Abstract
The G protein-coupled receptor (GPCR) kinases (GRKs) phosphorylate and desensitize agonist-occupied GPCRs. GRK2-mediated receptor phosphorylation is preceded by the agonist-dependent membrane association of this enzyme. Previous in vitro studies with purified proteins have suggested that this translocation may be mediated by the recruitment of GRK2 to the plasma membrane by its interaction with the free βγ subunits of heterotrimeric G proteins (Gβγ). Here we demonstrate that this mechanism operates in intact cells and that specificity is imparted by the selective interaction of discrete pools of Gβγ with receptors and GRKs. Treatment of Cos-7 cells transiently overexpressing GRK2 with a β-receptor agonist promotes a 3-fold increase in plasma membrane-associated GRK2. This translocation of GRK2 is inhibited by the carboxyl terminus of GRK2, a known Gβγ sequestrant. Furthermore, in cells overexpressing both GRK2 and Gβ1γ2, activation of lysophosphatidic acid receptors leads to the rapid and transient formation of a GRK/Gβγ complex. That Gβγ specificity exists at the level of the GPCR and the GRK is indicated by the observation that a GRK2/Gβγ complex is formed after agonist occupancy of the lysophosphatidic acid and β-adrenergic but not thrombin receptors. In contrast to GRK2, GRK3 forms a Gβγ complex after stimulation of all three GPCRs. This Gβγ binding specificity of the GRKs is also reflected at the level of the purified proteins. Thus the GRK2 carboxyl terminus binds Gβ1 and Gβ2 but not Gβ3, while the GRK3 fusion protein binds all three Gβ isoforms. This study provides a direct demonstration of a role for Gβγ in mediating the agonist-stimulated translocation of GRK2 and GRK3 in an intact cellular system and demonstrates isoform specificity in the interaction of these components.
Exposure of G protein-coupled receptors (GPCRs) to an agonist often results in rapid attenuation of receptor responsiveness, a process termed desensitization. Uncoupling of the receptor from its cognate heterotrimeric G protein underlies the rapid phase of this process and is mediated, at least in part, by receptor phosphorylation (reviewed in ref. 1). Two distinct classes of serine/threonine kinases phosphorylate GPCRs; the second messenger-dependent protein kinases (cAMP-dependent protein kinase and protein kinase C) and the second messenger-independent GPCR kinases (GRKs). On the basis of structural and functional similarities, the six known members of the GRK family have been divided into three subfamilies: (i) rhodopsin kinase (GRK1), (ii) the β-adrenergic receptor (βAR) kinase subfamily (GRK2 and GRK3), and (iii) The GRK4 subfamily (GRK4, GRK5, and GRK6) (reviewed refs. 2 and 3).
The members of the βAR kinase subfamily of GRKs (GRK2 and GRK3) exhibit an agonist-dependent association with the plasma membrane in cellular systems (4). Thus treatment of DDT1MF-2 or S49 cells with a β-agonist leads to a rapid translocation of GRK2 activity from the cytosol to the plasma membrane (4). Agonist-induced GRK2 translocation precedes receptor phosphorylation and desensitization (4). In vitro, using purified components in a reconstituted system, the βγ subunits of heterotrimeric G proteins (Gβγ) are required for association of GRK2 with lipid vesicles and GRK2-mediated βAR phosphorylation (5, 6). Furthermore, a direct association between purified GRK2 and Gβγ has been demonstrated in vitro (6). On the basis of these observations a model has been proposed in which Gβγ-mediated membrane association of GRK2 and GRK3 is required for localizing these enzymes to their receptor substrates and thus for their efficient function. In this model agonist occupancy of a GPCR leads to the activation of heterotrimeric G proteins and the release of free Gβγ dimer. The Gβγ subsequently interacts with GRK2 and/or GRK3 and serves to target these enzymes to their membrane-incorporated receptor substrates. In this study we experimentally test the validity of this model and show it to be operative in intact cells.
Mapping the Gβγ binding domain of GRK2 localizes it within the carboxyl terminus of the enzyme to a region encompassing the carboxyl-terminal portion of a pleckstrin homology (PH) domain (residues Trp-543 to Ser-670 in GRK2) (7, 8). Comparison of the amino acid sequence identity between GRK2 and GRK3 reveals these proteins to be 85% identical across their entire sequence (2, 9). Restricting sequence comparison to the Gβγ-binding domain, however, reveals a more limited 52% identity. The somewhat diverse Gβγ binding regions of GRK2 and GRK3 might potentially reflect differential abilities to bind distinct combinations of Gβγ subunits. To date, mammalian cDNA clones encoding 6 Gβ (10), and 12 Gγ (11) subunit proteins have been described. In this study an examination of GRK2/Gβγ and GRK3/Gβγ complex formation in a cellular system reveals important insights into the Gβγ binding specificities of not only the GRKs but also specific GPCRs.
EXPERIMENTAL PROCEDURES
Materials.
Tissue culture supplies and Lipofectamine were purchased from GIBCO/BRL. Fetal bovine serum and gentamicin were from Life Technologies. Protein G-Sepharose and glutathione-Sepharose 4B were from Pharmacia. The cDNAs for GRK2 and GRK3 were as described (12); cDNAs encoding Gβ1, Gβ2, Gβ3, and Gγ2 were provided by M. Simon (California Institute of Technology, Pasadena, CA). The glutathione S-transferase (GST) GRK carboxyl-terminal fusion proteins (GST-GRK2ct and GST-GRK3ct) and the GRK carboxyl-terminal peptide-encoding minigenes were constructed in our laboratory as previously described (7). Gβγ subunits were purified from bovine brain as described in ref. 13. Antibodies to Gβcommon subunits were from DuPont/New England Nuclear. Monoclonal antibodies reactive with GRK2/3 and GRK5 were generated in our laboratory and have been described previously (14). Horseradish peroxidase-coupled goat anti-rabbit antibodies and chemiluminescent detection reagents were from Amersham. All other chemicals were from Sigma.
Cell Culture and Transfection.
Cos-7 African green monkey kidney cells were maintained in Dulbeco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and 50 μg of gentamicin per ml at 37°C in a humidified 5% CO2/95% air atmosphere. Cells were harvested and reestablished in fresh medium 24 h before each experiment. Transient transfections were performed using Lipofectamine. Briefly, cells, at 80% confluence in 100-mm culture plates, were washed once with serum-free medium and incubated for 2 h at 37°C in 4.0 ml of serum-free medium containing 2 μg of each plasmid DNA and 6.0 μl of Lipofectamine per μg of DNA. The transfection medium was subsequently replaced with 10 ml of DMEM containing serum for an additional 24 h. Cells were starved overnight in medium containing 10 mM Hepes (pH 7.4) and 0.1% bovine serum albumin (BSA) prior to agonist stimulation.
Subcellular Fractionation.
Cell monolayers were scraped in 0.25 M sucrose solution prepared in 50 mM Tris·HCl, pH 8.0/5 mM EDTA (TE) containing protease inhibitors and disrupted by 15 strokes with a hand-driven Dounce homogenizer. Intact cells and nuclei were removed by centrifugation at 300 × g for 5 min. Crude membranes were obtained by centrifuging the resulting supernatant at 300,000 × g for 30 min. Pellets were resuspended in TE containing 5% sucrose, layered on top of a 5–50% continuous, nonlinear sucrose gradient (15, 16), and centrifuged at 100,000 × g for 100 min. Plasma membrane fractions were analyzed for their content of the GRK2 antigen by Western blotting (see below).
Immunoprecipitation.
Serum-starved transfected cells were stimulated for the specified times with the indicated agonist. Cells were washed twice with ice-cold phosphate-buffered saline (PBS) and lysed in 1.0 ml of 1% CHAPS-HEDN buffer {CHAPS, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid; HEDN, 10 mM Hepes/1 mM EDTA/1 mM dithiothreitol/100 mM NaCl} as described (17). Lysates were cleared by centrifugation at 15,000 × g for 15 min at 4°C. Supernatants were transferred to test tubes containing 15 μg of anti-GRK2/3 antibody and 50 μl of a 50% slurry of protein G-Sepharose beads. GRK2/3 immune complexes were allowed to form by rotating for 1 h at 4°C followed by three washes with CHAPS-HEDN buffer. Proteins were removed from the Sepharose beads with SDS/PAGE sample buffer, resolved by electrophoresis on 12% acrylamide gels, and transferred to nitrocellulose filters (Bio-Rad). The Gβ-specific antibodies were generated using the following peptides: Gβ1, NH2-Cys-Glu-Gly-Asn-Val-Arg-Val-Ser-Arg-Glu-Leu-Ala-Gly-His-Thr-Gly-Tyr; Gβ2, NH2-Cys-Glu-Gly-Asn-Val-Arg-Val-Ser-Arg-Glu-Leu-Pro-Gly-His-Thr-Gly-Tyr; and Gβ3, NH2-Cys-Ala-Glu-Leu-Val-Ser-Gly-Leu-Glu-Val-Val-Gly-Arg. Antibodies were used at the following dilutions: Gβcommon, 1:5000; Gβ1 and Gβ2, 1:500; Gβ3, 1:200; GRK2/3 and GRK5, 1:4000. Blots were developed with goat anti-rabbit IgG coupled to horseradish peroxidase and were visualized using enhanced chemiluminescence kits (ECL; Amersham).
Gβγ Purification.
Gβ1γ5 and Gβ3γ5 were purified according to the procedure of Kozasa and Gilman (18). Briefly, Sf9 cells were infected simultaneously with recombinant baculoviruses encoding 6Hisαi1, γ5, and either β1 or β3. The cholate-soluble particulate fractions of cell lysates were loaded onto a Ni–nitrilotriacetate (NTA)-agarose column and washed with 100 ml of 300 mM NaCl and 5 mM imidazole. Bound 6Hisαi1β1γ5 or 6Hisαi1β3γ5 heterotrimers were activated with AlF4− and high Mg2+ to release the free Gβγ dimers. The Gβ1γ5 or Gβ3γ5 obtained in this way was subsequently bound to hydroxylapatite and eluted with 200 mM potassium phosphate, pH 8.0. The proteins were subsequently dialyzed into 20 mM Hepes, pH 8.0/100 mM NaCl/2 mM dithithreitol/0.05% Lubrol-PX.
Gβγ Binding to GST-GRK2/3ct Proteins.
Binding of purified brain Gβγ, Gβ1γ5, or Gβ3γ5 to the carboxyl-terminal fusion peptides GRK2 and GRK3 was performed essentially as described (7). In brief, GST-GRK2ct and GST-GRK3ct, diluted in 250 μl of PBS to a final concentration of 500 nM, were coupled to glutathione-Sepharose 4B for 20 min at ambient temperature. Protein-bead complexes were washed three times in ice-cold PBS and resuspended in 250 μl of PBS containing Gβγ at a final concentration of 50 nM. Complexes were allowed to form for 20 min at room temperature and then washed three times with PBS. Complexed proteins were dissociated from Sepharose beads with SDS/PAGE sample buffer, resolved on 12% acrylamide gels, and transferred to nitrocellulose. Filters were blotted with Gβ-specific antibodies, developed with goat anti-rabbit IgG coupled to horseradish peroxidase, and visualized using enhanced chemiluminescence kits.
RESULTS AND DISCUSSION
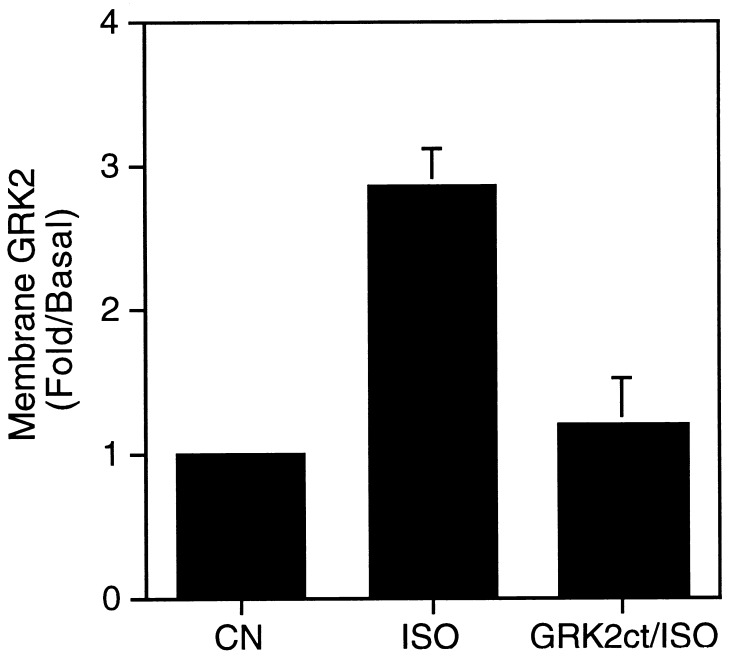
The agonist-dependent association of GRK2 with plasma membranes has been demonstrated in DDT1MF-2 and S49 cells (4). In this study Cos-7 cells transiently overexpressing GRK2 were used as a model system to investigate the mechanism of this translocation phenomenon. Gβγ has been shown to bind to and promote membrane association of GRK2 in vitro (5, 6), however, a Gβγ/GRK2 interaction has not previously been demonstrated in an intact cellular system. That agonist-dependent translocation of GRK2, from the cytosol to the plasma membrane, occurs in Cos-7 cells is shown in Fig. 1. The GRK2 content of purified plasma membranes prepared from cells transiently overexpressing GRK2 was assessed immunologically by Western blot analysis. Treatment of cells with the β-agonist isoproterenol increases the membrane content of GRK2 approximately 3-fold (Fig. 1). To identify a potential role of Gβγ in mediating this agonist-dependent membrane association, cells were cotransfected with GRK2 and a GRK2 carboxyl-terminal polypeptide (GRK2ct). The GRK2ct encodes the 195 carboxyl-terminal residues of GRK2, a region that encompasses the Gβγ binding domain (7, 8). The GRK2ct is extensively characterized as a sequestrant of Gβγ in both cellular systems (19) and when expressed as a GST fusion protein in vitro (6, 20). Agonist treatment of cells overexpressing both GRK2 and the GRK2ct showed no significant agonist-stimulated membrane association of GRK2 (Fig. 1). The ability of the GRK2ct to block the agonist-stimulated translocation of GRK2 to the plasma membrane directly implicates a role for Gβγ as a mediator of this process in intact cells.
Figure 1.
Agonist-dependent association of GRK2 with the plasma membrane is inhibited by the GRK2 carboxyl terminus (GRK2ct). Cos-7 cells transiently overexpressing GRK2 (CN, ISO) or GRK2 and the GRK2ct (GRK2ct/ISO) were incubated either in medium alone (CN) or medium containing 10 μM isoproterenol (ISO) for 5 min. Cells were harvested and plasma membranes were subjected to Western blot analysis using an anti-GRK2 monoclonal antibody. The intensity of the immunoreactive band corresponding to GRK2 was measured by laser densitometry. Data represent the mean ± SEM. of three independent experiments expressed as fold increase over basal. The basal value represents that amount of membrane-associated GRK2 observed in the absence of agonist (ISO) treatment.
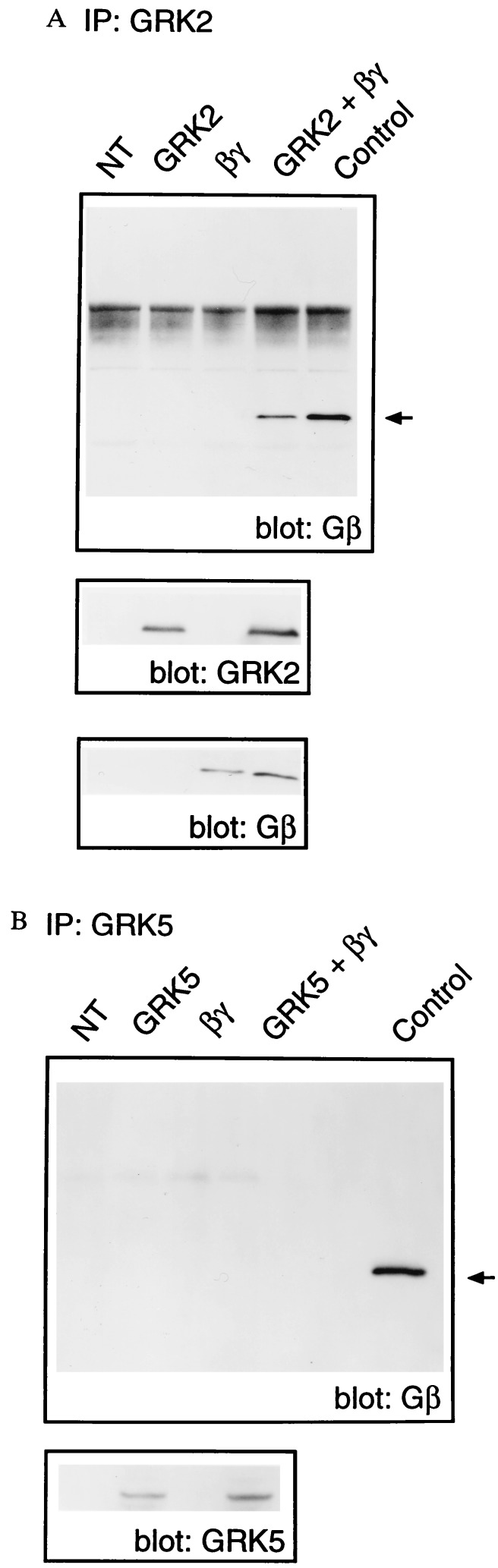
To directly demonstrate an interaction between GRK2 and Gβγ in a cellular system, GRK2 was immunoprecipitated from Cos-7 cells transiently overexpressing GRK2, Gβ1, and Gγ2 cDNAs. GRK2 immunoprecipitates were subsequently probed immunologically for the presence of Gβ (Fig. 2). In this cellular system overexpressing GRK2 and Gβ1γ2, Gβ coimmunoprecipitated with GRK2 in the absence of agonist stimulation (Fig. 2A). Indeed, following prolonged exposure of the Western blot a GRK2/Gβγ complex could be detected in the absence of Gβγ overexpression—i.e., a complex between overexpressed GRK2 and endogenous Gβγ could be detected (data not shown). GRK5, a member of the GRK family that does not bind Gβγ (21), was used to demonstrate the specificity of the GRK2/Gβγ interaction (Fig. 2B). No Gβγ was detected in the GRK5 immunoprecipitates even upon prolonged exposure of the Western blot (Fig. 2B).
Figure 2.
Gβγ binds to GRK2 but not GRK5 in intact cells. (A) Cos-7 cells were transfected with pcDNA-1 vector (NT), GRK2 (GRK2), Gβ1 and Gγ2 (βγ), or GRK2, Gβ1, and Gγ2 (GRK2 + βγ). Cells were solubilized in 1.0% CHAPS-HEDN, and GRK2 was immunoprecipitated using an anti-GRK2 monoclonal antibody. Immunoprecipitated proteins were subsequently subjected to Western blot analysis using an anti-Gβ antibody. Purified bovine brain Gβγ was included on the Western blot as a positive control (Control). (B) Immunoprecipitation of GRK5 from cells transfected with empty vector (NT), GRK5 (GRK5), Gβ1 and Gγ2 (βγ), or GRK5, Gβ1, and Gγ2 (GRK5 + βγ). The immunoprecipitations and Gβ Western blot analysis were performed as described for A with the exception that an anti-GRK5 antibody was used for immunoprecipitation. The lower blots in A and B show the GRK2, Gβ, and GRK5 contents of the Cos-7 cell lysates. The Western blots shown are representative of three independent experiments.
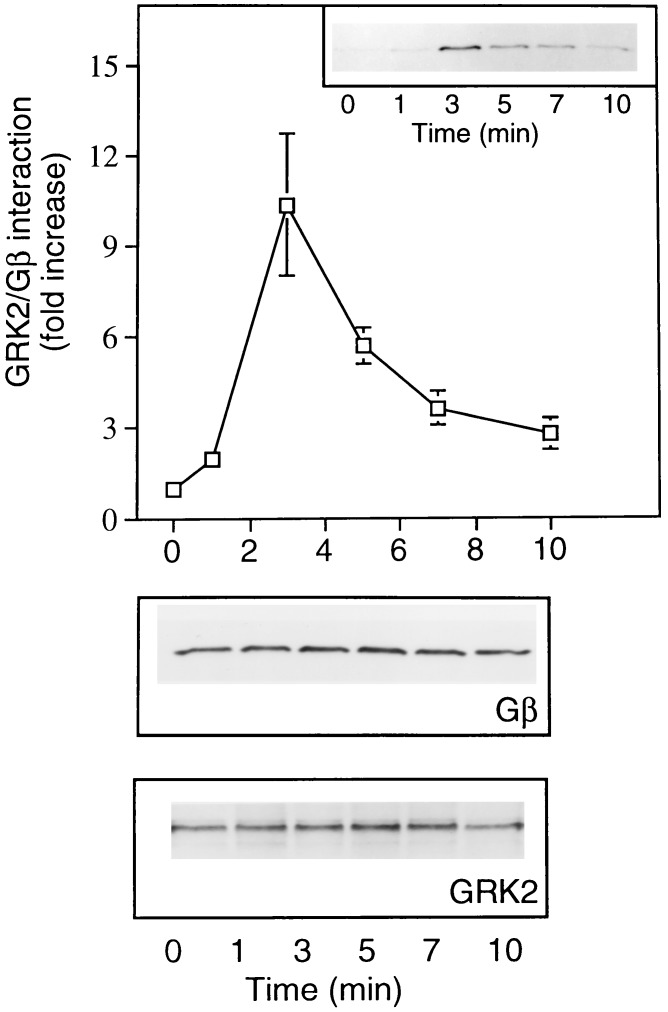
The observation that GRK2 translocation to the plasma membrane, following agonist treatment, is mediated by Gβγ binding (Fig. 1), coupled with the finding that overexpressed GRK2 and Gβ1γ2 associate in a cellular system (Fig. 2), led us to examine the effect of GPCR activation on GRK2/Gβγ complex formation. Cos-7 cells transiently transfected with GRK2, Gβ1, and Gγ2 cDNAs were treated with lysophosphatidic acid (LPA) to activate endogenous receptors. After receptor activation GRK2 was immunoprecipitated and GRK2/Gβγ complex formation was assessed by probing the GRK2 immunoprecipitates with a Gβ antibody. Activation of endogenous LPA receptors induced a rapid and transient increase in GRK2/Gβγ complex formation (Fig. 3 Upper). The amount of Gβ associated with GRK2 was maximal 3 min after stimulation and returned to basal levels within approximately 10 min. In contrast to the coimmunoprecipitated Gβ, the expression level of total Gβ protein remained constant over the entire time course examined (Fig. 3 Lower).
Figure 3.
(Upper) Time course of agonist-stimulated GRK2-Gβγ complex formation. Cos-7 cells transfected with GRK2, Gβ1, and Gγ2 were incubated overnight in serum-free media prior to LPA treatment. Following agonist treatment and at the indicated times cells were lysed and GRK2 was immunoprecipitated. The Gβ content of GRK2 immunoprecipitates was determined by Western blot analysis. Blots were quantified by laser densitometry. The data at each time point represent the mean ± SEM. of three separate determinations. The data are presented as fold increase in Gβ content relative to an unstimulated cell control. (Inset) Representative Western blot showing Gβ immunoreactivity. (Lower) Blots showing Gβ and GRK2 immunoreactivity present in equivalent protein aliquots of cell lysate.
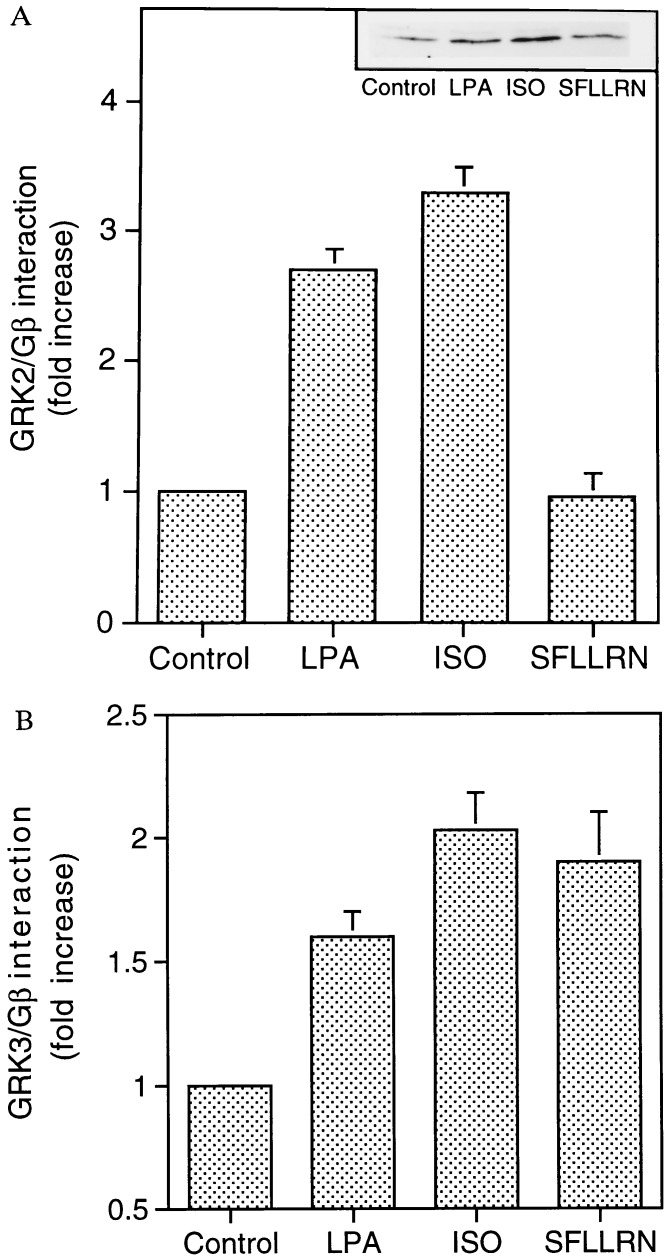
The increase in GRK2/Gβγ complex formation following agonist occupancy of a specific GPCR, coupled with the observation that a complex between endogenous Gβγ and overexpressed GRK2 could be detected in Cos-7 cells (Fig. 1), permitted an exploration of potential Gβγ specificity among different receptors and between GRK2 and GRK3. GRK/Gβγ complex formation was monitored after stimulation of three GPCRs endogenous to Cos-7 cells. The LPA receptor, βAR, and thrombin receptor were utilized as representative examples of, respectively, Gi-, Gs-, and Gq-coupled receptors. Overexpressed GRK2 or GRK3 was immunoprecipitated after a 3-min exposure to agonist (LPA, isoproterenol, or the thrombin peptide SFLLRN). Formation of the GRK/Gβγ complex was assessed by Western blot analysis using an anti-Gβ antibody. In cells overexpressing GRK2, stimulation of either the LPA receptor or βAR promoted an approximately 3-fold increase in GRK2/Gβγ complex formation. In contrast, stimulation of the thrombin receptor failed to promote a GRK2/Gβγ interaction (Fig. 4A). Stimulation of endogenous thrombin receptors in the Cos-7 cells, however, was sufficient to activate phospholipase C as measured by inositol phosphate accumulation (data not shown). A somewhat different pattern of Gβγ association was observed in cells overexpressing GRK3. Activation of LPA receptor, βAR, or thrombin receptor promoted GRK3/Gβγ complex formation. (Fig. 4B).
Figure 4.
Agonist-specific formation of GRK/Gβγ complexes. Cos-7 cells transfected with either GRK2 (A) or GRK3 (B) were starved overnight in serum-free medium prior to exposure to lysophosphatidic acid (LPA; 10 μM), isoproterenol (ISO; 10 μM), or the thrombin receptor agonist SFLLRN (100 μM). GRK2 or GRK3/Gβγ complex formation was assessed by coimmunoprecipitation. The data represent mean ± SEM values from four independent experiments and are expressed as fold increase over unstimulated (control) cells. (Inset) Immunoblot of a representative experiment.
Membrane recruitment of the GRKs has been shown to be required for their efficient function (5, 6). Here we demonstrate in a cellular system that this process is mediated by interaction with Gβγ (Fig. 1). That agonist occupancy of the thrombin receptor promotes formation of a GRK3/Gβγ complex but fails to stimulate association of GRK2 with Gβγ would thus suggest that GRK3 but not GRK2 can phosphorylate and thus desensitize the thrombin receptor. Indeed, GRK3 has been shown to be markedly more effective than GRK2 at inhibiting thrombin receptor signaling when coexpressed in Xenopus oocytes (22).
The differential ability of thrombin receptor activation to promote a GRK3/Gβγ but not a GRK2/Gβγ interaction provides a potential basis for receptor specificity of the GRKs. Additionally, however, this result has implications concerning the Gβγ specificities of both GRKs and GPCRs. Activation of LPA receptor and βAR promotes GRK/Gβγ complex formation for both GRK2 and GRK3. Thrombin receptor activation, however, promotes specifically GRK3/Gβγ complex formation. These results suggest (i) that activation of a particular GPCR releases a specific pool of Gβγ dimers and (ii) that the carboxyl-terminal Gβγ binding domains of GRK2 and GRK3 differentially bind specific Gβγ combinations. The finding that Gβγ isoforms display preference for particular GPCRs is consistent with previously published data collected by utilizing antisense oligonucleotides directed against distinct Gβ and Gγ subunits (23, 24). In a cellular system the expression of a particular Gβ or Gγ antisense oligonucleotide can be shown to selectively and specifically inhibit signal transduction mediated by a GPCR. In this way Gβ1γ3 and Gβ3γ4 have been shown to couple, respectively, the somatostatin and muscarinic acetylcholine receptors to voltage-dependent Ca2+ channels (23, 24). The differential coupling of distinct Gβγ isoforms to a particular receptor has also been proposed on the basis of in vitro phosphorylation studies using GRK2 (25, 26). Distinct Gβγ dimers have been shown to differentially enhance GRK2-mediated phosphorylation of rhodopsin and βAR (25, 26). Thus, when purified proteins are used, a GRK2/Gβ2γ2 complex preferentially interacts with and phosphorylates rhodopsin, whereas a GRK2/Gβ1γ2 complex preferentially phosphorylates βAR (25, 26).
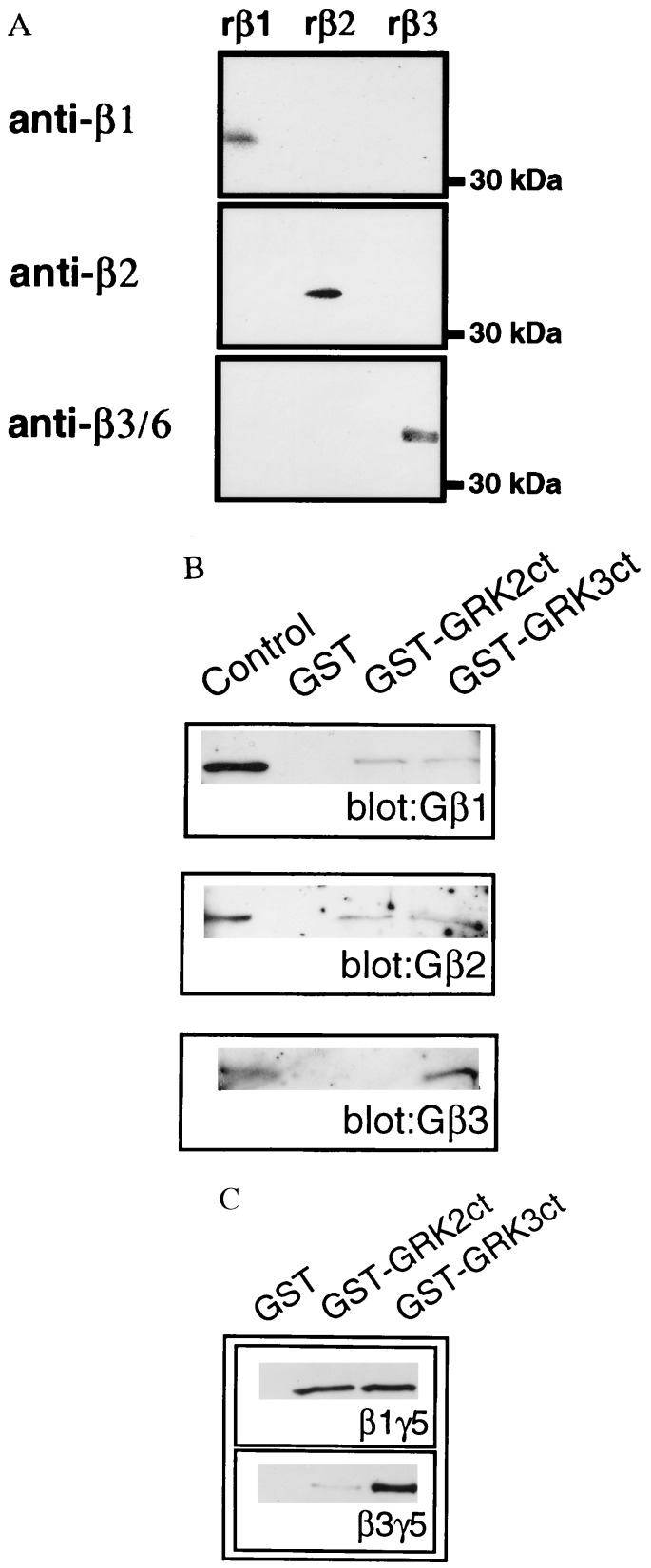
The ability of thrombin receptor activation to specifically induce formation of a GRK3/Gβγ complex represents the first (to our knowledge) example of specificity of Gβγ isoform binding between GRK2 and GRK3. In an attempt to elucidate the specific Gβ isoforms binding to these two enzymes, GST fusion proteins encompassing the Gβγ-binding domains of GRK2 and GRK3 were incubated with a purified preparation of Gβγ. The GST-GRK fusion proteins bound to glutathione-Sepharose were incubated with purified bovine brain Gβγ, washed, and analyzed immunologically for bound Gβ using Gβ1-, Gβ2-, or Gβ3-specific antisera. The specificity of the Gβ antisera is demonstrated in Fig. 5A, using recombinant Gβ1, Gβ2, or Gβ3 subunits expressed in and purified from Sf9 cells. Western blot analysis of purified Gβ preparations revealed that each Gβ-specific antibody recognizes only the corresponding Gβ subunit. Although untested, the anti-Gβ3 antiserum is likely to detect Gβ6 in addition to Gβ3, since the region in Gβ3 used to generate the antibody is identical to that in Gβ6. Because Northern blot analysis shows Gβ6 to be absent from brain (27), the Gβ3 antisera can thus be used to unambiguously identify Gβ3 in the purified bovine brain-derived Gβγ.
Figure 5.
Gβγ binding specificity of GRK2 and GRK3 carboxyl termini. (A) Recombinant Gβ1, Gβ2, or Gβ3 subunits from Sf9 cells were subjected to Western blot analysis using Gβ-specific antibodies. (B) Purified GST, GST-GRK2ct, or GST-GRK3ct proteins were incubated with purified bovine brain Gβγ in the presence of glutathione-Sepharose beads. Protein–bead complexes were washed and analyzed for Gβ isoforms by Western blotting using the specific Gβ1, Gβ2, or Gβ3 antibodies. (C) GST-GRK2ct or GST-GRK3ct proteins were incubated with purified recombinant Gβ1γ5 or Gβ3γ5 and analyzed for complex formation as previously described. Blots were developed using Gβcommon antibodies. A Western blot representative of at least three independent experiments is shown.
Western blotting of the Gβ retained by the GST-GRKct constructs reveals distinct patterns of Gβγ binding. Thus, although Gβ1 and Gβ2 bind to both the GRK2 and GRK3 carboxyl termini, Gβ3 is specifically retained only by GST-GRK3 (Fig. 5B). These results directly demonstrate specificity of Gβγ isoform binding to GRK2 and GRK3 and suggest that Gβ3 preferentially binds to GRK3. Indeed, when Gβ1γ5 and Gβ3γ5 purified from Sf9 cells are used, Gβ3γ5 is shown to preferentially bind to GRK3 (Fig. 5C). Thus although purified Gβ1γ5 binds with equal affinity to both the GRK2 and GRK3 carboxyl termini, purified Gβ3γ5 binds exclusively to GRK3 (Fig. 5C).
The specific interaction of GRK3 with Gβ3 raises the possibility that this Gβ subunit is coupled to the thrombin receptor. Unfortunately, the low titer of the Gβ-specific antibodies prevented a direct test of this hypothesis. Thus, although Cos-7 cell endogenous Gβ can be detected by using the Gβcommon antibody, the Gβ3-specific antisera failed to detect the Gβ3 protein.
The results presented in this study provide what we believe to be the first evidence to support a role for Gβγ in mediating agonist-dependent membrane association of GRK2 and GRK3 in an intact cellular system. Furthermore, examination of GRK/Gβγ complex formation after agonist occupancy of three different GPCRs (LPA receptor, βAR, and thrombin receptor) has extended our current conception of this model. Thus activation of specific GPCRs is postulated to lead to the release of a specific pool of Gβγ dimers (Fig. 4). GRK2 and GRK3 exhibit differential affinities for distinct Gβγ dimers (Fig. 5), thus GPCR activation could lead to the recruitment of a specific GRK isoform to the membrane. Since the membrane localization of the GRKs is required for their efficient phosphorylation of GPCRs, this model provides the basis for receptor specificity of the GRKs.
Acknowledgments
We thank Drs. R. Premont, W. Koch, and A. Gilman for providing the GRK2, GST-GRK2/3ct, and 6Hisαi1 DNAs, respectively. This work was supported by National Institutes of Health Grants 16037 (to R.J.L.) and 39867 (to J.D.R.).
ABBREVIATIONS
- GPCR
G protein-coupled receptor
- GRK
GPCR kinase
- GRKct
GRK carboxyl terminus
- βAR
β-adrenergic receptor
- CHAPS
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid
- GST
glutathione S-transferase
- LPA
lysophosphatidic acid
References
- 1.Freedman N J, Lefkowitz R J. Recent Prog Horm Res. 1996;51:319–353. [PubMed] [Google Scholar]
- 2.Inglese J, Freedman N J, Koch W J, Lefkowitz R J. J Biol Chem. 1993;268:23735–23738. [PubMed] [Google Scholar]
- 3.Premont R T, Inglese J, Lefkowitz R J. FASEB J. 1995;9:175–182. doi: 10.1096/fasebj.9.2.7781920. [DOI] [PubMed] [Google Scholar]
- 4.Strasser R H, Benovic J L, Caron M G, Lefkowitz R J. Proc Natl Acad Sci USA. 1986;83:6362–6366. doi: 10.1073/pnas.83.17.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitcher J A, Inglese J, Higgins J B, Arriza J L, Casey P J, Kim C, Benovic J L, Kwatra M M, Caron M G, Lefkowitz R J. Science. 1992;257:1264–1267. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- 6.Pitcher J A, Touhara K, Payne S E, Lefkowitz R J. J Biol Chem. 1995;270:11707–11710. doi: 10.1074/jbc.270.20.11707. [DOI] [PubMed] [Google Scholar]
- 7.Koch W J, Inglese J, Stone W C, Lefkowitz R J. J Biol Chem. 1993;268:8256–8260. [PubMed] [Google Scholar]
- 8.Touhara K, Koch W J, Hawes B E, Lefkowitz R J. J Biol Chem. 1995;270:17000–17005. doi: 10.1074/jbc.270.28.17000. [DOI] [PubMed] [Google Scholar]
- 9.Benovic J L, Onorato J J, Arriza J L, Stone W C, Lohse M, Jenkins N A, Gilbert D J, Copeland N G, Caron M G, Lefkowitz R J. J Biol Chem. 1991;266:14939–14946. [PubMed] [Google Scholar]
- 10.Watson A J, Aragay A M, Slepak V Z, Simon M I. J Biol Chem. 1996;271:28154–28160. doi: 10.1074/jbc.271.45.28154. [DOI] [PubMed] [Google Scholar]
- 11.Ray K, Kunsch C, Bonner L M, Robishaw J D. J Biol Chem. 1995;270:21765–21771. doi: 10.1074/jbc.270.37.21765. [DOI] [PubMed] [Google Scholar]
- 12.Pei G, Tiberi M, Caron M G, Lefkowitz R J. Proc Natl Acad Sci USA. 1994;91:3633–3636. doi: 10.1073/pnas.91.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casey P J, Graziano M P, Gilman A G. Biochemistry. 1989;28:611–616. doi: 10.1021/bi00428a029. [DOI] [PubMed] [Google Scholar]
- 14.Oppermann M, Diverse-Pierluissi M, Drazner M H, Dyer S L, Freedman N J, Peppel K C, Lefkowitz R J. Proc Natl Acad Sci USA. 1996;93:7649–7654. doi: 10.1073/pnas.93.15.7649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kassis S, Sullivan M. J Cyclic Nucleotide Protein Phosphorylation Res. 1986;11:35–46. [PubMed] [Google Scholar]
- 16.Krueger K M, Daaka Y, Pitcher J A, Lefkowitz R J. J Biol Chem. 1997;272:5–8. doi: 10.1074/jbc.272.1.5. [DOI] [PubMed] [Google Scholar]
- 17.Krapivinsky G, Krapivinsky L, Wickman K, Clapham D E. J Biol Chem. 1995;270:29059–29062. doi: 10.1074/jbc.270.49.29059. [DOI] [PubMed] [Google Scholar]
- 18.Kozasa T, Gilman A G. J Biol Chem. 1995;270:1734–1741. doi: 10.1074/jbc.270.4.1734. [DOI] [PubMed] [Google Scholar]
- 19.Koch W J, Hawes B E, Inglese J, Luttrell L M, Lefkowitz R J. J Biol Chem. 1994;269:6193–6197. [PubMed] [Google Scholar]
- 20.Touhara K, Inglese J, Pitcher J A, Shaw G, Lefkowitz R J. J Biol Chem. 1994;269:10217–10220. [PubMed] [Google Scholar]
- 21.Premont R T, Koch W J, Inglese J, Lefkowitz R J. J Biol Chem. 1994;269:6832–6841. [PubMed] [Google Scholar]
- 22.Ishii K, Chen J, Ishii M, Koch W J, Freedman N J, Lefkowitz R J, Coughlin S R. J Biol Chem. 1994;269:1125–1130. [PubMed] [Google Scholar]
- 23.Kleuss C, Scherubl H, Hescheler J, Schultz G, Wittig B. Science. 1993;259:832–834. doi: 10.1126/science.8094261. [DOI] [PubMed] [Google Scholar]
- 24.Kleuss C, Scherubl H, Hescheler J, Schultz G, Wittig B. Nature (London) 1992;358:424–426. doi: 10.1038/358424a0. [DOI] [PubMed] [Google Scholar]
- 25.Muller S, Hekman M, Lohse M J. Proc Natl Acad Sci USA. 1993;90:10439–10443. doi: 10.1073/pnas.90.22.10439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller S, Straub A, Schroder S, Bauer P H, Lohse M J. J Biol Chem. 1996;271:11781–11786. doi: 10.1074/jbc.271.20.11781. [DOI] [PubMed] [Google Scholar]
- 27.Ray K, Robishaw J D. Gene. 1994;149:337–340. doi: 10.1016/0378-1119(94)90172-4. [DOI] [PubMed] [Google Scholar]