar]
- 44.Dauder S, Young G, Hass L, Bern H A. Gen Comp Endocrinol. 1990;77:368–377. doi: 10.1016/0016-6480(90)90226-c. [DOI] [PubMed] [Google Scholar]
- 45.Auperin B, Rentier-Delrue F, Martial J A, Prunet P. J Endocrinol. 1995;145:213–220. doi: 10.1677/joe.0.1450213. [DOI] [PubMed] [Google Scholar]
- 46.Sakamoto T, Hirano T. J Endocrinol. 1991;130:425–433. doi: 10.1677/joe.0.1300425. [DOI] [PubMed] [Google Scholar]
- 47.Johnsson J I, Björnsson B T. Anim Behav. 1994;48:177–186. [Google Scholar]
- 48.Foster A R, Houlihan D F, Gray C, Medale F, Fauconneau B, Kaushik S J, Le Bail P-Y. Gen Comp Endocrinol. 1991;82:111–120. doi: 10.1016/0016-6480(91)90302-m. [DOI] [PubMed] [Google Scholar]
- 49.Harvey S, Hull K L. In: Growth Hormone. Harvey S, Scanes C G, Daughaday W H, editors. London: CRC; 1995. pp. 303–335. [Google Scholar]
- 50.Sandra O, Sohm F, de Luze A, Prunet P, Edery M, Kelley P A. Proc Natl Acad Sci USA. 1995;92:6037–6041. doi: 10.1073/pnas.92.13.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rand-Weaver M, Kawauchi H, Ono M. In: The Endocrinology of Growth, Development and Metabolism in Vertebrates. Schreibman M P, Scanes C G, Pang P K T, editors. San Diego: Academic; 1993. pp. 13–42. [Google Scholar]
- 52.Borski R J, Yoshikawa J, Madsen S S, Nishioka R S, Zabetian C, Bern H A, Grau E G. Gen Comp Endocrinol. 1994;95:483–494. doi: 10.1006/gcen.1994.1148. [DOI] [PubMed] [Google Scholar]
- 53.Rand-Weaver M, Kawauchi H. In: Biochemistry and Molecular Biology of Fishes: Analytical Techniques. Hochachka P W, Mommsen T P, editors. Vol. 2. New York: Elsevier; 1993. pp. 39–56. [Google Scholar]