Abstract
During heat shock, structural changes in proteins and membranes may lead to cell death. While GroE and other chaperone proteins are involved in the prevention of stress-induced protein aggregation and in the recovery of protein structures, a mechanism for short-term membrane stabilization during stress remains to be established. We found that GroEL chaperonin can associate with model lipid membranes. Binding was apparently governed by the composition and the physical state of the host bilayer. Limited proteolysis of GroEL oligomers by proteinase K, which removes selectively the conserved glycine- and methionine-rich C terminus, leaving the chaperonin oligomer intact, prevented chaperonin association with lipid membranes. GroEL increased the lipid order in the liquid crystalline state, yet remained functional as a protein-folding chaperonin. This suggests that, during stress, chaperonins can assume the functions of assisting the folding of both soluble and membrane-associated proteins while concomitantly stabilizing lipid membranes.
GroESL chaperonins belong to a major class of molecular chaperones involved in the folding of proteins or of stress-destabilized polypeptides (for a review, see ref. 1). Group I chaperonins, comprising the highly conserved GroEL from Escherichia coli, or cpn60-like complexes (2), are generally considered as soluble proteins that function in the cytoplasm of prokaryotes and in the matrix compartment of mitochondria and chloroplasts. However, several reports suggest the existence of an additional membrane-associated pool for a number of GroEL homologues.
In Mycobacterium leprae and Coxiella burnetii a fraction of GroEL chaperonins sediments with the insoluble pellet after cell lysis (3, 4). Early findings that GroEL was localized at the cytoplasmic membrane in the photosynthetic prokaryote Chromatium vinosum led to the speculation that membrane-associated chaperonins assist in the post-translational assembly of oligomeric proteins in the membrane (5). Binding of a chloroplast 60-kDa heat shock protein (Hsp60) to the thylakoid membrane was also suggested in Vigna sinensis (6). Analysis of heat-treated Synechocystis PCC6803 cells revealed that the two GroEL homologues distribute both in the soluble and in the thylakoid membrane fractions (7–9). After a sublethal heat treatment of cyanobacterial cells, an increase of the membrane-associated GroEL fraction was observed concomitantly with an increase in the heat stability of the photosynthetic electron transport machinery (7). Localization of chaperonins in the thylakoid region was also demonstrated in the nitrogen-fixing cyanobacterium Anabaena PCC7120 (10). Temperature was also shown to control the subcellular distribution of Hsp60 species in Borrelia burgdorferi (11), where Hsp60 and Hsp70 are primarily involved in the processing of flagellin.
Although in mammalian cells the majority of mitochondrial Hsp60 is localized in the matrix compartment of the organelle, highly specific immunolabeling also locates mitochondrial Hsp60 in the mitochondrial outer membrane, plasma membrane, endoplasmic reticulum, and peroxisomes (12). In chloroplasts, stromal Cpn60 transiently associates with an integral membrane protein, the import intermediate-associated protein (IAP100) (13). Cell surface translocation of GroEL is also suggested to be involved in processing of proteins for antigenic presentation (14).
Whereas all the above-cited data point to the association of chaperonins with membranes, the mechanism and function of this association remain unclear. This report presents evidences that the soluble chaperonin GroEL from E. coli has high affinity for model lipid membranes, and the conserved C terminus of GroEL is involved in membrane binding. Fluorescence anisotropy measurements on large unilamellar vesicles revealed that the interaction of GroEL14 oligomers with lipid membrane increases the molecular order of the lipid bilayer. The physiologically relevant GroEL14GroES7 and GroEL14(GroES7)2 chaperonin heterooligomers penetrate into the hydrophobic core of the lipid bilayer, while protein folding and ATPase activities remain remarkably unchanged as compared with the soluble state.
MATERIALS AND METHODS
Materials.
Cardiolipin from E. coli (ECCL), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), 1,2-dioleoyl-sn-glycero-3-phosphoglycerol (DOPG), 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), and NADH were purchased from Sigma; 1,6-diphenyl-1,3,5-hexatriene (DPH) and 1-(4-trimethylammoniumphenyl)-6-phenyl-1,3,5-hexatriene (TMA-DPH) were from Molecular Probes.
GroEL and GroES purification.
The E. coli chaperonin proteins were overexpressed in E. coli carrying the plasmid pSESac2 as in ref. 15. Cells were collected and resuspended in distilled water containing 5 μg/ml leupeptin and 1 mM phenylmethanesulfonyl fluoride (PMSF) and disrupted with a Bead-Beater (Biospec Products, Bartlesville, OK), using 0.1-mm glass beads 10 times for 20 sec, with 40-sec cooling intervals on ice. The soluble proteins of 30,000 × g (15 min) supernatant were heat-treated (60°C for 7 min) and acidified (pH 4.5 with 10% acetic acid) following centrifugation after each treatment to remove insoluble proteins. The final supernatant was neutralized with solid Tris base, then the proteins were precipitated by adding ammonium sulfate to a final concentration of 67% saturation. After centrifugation (30,000 × g, 15 min), the pellet was resuspended and dialyzed against 10 mM Tris·HCl, pH 7.5, for 18 hr. The protein solution was applied on a Superose 6 HR 10/30 size exclusion HPLC column (Pharmacia) and eluted with 50 mM Tris·HCl, pH 7.5/0.05% NaN3 at 0.4 ml/min. Fractions containing either the oligomeric 800-kDa GroEL14 or the 70-kDa GroES7 were applied on a ResourceQ ion-exchange column (Pharmacia) and eluted with a 0–1 M KCl linear gradient in 50 mM Tris·HCl, pH 8.0. GroEL14 and GroES7 were detected as single peaks (A280) eluting at 360 mM and 280 mM KCl, respectively. The fractions were concentrated and washed by using a Centricon 30 (Amicon) and were stored at −80°C in 50 mM triethanolamine (TEA) hydrochloride, pH 7.5/0.02% NaN3. Both GroEL and GroES were at least 95% pure and in an oligomeric state, as judged by SDS and nondenaturing polyacrylamide gel electrophoresis, respectively.
Monolayer Experiments.
Monolayer experiments were carried out essentially as in ref. 16 with a KSV3000 Langmuir Blodget instrument (KSV Instruments, Helsinki), by using a Teflon dish with a volume of 6.5 ml and a surface area of 9 cm2 at 23°C in buffer A (50 mM TEA·HCl, pH 7.5/10 mM MgCl2/100 mM KCl). Surface pressure was measured by the Wilhelmy method, using a platinum plate. Monomolecular lipid layers of 75% DOPE, 20% DOPG, and 5% ECCL (wt %) were spread from CHCl3 lipid solution to give the desired initial surface pressure on a subphase of buffer A. The subphase was continuously stirred with a magnetic bar. C-terminally truncated GroEL (GroELΔC) was prepared as in ref. 17. GroEL oligomers (10 μM) were incubated for 10 min in buffer A containing 1 mM ADP. The mixture was further incubated in the presence of proteinase K (30 μg/ml) for 10 min and 65 μl was injected underneath a monolayer spread on 6.5 ml of buffer A containing 1 mM ADP and 2 μM leupeptin to stop the protease action. In the control experiment, the conditions were the same except the protease inhibitor was present throughout the incubation. The samples injected into the subphase were analyzed by SDS/PAGE (8% gels).
Vesicle Preparation.
Large unilamellar vesicles (LUVs) were prepared in buffer A by the extrusion technique using a Liposofast extruder (Avestin, Ottawa, Canada), with two stacked polycarbonate filters with a pore size of 400 nm, as previously described (18). Concentrations of LUVs were expressed on lipid phosphorus basis.
Chaperonin Binding Experiments.
GroEL (3.5 μM) and GroES (7 μM) were preincubated with ADP (1.5 mM) or 5′-adenylyl imidodiphosphate (AMP-PNP) (3 mM) in buffer A for 10 min at 23°C. Aliquots (270 μl) were added to 30 μl of solution containing increasing amounts of DOPG LUVs and 1.5 mM ADP or 3 mM AMP-PNP, followed by 30 min of incubation at 23°C. Membrane-bound proteins were separated by centrifugation at 250,000 × g for 30 min at 25°C. Pellet and supernatant fractions were analyzed by SDS and nondenaturing PAGE (on 15% and 6% gels, respectively) and were visualized by staining with Coomassie brilliant blue.
Steady-State Fluorescence Anisotropy.
LUVs were labeled by adding DPH or TMA-DPH probe directly to the lipids in organic solvent before drying of the lipid film. The lipid-to-probe molar ratio in the liposome solution was 1000:1. Fluorescence anisotropy was measured on a T-format fluorescence spectrometer (Quanta Master QM-1, Photon Technology International, Princeton, NJ) as described in ref. 19. Excitation and emission wavelengths were 360 and 430 nm, respectively (5-nm slits). Temperature was controlled by a circulating water bath and measured directly in the cuvettes by a platinum probe. Both heating and cooling rates were 0.2°C/min. GroEL (1.5 μM) was added to the LUV solution (50 μM) with continuous stirring.
ATPase Activity.
The hydrolysis of ATP by chaperonins was measured by following the time-dependent release of 32Pi by adsorption of the unhydrolyzed [γ-32P]ATP on activated charcoal in 20 mM phosphoric acid as in ref. 20. GroEL (3.5 μM) was incubated with or without 400 μM DOPG LUVs in the absence or presence of 7 μM GroES at 23°C for 30 min. Rates of ATPase activity of GroEL were measured at 23°C for 0–10 min following the addition of ATP to a final concentration of 1 mM in buffer A.
Chaperonin Protein Refolding Activity.
Mitochondrial malate dehydrogenase (MDH) (0.25 μM) was thermally denatured in buffer A for 20 min at 47°C in the presence of GroEL (3.5 μM) and GroES (7 μM) (21). This mixture was incubated with or without 400 μM DOPG LUVs at 23°C for 30 min. Chaperonin-assisted MDH refolding was initiated by the addition of 1 mM ATP, 2 mM phosphoenolpyruvate, and 20 ng/ml pyruvate kinase (Sigma). The activity of mitochondrial MDH from pig heart (Boehringer Mannheim) was assayed at 25°C in 150 mM potassium phosphate buffer, pH 7.5/10 mM DTT/0.5 mM oxaloacetate/0.28 mM NADH (22). The time-dependent oxidation of NADH by MDH was monitored at 340 nm. At 23°C, rates of MDH refolding were linear between 3 and 12 min.
Other Methods.
Chaperonin concentrations were determined by the Bradford protein assay (Bio-Rad), using GroEL and GroES standard solutions, whose respective concentrations were determined by total amino acid analysis (20) and were expressed in terms of the individual GroES and GroEL protomers and not of the GroES7 and GroEL14 oligomers. Lipid phosphorus was determined according to ref. 23.
RESULTS
Interaction of GroEL Chaperonin with Phospholipid Monolayers.
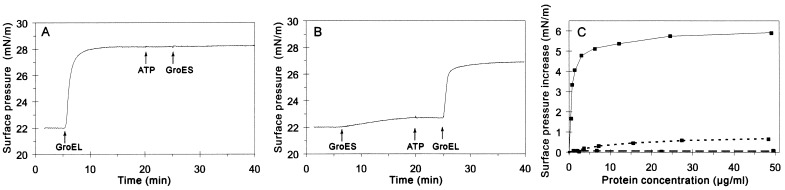
The interaction of GroEL with lipid membrane was studied by simulation of one side of the bilayer with injection of the protein into the aqueous phase beneath a phospholipid monolayer. The consequent change in surface pressure was measured while keeping the surface area constant. To minimize the possibility of incomplete mixing and the formation of patches of pure protein in the monolayer, lipids were spread to a surface pressure greater than the equilibrium spreading pressure of the protein at an air–water interface, 21 mN/m for a GroEL concentration of 3.5 μM in the subphase. Thus, an increase in surface pressure indicated that GroEL specifically interacted with the lipids in the monolayer. GroEL was injected into the subphase underneath a stable monolayer mimicking the lipid composition of E. coli inner membrane [75% DOPE/20% DOPG/5% ECCL (24)] at an initial surface pressure of 22 mN/m and constant area (Fig. 1A). The surface pressure increased and equilibrated after about 15 min at 28 mN/m, indicating the insertion of the protein into the monolayer. Neither the addition of ATP at 1.5 mM nor the addition of the cochaperonin GroES at 7 μM resulted in a further increase of pressure. In the absence of GroEL, injection of GroES alone caused only a minor increase in surface pressure. The presence of the GroES cochaperonin slightly reduced the GroEL-induced pressure increase, which equilibrated at around 27 mN/m (Fig. 1B).
Figure 1.
Effect of chaperonins on the surface pressure of lipid monolayers. GroEL14 or GroES7 oligomers were injected underneath the monolayer and the surface pressure was measured. (A) At time points indicated by arrows, 3.5 μM GroEL, 1.5 mM ATP, and 7 μM GroES were added. (B) The same as in A, with reverse order of events. (C) Surface pressure increases as a function of GroEL concentration in the subphase. Different amounts of GroEL (—), GroES (- - -), or MDH (– – –) were injected into the subphase. The increase in surface pressure was measured after pressure equilibration.
The increase in the surface pressure was dependent on the GroEL concentration (Fig. 1C). The ability of a surface-active molecule to penetrate a lipid monolayer depends on the initial surface pressure (25). The critical pressure for insertion (CPI) is the pressure above which the penetrating molecule can no longer insert into the monolayer. CPI is obtained by measuring the dependence of the pressure increase on the initial surface pressure of the monolayer, extrapolated to a pressure increase of zero. The CPI value of GroEL, determined at saturating protein concentration (25 μg/ml), was estimated to be 29 mN/m with monolayers of 75% DOPE/20% DOPG/5% ECCL. As the surface pressure of biological membranes is thought to be in a similar range (26), GroEL interaction with membrane in the cell is thus conceivable. CPI values similar to the value of GroEL were reported for membrane-associated proteins such as colicin A, rat apolipoprotein AI, LamB signal peptide of E. coli (27), and mitochondrial creatine kinase (28). It is noteworthy that the extent of surface pressure increase was much lower for GroES than for GroEL. Moreover, MDH was unable to penetrate into the monolayer at the applied initial surface pressure (Fig. 1C), although it was as efficiently refolded by membrane-bound as by soluble chaperonins (see below).
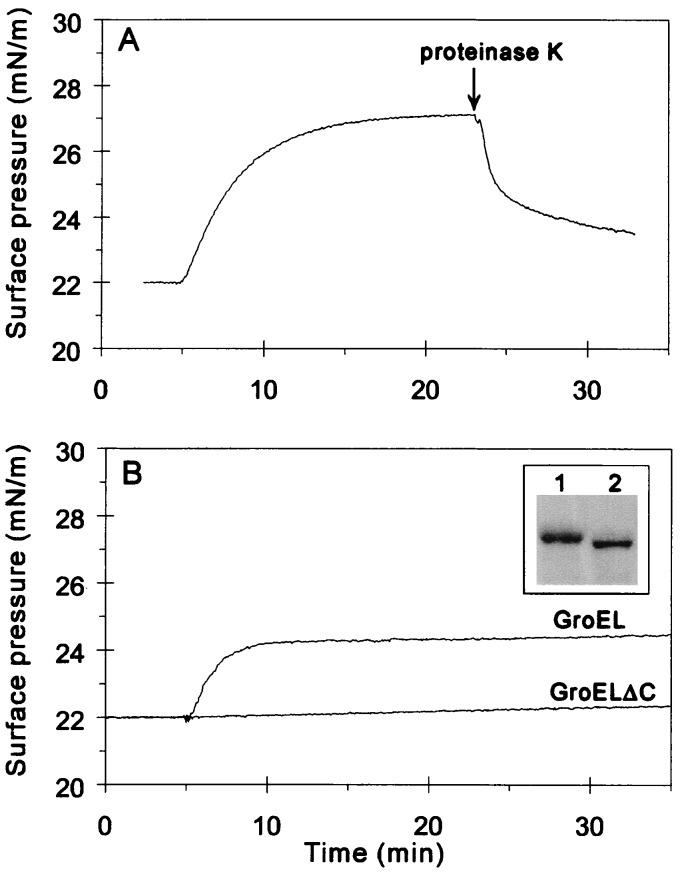
GroEL Dissociates from Monolayers upon Limited Proteolysis by Proteinase K.
The ability of GroEL to resist hydrolysis by proteinase K (29) was exploited to obtain further insight into the mechanism of interaction between GroEL and the lipid monolayer. GroEL-induced surface pressure was rapidly decreased upon exposing GroEL-bound lipid monolayers to proteinase K (Fig. 2A). The injection of GroEL/proteinase K solutions that had been first incubated with or without protease inhibitors (Fig. 2B Inset, lanes 1 and 2, respectively) confirmed that proteinase K treatment prevents GroEL oligomers from penetrating the lipid monolayer (Fig. 2B). Neither proteinase K (16) nor ADP and protease inhibitors caused visible effects on the lipid surface pressure (data not shown). Although proteolysis reduced the total GroEL concentration (Fig. 2B Inset), this concentration drop does not explain the abolished interaction (see titration experiment in Fig. 1C). Therefore, this effect can be primarily attributed to the truncation of GroEL.
Figure 2.
Effect of proteinase K treatment on the interaction of GroEL with lipid monolayers. (A) GroEL was added to the subphase at 5 min at a final concentration of 0.1 μM. Proteinase K (final concentration, 30 μg/ml) was injected at the time indicated by the arrow. (B) GroEL or GroELΔC, prepared as detailed in Materials and Methods, was injected underneath the monolayer at 5 min. (Inset) Analysis of the samples injected into the subphase by SDS/PAGE on an 8% gel (lane 1, GroEL; lane 2, GroELΔC).
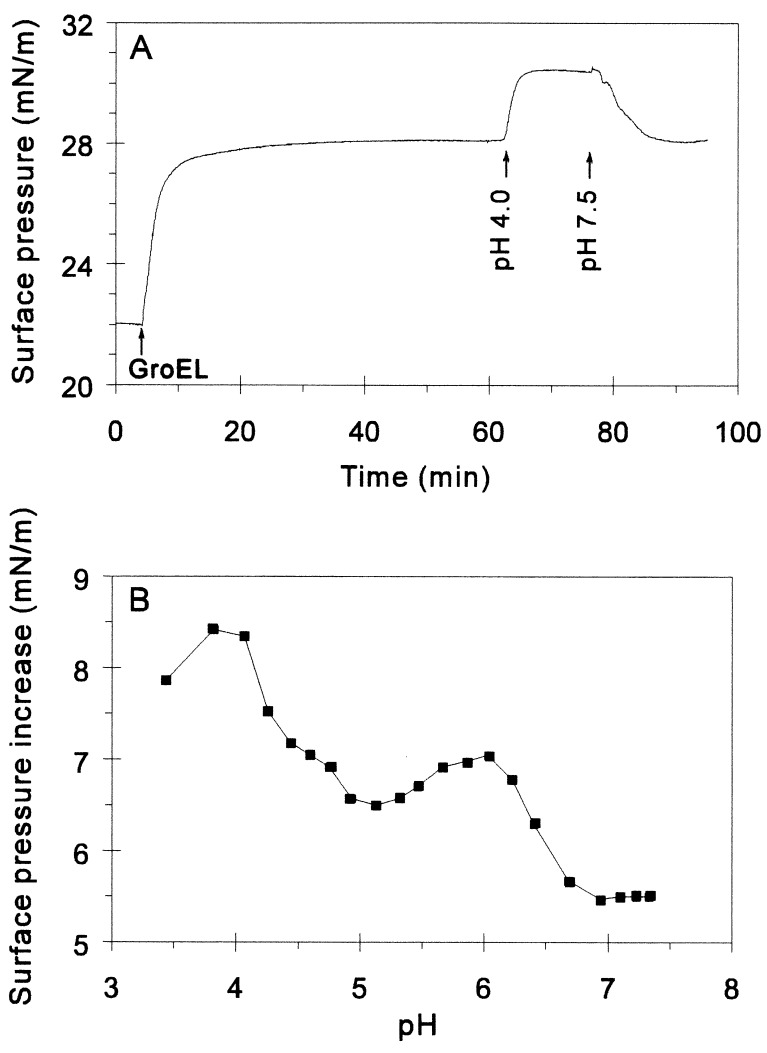
Effect of pH on the GroEL–Lipid Interaction.
The hydrophobic exposure of GroEL14 can be increased experimentally upon decreasing the pH (30). When the pH was lowered to 4.0 by addition of HCl to the subphase containing GroEL, a marked increase in surface pressure was observed (Fig. 3A). The effect was reversible, as the pressure was returned to the original level upon restoration of the initial pH with NaOH. Fig. 3B shows the pH titration curve for GroEL-induced surface pressure increase. Two main transition regions were identified, centering at around pH 6.5 and 4.5. Whereas lowering the pH below 4 caused irreversible effects, above pH 4, the reported effects were reversible (data not shown).
Figure 3.
Effect of pH on GroEL–monolayer interaction. (A) At the indicated time, 3.5 μM GroEL was added to the subphase. After pressure equilibration, the pH of the subphase was lowered to 4 with HCl. Where indicated, the pH was readjusted to 7.5 with NaOH. (B) pH-dependent surface pressure increase caused by the addition of 0.35 μM GroEL.
Chaperonin Binding to LUVs.
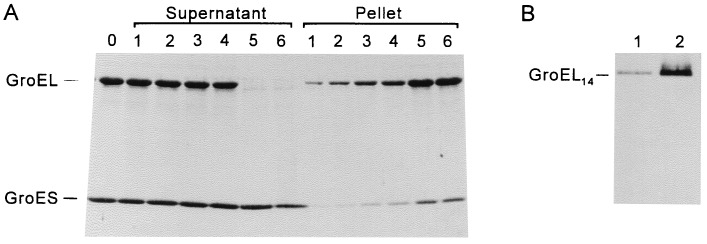
Binding of chaperonins to lipid bilayers was studied by using LUVs of DOPG as model membranes. In the absence of vesicles, only a minor amount of chaperonins was sedimented at 250,000 × g (Pellet lane 1, Fig. 4A). As the vesicle concentration was increased prior to centrifugation, increasing amounts of GroEL and GroES were found in the membrane pellet, at the expense of the supernatant. As judged by scanning of the Coomassie brilliant blue-stained SDS gels (data not shown), GroES sedimented together with GroEL in the presence of ADP at a constant GroES/GroEL molar ratio of ≈0.5. This is anticipated from the asymmetric GroEL14GroES7 heterooligomers that form in the presence of ADP (31). Since the initial GroES/GroEL ratio in the supernatant was 2, this demonstrates that GroES7 cosedimentation with membranes depends on the extent of GroES7 binding to GroEL14. The GroES/GroEL ratio in the membrane pellet was further increased in the presence of 3 mM 5′-adenylyl imidodiphosphate, as expected from the domination of symmetric GroEL14(GroES7)2 heterooligomers that form under such conditions (31). At a lipid concentration of ≈400 μM, 3.5 μM GroEL was entirely sedimented with the membranes (Pellet lanes 5 and 6 in Fig. 4A), while excess unbound GroES remained constant in the supernatant. Analysis on nondenaturing PAGE confirmed that the membrane-associated GroEL was in the native tetradecameric form as expected from the observed binding of GroES7 to membrane-bound GroEL14 (Fig. 4B).
Figure 4.
Binding of chaperonins to LUVs. (A) Binding experiments were carried out as described in the text. GroEL at 3.5 μM and GroES at 7 μM were incubated in the presence of 1.5 mM ADP (lane 0). This mixture was added to LUVs made of DOPG at increasing lipid concentrations. After incubation at 23°C for 30 min samples were centrifuged at 250,000 × g. Supernatants and pellets were analyzed by SDS/PAGE (15% gel). Lipid concentrations were 0, 140, 170, 225, 420, and 560 μM in samples 1–6, respectively. (B) Membrane-bound GroEL is the native 14-mer. GroEL (3.5 μM) was added to 0 μM (lane 1) or 400 μM (lane 2) DOPG vesicles in buffer A. After 30 min of incubation at 23°C, samples were centrifuged. Pellets were analyzed by nondenaturing PAGE (6% gel).
Membrane Binding Does Not Interfere with Chaperonin Activity.
The rate of GroEL ATP hydrolysis was unaffected by the interaction of GroEL14 with lipid membranes (Table 1). Moreover, the ATPase activity of GroEL14 was similarly inhibited by GroES7 (≈50%) in both the membrane-bound and the soluble forms. Thus, GroES7 binding and activity is not affected by the interaction of GroEL14 with membranes. The protein folding activity further confirmed that membrane binding affects neither the GroES binding nor the chaperonin activity of GroEL. Hence, corroborating the ATPase results, rates of chaperonin-assisted refolding of thermally denatured MDH were identical in the absence or presence of membranes in saturating concentration (400 μM) (Table 1). As under the same conditions without membranes, efficient chaperonin-assisted MDH refolding requires the transient formation of MDH–GroEL14(GroES7)2 complexes (21, 22, 32), and membrane insertion of GroEL14 or of the MDH–GroEL14 complex does not interfere with the binding of both GroES7 cochaperonins.
Table 1.
Rate of ATP hydrolysis and chaperonin-assisted refolding of heat-denatured MDH in the presence or absence of membrane vesicles
| Reaction, rate | Without membranes
|
With membranes
|
||
|---|---|---|---|---|
| Without GroES | With GroES | Without GroES | With GroES | |
| ATP hydrolysis, (mol ATP)·min−1·(mol GroEL)−1 | 3.0 ± 0.2 | 1.7 ± 0.2 | 2.9 ± 0.2 | 1.4 ± 0.1 |
| MDH refolding, (mmol MDH)·min−1·(mol GroEL)−1 | 1.16 ± 0.1 | 1.15 ± 0.1 | ||
The data shown are mean values, and the standard deviations are calculated from three independent experiments.
GroEL Increases the Molecular Order of Lipid Bilayers.
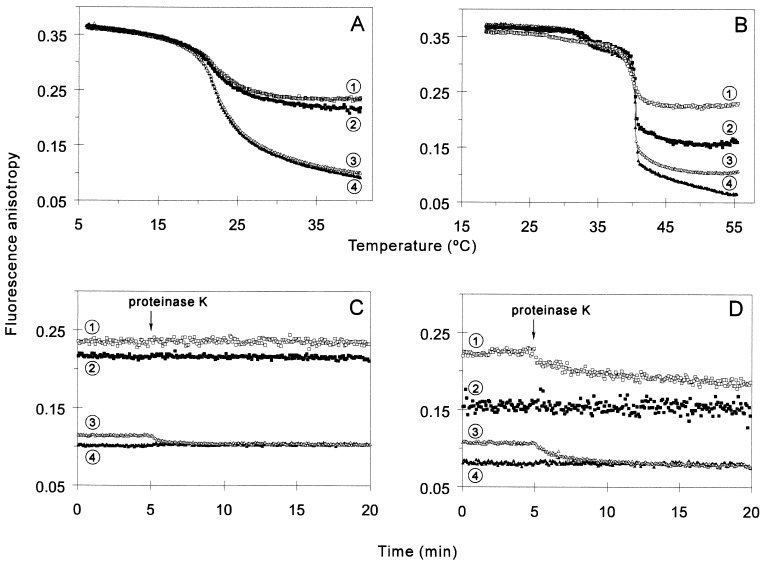
To assess the effect of GroEL14 on the molecular order of lipid bilayers, fluorescence anisotropy measurements were carried out with LUVs labeled with the hydrophobic probes DPH or TMA-DPH. The fluorescence anisotropy of DPH embedded in lipid membranes is a measure of the motional order within the hydrophobic core of the membrane, while TMA-DPH reports movements in the headgroup region (33). Regardless of the probe position, GroEL14 preincubated with either DMPC or DPPC LUVs had no effect on the order of the membrane below the temperature of the main gel-to-liquid-crystalline phase transition [DMPC Tc = 23°C; DPPC Tc = 41°C (34)]. While the presence of the chaperonin did not influence the Tc values (Fig. 5A and B), the increase in acyl chain motion caused by the rise of the temperature was significantly reduced in the presence of GroEL, indicating a higher lipid order. The chaperonin had a more pronounced effect on the fluorescence anisotropy of TMA-DPH in both lipid systems. Thus, membrane perturbations caused by GroEL penetration dominated in the head group region, as compared with the hydrophobic core. Irrespective of the length of the lipid acyl chain, the GroEL-dependent increase in molecular order in the membrane region sampled by DPH was abolished by proteinase K digestion. In contrast, the protease was less effective in the restoration of the lipid order monitored by TMA-DPH (Fig. 5 C and D). Whereas TMA-DPH anisotropy was partially reestablished for DPPC membranes, the proteolysis of GroEL was seemingly ineffective with DMPC. GroEL14 insertion caused a higher degree of membrane order increase (or “fluidity” decrease) in the DPPC vesicles at all depths of the membrane. Therefore, the vertical positioning of GroEL14 within a bilayer is strictly controlled by the length of acyl chain in the host lipid. The ordering effect of GroEL on membranes proved to be reversible, as it was not apparent after the samples had been cooled and subsequently analyzed below the phase transition temperature of the lipids (data not shown).
Figure 5.
Effect of GroEL on the membrane physical state tested on LUVs. (A and B) Steady-state fluorescence anisotropy of TMA-DPH (squares, curves 1 and 2) or DPH (triangles, curves 3 and 4) embedded in LUVs made of DMPC (A) or DPPC (B) was measured as a function of temperature in the presence (open symbols, curves 1 and 3) or in the absence (filled symbols, curves 2 and 4) of 1.5 μM GroEL in buffer A. (C and D) Effect of proteinase K treatment on the fluorescence anisotropy of TMA-DPH (squares, curves 1 and 2) or DPH (triangles, curves 3 and 4) in the presence (open symbols, curves 1 and 3) or in the absence (filled symbols, curves 2 and 4) of 1.5 μM GroEL preincubated with 50 μM LUVs of DMPC (C) or DPPC (D), at 35.6°C and 49°C, respectively. Proteinase K (30 μg/ml, final concentration) was added at the indicated time.
DISCUSSION
The results presented here demonstrate that the GroEL14 oligomer is able to penetrate efficiently into both monolayers and bilayers (LUVs) of various phospholipids. The binding of the chaperonin to lipid membrane—i.e., the formation of a “lipochaperonin”—is apparently governed by the composition and physical state of the host bilayer (Fig. 5), and the presence of the C-terminal tail of GroEL. As temperature defines membrane fluidity and induces minor reversible changes in the structure of the GroEL14 oligomer (35), it may therefore control the GroEL–lipid interactions. We showed that the active GroEL–GroES chaperonin heterooligomers are able to interact with lipids and, furthermore, preserve their ATPase and protein folding activity while bound to the membrane. Since the site of interaction with lipid membranes does not overlap that of GroES7 binding on both ends of the GroEL14 cylinder, membrane binding is likely to occur on the external envelope, rather then on the ends, of the GroEL14 cylinder. This implies that the interaction of GroEL is not isotropic, where chaperonin particles would lie on their side partially embedded and possibly rolling on the membrane surface.
Limited proteolysis of GroEL14 oligomers by proteinase K prevented chaperonin association with lipid membranes (Fig. 2). Mass spectrometry has shown that under the same conditions as in the present study, limited proteinase K treatment removes the last 16 amino acids of the C terminus of the GroEL subunits (17). While the function of this short C-terminal segment of GroEL is unknown, its removal has no destabilizing effect on the GroEL14 oligomer in vitro, nor does it compromise the “classical” chaperonin function of GroEL in vivo (36–38). This motif has been conserved through evolution, and similar sequences are found in most members of the Hsp60 family (36). Our results suggest that the conserved glycine- and methionine-rich C terminus of GroEL, reported to be a flexible tail possibly located in the central channel of the GroEL14 cylinder (39), may be responsible for membrane targeting. It is tempting to speculate that this C-terminal segment functions as a switch under conditions that perturb the membrane, such as during cell division, or under stress, that makes GroEL able to recognize membrane defects. The C-terminal segment may act directly as an anchor or through an unknown mechanism rendering GroEL14 competent for lipid association.
The hydrophobic exposure of GroEL14 can be induced experimentally by decreasing the pH (30), and it may thus increase the affinity of GroEL toward membranes. Indeed, lowering the pH resulted in a higher level of GroEL-induced surface pressure increase. However the observed pH profile (Fig. 3) was not parallel to the pH-dependent hydrophobicity profile of the protein (30). The elevation in surface pressure detected between pH 6 and 7 (Fig. 3B) could be of physiological relevance. For instance, a rapid decrease in the intracellular pH is one of the immediate effect of mild heat shock (40). Consequently, temperature-induced acidification could result in a redistribution of soluble and membrane-associated chaperonins. Whether initiated by high temperature or by pH-induced alterations, membrane binding could increase the concentration of chaperonins in the vicinity of particularly sensitive membrane-associated protein complexes.
Increased microviscosity of lipid membranes upon GroEL binding could also be of physiological significance. It is generally assumed that under thermal stress, damages result from changes in the interactions between lipids and proteins, and from the increased fluidity of membranes. Indeed, an increase in the lipid order, such as in warmth-adapted cells, can prevent heat-induced membrane disorganization (34, 41, 42). Besides long-term warmth adaptation, which confers increased membrane order by changes of lipid composition (43), cells need a means for rapid adjustment of their hyperfluidized lipid matrix. The molecular basis of the fast heat protection is unknown. Due to the time required for changes in lipid composition, the involvement of lipids in short-term adaptation can be excluded. To ensure such protection, one could assume the implication of heat-shock proteins localized in the cytosol with the capability to associate with lipids and counterbalance the abnormal membrane fluidity induced by high temperature. Furthermore, it was shown that the physical state of cell membranes is involved in the sensing and signaling of temperature stress (44, 45). Thus, while lipid interaction with chaperonins rigidifies membranes, this modulation may lead to a down-regulation of the transcriptional activity of heat-shock genes, simultaneously.
Apart from the role in membrane stabilization, GroEL may also function as lipochaperonin that can prevent the irreversible thermal aggregation and assist the refolding of membrane proteins. Moreover, water-soluble proteins could also be rescued by lipochaperonins under stress conditions. It has been shown that during mild denaturation acetylcholine esterase, while assuming the features of the molten globule state, becomes membrane bound (46). It is tempting to speculate that membrane may act as a hydrophobic solvent for molten globule species of stress-destabilized soluble proteins, and thus reduce their propensity to aggregate. The concomitant binding of GroE chaperonins to the same membrane would increase the chances for unstable protein folding intermediates to refold correctly.
The novel properties of GroEL highlighted above are fully consistent with previous reports on the association between membranes and chaperonins in the cell. Our finding on the existence of lipochaperonin may open a new dimension in research focusing on the cellular role of chaperonins.
Acknowledgments
This research was supported by the Hungarian National Scientific Research Foundation (T 012975 to L.V. and T 016392 to I.H.)
ABBREVIATIONS
- DPH
1,6-diphenyl-1,3,5-hexatriene
- LUV
large unilamellar vesicle
- MDH
malate dehydrogenase
- TEA
triethanolamine
- TMA-DPH
1-(4-trimethylammoniumphenyl)-6-phenyl-1,3,5-hexatriene
- ECCL
cardiolipin from E. coli
- DOPE
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine
- DOPG
1,2-dioleoyl-sn-glycero-3-phosphoglycerol
- DMPC
1,2-dimyristoyl-sn-glycero-3-phosphocholine
- DPPC
1,2-dipalmitoyl-sn-glycero-3-phosphocholine
References
- 1.Parsell D A, Lindquist S. Annu Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- 2.Kubota H, Hynes G, Willison K R. Eur J Biochem. 1995;230:3–16. doi: 10.1111/j.1432-1033.1995.tb20527.x. [DOI] [PubMed] [Google Scholar]
- 3.Gillis T P, Miller R A, Young D B, Khanolkar S R, Buchanan T M. Infect Immunol. 1985;49:371–377. doi: 10.1128/iai.49.2.371-377.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vodkin M H, Williams J C. J Bacteriol. 1988;170:1227–1234. doi: 10.1128/jb.170.3.1227-1234.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McFadden B A, Torres-Ruiz J A, Franceschi V R. Planta. 1989;178:297–302. doi: 10.1007/BF00391857. [DOI] [PubMed] [Google Scholar]
- 6.Krishnasamy S, Mannar Mannan R, Krishnan M, Gnanam A. J Biol Chem. 1988;263:5104–5109. [PubMed] [Google Scholar]
- 7.Kovács E, Török Z, Horváth I, Vígh L. Plant Physiol Biochem. 1994;32:285–293. [Google Scholar]
- 8.Lehel C, Wada H, Kovács E, Török Z, Gombos Z, Horváth I, Murata N, Vígh L. Plant Mol Biol. 1992;18:327–336. doi: 10.1007/BF00034959. [DOI] [PubMed] [Google Scholar]
- 9.Lehel C, Los D, Wada H, Györgyei J, Horváth I, Kovács E, Murata N, Vígh L. J Biol Chem. 1993;268:1799–1804. [PubMed] [Google Scholar]
- 10.Jäger K M, Bergman B. Planta. 1990;183:120–125. doi: 10.1007/BF00197575. [DOI] [PubMed] [Google Scholar]
- 11.Scorpio A, Johnson P, Laquerre A, Nelson D R. J Bacteriol. 1994;176:6449–6456. doi: 10.1128/jb.176.21.6449-6456.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soltys B J, Gupta R S. Exp Cell Res. 1996;222:16–27. doi: 10.1006/excr.1996.0003. [DOI] [PubMed] [Google Scholar]
- 13.Kessler F, Blobel G. Proc Natl Acad Sci USA. 1996;93:7684–7689. doi: 10.1073/pnas.93.15.7684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blander S J, Horwitz M A. J Clin Invest. 1993;91:717–721. doi: 10.1172/JCI116253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azem A, Diamant S, Goloubinoff P. Biochemistry. 1994;33:6671–6675. doi: 10.1021/bi00187a037. [DOI] [PubMed] [Google Scholar]
- 16.Török Z, Demel R A, Leenhouts J M, De Kruijff B. Biochemistry. 1994;33:5589–5594. doi: 10.1021/bi00184a030. [DOI] [PubMed] [Google Scholar]
- 17.Martin J, Mayhew M, Langer T, Hartl F-U. Nature (London) 1993;366:228–233. doi: 10.1038/366228a0. [DOI] [PubMed] [Google Scholar]
- 18.Hope M J, Bally M B, Webb G, Cullis P R. Biochim Biophys Acta. 1983;812:55–65. doi: 10.1016/0005-2736(85)90521-8. [DOI] [PubMed] [Google Scholar]
- 19.Schlame M, Horváth I, Török Z, Horváth L I, Vígh L. Biochim Biophys Acta. 1990;1045:1–8. doi: 10.1016/0005-2760(90)90196-5. [DOI] [PubMed] [Google Scholar]
- 20.Diamant S, Azem A, Goloubinoff P. Biochemistry. 1995;34:273–277. doi: 10.1021/bi00001a033. [DOI] [PubMed] [Google Scholar]
- 21.Török Z, Vígh L, Goloubinoff P. J Biol Chem. 1996;271:16180–16186. doi: 10.1074/jbc.271.27.16180. [DOI] [PubMed] [Google Scholar]
- 22.Diamant S, Azem A, Weiss C, Goloubinoff P. J Biol Chem. 1995;270:28387–28391. doi: 10.1074/jbc.270.47.28387. [DOI] [PubMed] [Google Scholar]
- 23.Rouser G, Fleischer S, Yamamoto A. Lipids. 1970;5:494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- 24.Kusters R, Dowhan W, de Kruijff B. J Biol Chem. 1991;266:8659–8662. [PubMed] [Google Scholar]
- 25.Verger R, Pattus F. Chem Phys Lipids. 1982;30:189–227. doi: 10.1016/0009-3084(76)90023-2. [DOI] [PubMed] [Google Scholar]
- 26.Demel R A, Van Kessel W S M, Zwaal R F A, Roelofsen B, van Deenen L L M. Biochim Biophys Acta. 1975;406:97–107. doi: 10.1016/0005-2736(75)90045-0. [DOI] [PubMed] [Google Scholar]
- 27.Briggs M S, Gierasch L M, Zlotnik A, Lear J D, DeGrado W F. Science. 1985;228:1096–1098. doi: 10.1126/science.3158076. [DOI] [PubMed] [Google Scholar]
- 28.Rojo M, Hovius R, Demel R, Wallimann T, Eppenberger H M, Nicolay K. FEBS Lett. 1991;281:123–129. doi: 10.1016/0014-5793(91)80374-c. [DOI] [PubMed] [Google Scholar]
- 29.Langer T, Pfeifer G, Martin J, Baumeister W, Hartl F-U. EMBO J. 1992;11:4757–4765. doi: 10.1002/j.1460-2075.1992.tb05581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gibbons D L, Horowitz P M. J Biol Chem. 1995;270:7335–7340. doi: 10.1074/jbc.270.13.7335. [DOI] [PubMed] [Google Scholar]
- 31.Azem A, Kessel M, Goloubinoff P. Science. 1994;265:654–656. doi: 10.1126/science.7913553. [DOI] [PubMed] [Google Scholar]
- 32.Azem A, Diamant S, Kessel M, Weiss C, Goloubinoff P. Proc Natl Acad Sci USA. 1995;92:12021–12025. doi: 10.1073/pnas.92.26.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitagawa S, Matsubayashi M, Kotani K, Usui K, Kametani F. J Membr Biol. 1991;119:221–227. doi: 10.1007/BF01868727. [DOI] [PubMed] [Google Scholar]
- 34.Quinn P J, Joó F, Vígh L. Prog Biophys Mol Biol. 1989;53:71–103. doi: 10.1016/0079-6107(89)90015-1. [DOI] [PubMed] [Google Scholar]
- 35.Hansen J E, Gafni A. J Biol Chem. 1994;269:6286–6289. [PubMed] [Google Scholar]
- 36.McLennan N F, Girshovich A S, Lissin N M, Charters Y, Masters M. Mol Microbiol. 1993;7:49–58. doi: 10.1111/j.1365-2958.1993.tb01096.x. [DOI] [PubMed] [Google Scholar]
- 37.McLennan N F, McAteer S, Masters M. Mol Microbiol. 1994;14:309–321. doi: 10.1111/j.1365-2958.1994.tb01292.x. [DOI] [PubMed] [Google Scholar]
- 38.Weissman J S, Hohl C M, Kovalenko O, Kashi Y, Chen S X, Braig K, Saibil H R, Fenton W A, Horwich A L. Cell. 1995;83:577–587. doi: 10.1016/0092-8674(95)90098-5. [DOI] [PubMed] [Google Scholar]
- 39.Braig K, Otwinowski Z, Hegde R, Boisvert D C, Joachimiak A, Horwich A L, Sigler P B. Nature (London) 1994;371:578–586. doi: 10.1038/371578a0. [DOI] [PubMed] [Google Scholar]
- 40.Weitzel G, Pilatus U, Rensing L. Exp Cell Res. 1987;170:64–79. doi: 10.1016/0014-4827(87)90117-0. [DOI] [PubMed] [Google Scholar]
- 41.Vígh L, Gombos Z, Horváth I, Joó F. Biochim Biophys Acta. 1989;979:361–364. [Google Scholar]
- 42.Mejía R, Gómez-Eichelmann M C, Fernández M S. Biochim Biophys Acta. 1995;1239:195–200. doi: 10.1016/0005-2736(95)00152-s. [DOI] [PubMed] [Google Scholar]
- 43.Hazel J R. Annu Rev Physiol. 1995;57:19–43. doi: 10.1146/annurev.ph.57.030195.000315. [DOI] [PubMed] [Google Scholar]
- 44.Vígh L, Los D A, Horváth I, Murata N. Proc Natl Acad Sci USA. 1993;90:9090–9094. doi: 10.1073/pnas.90.19.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carratù L, Franceschelli S, Pardini C L, Kobayashi G S, Horváth I, Vígh L, Maresca B. Proc Natl Acad Sci USA. 1996;93:3870–3875. doi: 10.1073/pnas.93.9.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin I, Silman I, Weiner L M. Protein Sci. 1996;5:42–51. doi: 10.1002/pro.5560050106. [DOI] [PMC free article] [PubMed] [Google Scholar]