Abstract
Angiogenin stimulates both [3H]thymidine incorporation and proliferation of human endothelial cells in sparse cultures. Under these conditions, a 170-kDa cell surface protein can be detected that binds angiogenin specifically. Angiogenin-stimulated cell growth is concentration-dependent and is completely inhibited by an anti-angiogenin monoclonal antibody, but not by a nonimmune control antibody. It is not affected by the nonangiogenic homolog, RNase A, nor by other angiogenic proteins, such as basic fibroblast growth factor and its antibody. Results suggest that under specific conditions, endothelial cells express an angiogenin receptor that may mediate angiogenin-stimulated DNA synthesis and proliferation and play an important role in angiogenin-induced angiogenesis.
Keywords: angiogenesis, proliferation, [3H]thymidine incorporation, cell density, crosslinking
Angiogenin is a potent inducer of angiogenesis (1), a complex process of blood vessel formation that consists of several separate but interconnected steps at the cellular and biochemical level: (i) activation of endothelial cells by the action of an angiogenic stimulus; (ii) invasion of activated endothelial cells into the surrounding tissues and migration toward the source of the angiogenic stimulus; and (iii) proliferation and differentiation of endothelial cells to form a new microvasculature (2, 3). Angiogenin has been demonstrated to induce most of the individual events in the process of angiogenesis, including binding to endothelial cells (4), stimulating second messengers (5), mediating cell adhesion (6), activating cell-associated proteases (7), inducing cell invasion (8), and organizing the formation of tubular structures from cultured endothelial cells (9). Angiogenin has also been shown to undergo nuclear translocation in endothelial cells via receptor-mediated endocytosis (10) and nuclear localization sequence-assisted nuclear import (11).
A unique feature of angiogenin is that although it was originally isolated from the conditioned medium of human adenocarcinoma cells (1) based solely on its angiogenic activity on the chicken chorioallantoic membrane, it actually is a constituent of human plasma and normally circulates at a concentration of 250 to 360 ng/ml (12, 13). Angiogenesis is a highly controlled process under usual physiological conditions. Abnormal angiogenesis can have devastating consequences as in many pathological conditions, such as arthritis, diabetic retinopathy, and tumor growth. To avoid unwanted, rampant angiogenesis, circulating angiogenin must be poised for action without actively stimulating neovascularization. On the other hand, extravascular angiogenin does stimulate the formation of blood vessels, as evidenced by the angiogenesis seen in the chicken embryo chorioallantoic membrane assay (1) and in the cornea and meniscus of the knee of the rabbit (14) and by the ability of angiogenin antagonists to inhibit the establishment of cell tumors implanted in nude mice (15).
A conceivable mechanism in the regulation of the activity of angiogenin would act at the cellular level and control the expression and manifestation of its receptors. We have previously identified an angiogenin-binding protein located on the endothelial cell surface (16), characterized it as a dissociable smooth muscle-type α-actin (17), and showed that it is involved in angiogenin-induced angiogenesis by stimulating cell-associated proteolytic activities and endothelial cell invasion (8). However, a classical transmembrane cellular receptor for angiogenin has remained elusive.
The mitogenic activity of angiogenin toward endothelial cells has been another open question. Most known angiogenic factors, such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), acidic fibroblast growth factor (aFGF), and epidermal growth factor are all endothelial cell mitogens (18). But the mitogenic activity of angiogenin has not been established, except for an early study showing that bovine angiogenin stimulates [3H]thymidine uptake and proliferation of bovine brain capillary endothelial cells, but not of bovine aortic arch endothelial cells (19). It has been reported that initial vascular sprouting and elongation can be achieved merely by migration and redistribution of existing endothelial cells from the limbal vessels and does not require proliferation of endothelial cells (20). However, subsequent vascular ingrowth will not progress without cell proliferation. Hence, true angiogenesis and establishment of an extensive vascular network requires proliferation of endothelial cells.
One possible reason for the lack of an endothelial cell response to angiogenin is that cultured cells do not mimic the cells in vivo. Based on the hypothesis that endothelial cells may respond to angiogenin only under specific conditions, we used [3H]thymidine incorporation and proliferation assays to search for the appropriate circumstances under which the protein might actually stimulate the growth of human endothelial cells. We found that angiogenin indeed stimulates both DNA synthesis and proliferation of human endothelial cells when they are in very sparse cultures. Moreover, under these conditions, we could detect a 170-kDa putative receptor for angiogenin on the surface of human endothelial cells.
MATERIALS AND METHODS
Materials.
Human angiogenin (Met-1) was isolated from an Escherichia coli expression system (21) and was provided by R. Shapiro (Harvard Medical School); the anti-human angiogenin monoclonal antibody 26-2F (22) was provided by K. A. Olson (Harvard Medical School); bFGF, aFGF, anti-aFGF polyclonal antibody, fibronectin, monomeric avidin–Sepharose, streptavidin–alkaline phosphatase, and Biodyne B nylon membranes were from Promega; the 165-aa form of VEGF was from R & D Systems; carrier-free Na125I (17.4 Ci/g; 1 Ci = 37 GBq) and methyl-[3H]thymidine (6.7 Ci/mmol) were from DuPont/NEN; excellulose GF-5 desalting columns and Iodo-Beads iodination reagent were from Pierce; (disulfosuccinimidyl)suberate (Sulfo-DSS) was from Calbiochem; sulfosuccinimidyl-6-(biotinamido)hexanoate was from Vector Laboratories; complete protease inhibitor cocktail tablets were from Boehringer Mannheim; and anti-bFGF monoclonal antibody, biotin, RNase A, and DNase I were from Sigma.
Cell Culture.
Human umbilical venous endothelial (HUVE), human umbilical arterial endothelial (HUAE), and human microvascular endothelial (HME) cells were purchased from Cell Systems (Kirkland, WA) as primary cultures isolated from human umbilical veins, arteries, and human foreskin dermal tissues, respectively. HUAE and HME cells were cultured on attachment factor (Cell Systems)-coated flasks in endothelial cell growth medium (Cell Systems) containing 10% fetal bovine serum (FBS). HUVE cells were cultured on fibronectin-coated dishes in human endothelial serum-free medium (HE-SFM; GIBCO/BRL–Life Technologies) containing 20 ng/ml bFGF or on uncoated dishes but in HE-SFM plus 10% FBS and 20 ng/ml bFGF. Cells between passages 3 and 15 inclusive were used for all experiments. Cell numbers were determined with a Coulter counter, and cell viability was measured by trypan blue dye exclusion assay.
Iodination of Angiogenin.
125I-labeled angiogenin was prepared with the use of Iodo-Beads. One Iodo-bead was added to 175 μl of 0.114 M phosphate buffer (pH 6.5), containing 0.5 mCi of Na125I and incubated at room temperature for 5 min. Angiogenin, 50 μg in 25 μl of H2O, was added, and the mixture was incubated at room temperature for another 15 min. The reaction was terminated by removing the Iodo-Bead, and iodinated angiogenin was separated from free iodine by a GF-5 desalting column equilibrated in 0.1 M phosphate buffer (pH 6.5). Fractions of 0.5 ml were collected, and the radioactivity in each fraction was determined with a gamma counter.
[3H]Thymidine Incorporation.
HUVE and HME cells were seeded in either 24-well plates or 35-mm dishes at a density of 6 × 103–1.5 × 104 cells per cm2 and cultured in HE-SFM containing 10% FBS and 20 ng/ml bFGF at 37°C under humidified air containing 5% CO2. Six replicates were used for each sample. After 24 hr, the cells were washed three times with prewarmed HE-SFM and serum-starved in HE-SFM for 18 hr. The culture medium was removed, and the cells were incubated in HE-SFM with test samples in the presence of 1 μCi/ml [3H]thymidine for 14 hr. At the end of the incubation, the cells were washed three times with PBS, precipitated with 10% trichloroacetic acid at room temperature for 30 min, washed 2 times with ethanol, and solubilized with 0.2 M NaOH plus 0.2% SDS. After neutralization with 1/5 volume of 1 M HCl, the radioactivity was determined by liquid scintillation counting.
Cell Proliferation.
HUVE and HME cells were seeded in either fibronectin- or attachment factor (Cell Systems)-coated 35-mm dishes in HE-SFM at 4–8 × 103 cells per cm2. Test samples (10 μl) were added immediately after the cells were seeded. When combinations of samples were tested, they were premixed and always adjusted to a final volume of 10 μl with HE-SFM before addition to the cells. The cells were incubated at 37°C in humidified air containing 5% CO2 for 48 hr. At the end of this time, the medium was aspirated, and the cells were washed once with 1 ml of PBS and detached with 0.25 ml of trypsin-versene (0.05%) solution. Cell numbers were determined with a Coulter counter.
Binding and Crosslinking of 125I-Labeled Angiogenin to the Endothelial Cell Surface.
Endothelial cells (HUVE, HUAE, HME), seeded at 5 × 103 cells per cm2, were cultured in HE-SFM plus 10% FBS and 20 ng/ml bFGF at 37°C under 5% humidified CO2 for 24 hr and starved in HE-SFM for another 24 hr. The cells were then cooled to 4°C, washed twice with PBS, and incubated with 50 ng/ml 125I-labeled angiogenin in PBS at 4°C for 30 min. At the end of this time, the supernatant was removed and 1 ml of 50 ng/ml fresh 125I-labeled angiogenin in PBS was readded to the cells and incubated at 4°C for another 30 min. Unbound 125I-labeled angiogenin from the second incubation was removed by washing three times with PBS. Bound 125I-labeled angiogenin was crosslinked to the cell surface by treatment with 0.1 mM Sulfo-DSS in PBS at 4°C for 10 min. Unreacted Sulfo-DSS was quenched by 5 mM Tris·HCl (pH 7.5). The cells were then washed twice with PBS and solubilized in 100 μl of 1× SDS/PAGE sample buffer. The entire sample was subject to SDS/PAGE and autoradiography. In competition experiments, the competitors were premixed with 125I-labeled angiogenin and were present in both incubations.
Isolation of the Putative Angiogenin Receptor from the Endothelial Cell Surface.
HUVE cells, at passage 5, were plated at 5 × 103 cells per cm2 in a T162 flask and cultured in HE-SFM plus 10% FBS and 20 ng/ml bFGF at 37°C for 24 hr. The culture medium was removed and the cells were washed once with prewarmed HE-SFM and incubated in HE-SFM for another 18 hr. The cells were cooled at 4°C for 10 min, washed three times with cold PBS, and biotinylated by incubating with 15 ml of 0.5 mg/ml sulfosuccinimidyl-6-(biotinamido)hexanoate in PBS at 4°C for 30 min with gentle shaking. Unreacted reagent was removed by washing the cells three times with PBS. Biotinylated membrane molecules were solubilized with 0.5% Triton X-100 in 0.1 M phosphate buffer (pH 7.2), containing 1× complete protease inhibitor cocktail at 4°C for 30 min. The solubilizates were centrifuged to remove cell debris and subjected to chromatography on a monomeric avidin–Sepharose column. Biotinylated molecules were eluted from the column by 0.1 M biotin in 0.1 M phosphate buffer (pH 7.2) and were successively adsorbed by DNase I–Sepharose and RNase A–Sepharose. The flow-through fraction was then applied to an angiogenin–Sepharose column and washed with five volumes of H2O. The bound materials were eluted from the column with 0.1% trifluoroacetic acid, lyophilized, and analyzed by SDS/PAGE and Western blotting with alkaline phosphatase-labeled streptavidin.
RESULTS
Angiogenin Stimulates [3H]Thymidine Incorporation and Cell Proliferation of Human Endothelial Cells.
We have defined conditions under which human endothelial cells respond to exogenous angiogenin in both [3H]thymidine incorporation and cell proliferation assays (Table 1). To obtain statistically significant data, 5 and 15 independent experiments with HUVE cells were carried out for [3H]thymidine incorporation and cell proliferation, respectively. Results showed that angiogenin stimulated HUVE cells to increase [3H]thymidine incorporation and proliferation by 33 and 34%, respectively. In 14 and 9 independent experiments with HME cells, angiogenin stimulated an average increase of 19 and 25% in [3H]thymidine incorporation and cell proliferation, respectively. Student–Fisher t test showed that the increases of [3H]thymidine incorporation and cell proliferation induced by angiogenin in both HUVE and HME cells are statistically significant, as indicated by P values.
Table 1.
Angiogenin stimulates [3H]thymidine incorporation and proliferation of human endothelial cells
| Cells | [3H]thymidine incorporation
|
Cell proliferation
|
||||
|---|---|---|---|---|---|---|
| % increase | P value | n | % increase | P value | n | |
| HUVE | 33 ± 4.7 | <0.005 | 5 | 34 ± 3.0 | <0.0005 | 15 |
| HME | 19 ± 2.9 | <0.005 | 14 | 25 ± 2.3 | <0.0005 | 9 |
Data shown are the results of treatment with 1 μg/ml angiogenin in 5 and 15 independent experiments for HUVE and 14 and 9 experiments for HME cells in [3H]thymidine incorporation and proliferation assays, respectively.
Angiogenin-Stimulated Cell Growth Is Concentration-Dependent.
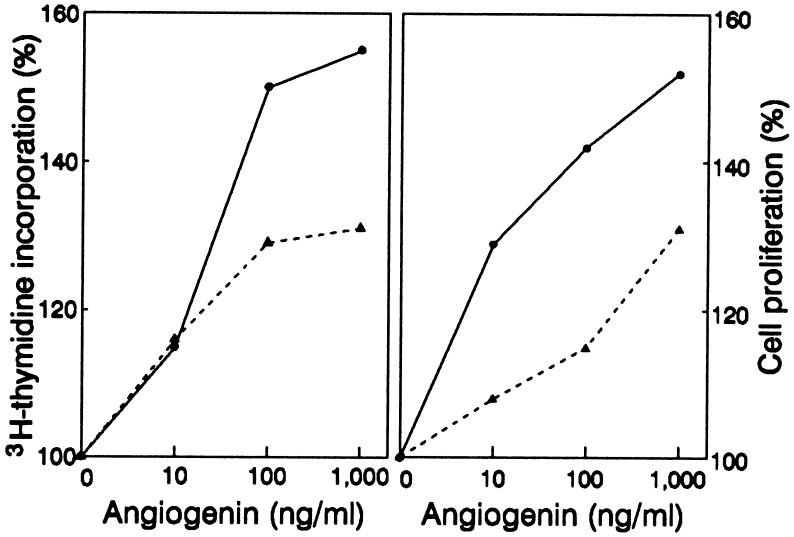
The ability of angiogenin to stimulate endothelial cell growth was concentration-dependent, as shown by a representative experiment in Fig. 1. In the absence of angiogenin, HUVE and HME cells (1 × 104) incorporated 5,600 ± 400 and 8,400 ± 600 cpm of [3H]thymidine, respectively, whereas in the presence of 10 ng/ml of angiogenin, the results were 6,400 ± 600 and 9,700 ± 300 cpm (i.e., 14 and 15% increases), respectively. At 100 ng/ml, angiogenin stimulated a 48% (8,300 ± 400) and a 30% (10,900 ± 400) increase of [3H]thymidine incorporation in HUVE and HME cells, respectively (Fig. 1 Left). Raising the angiogenin concentration to 1 μg/ml enhanced [3H]thymidine uptake by 54% (8,600 ± 700 cpm) and 32% (11,100 ± 300 cpm) for HUVE and HME cells, respectively, not significantly different from results seen at 100 ng/ml. A similar pattern of concentration dependency was observed for angiogenin-stimulated cell proliferation (Fig. 1 Right). Almost no proliferation occurred when cells were cultured in HE-SFM in the absence of angiogenin. The recovery from 50,000 cells was 47,900 ± 1,500 and 53,700 ± 1,800 after 48 hr for HUVE and HME cells, respectively. In the presence of 10, 100, and 1000 ng/ml of angiogenin, proliferation was increased by 29% (61,600 ± 1,800), 42% (67,800 ± 2,900), and 52% (72,600 ± 2,400) for HUVE cells and by 8% (57,800 ± 1,300), 15% (61,700 ± 1,600), and 31% (70,300 ± 1,900) for HME cells, respectively. A 30% increase of HME cell proliferation was observed with 10 μg/ml angiogenin in a separate experiment, indicating that angiogenin activity in HME cells reached saturation at 1 μg/ml. The data in Table 1 and Fig. 1 for 1 μg/ml angiogenin are somewhat different, because Table 1 lists the mean of results from all experiments carried out in the course of investigation, whereas Fig. 1 shows the data from a single exemplary experiment.
Figure 1.
Dose-dependent stimulation of [3H]thymidine incorporation and proliferation of endothelial cells by angiogenin. For [3H]thymidine incorporation (Left), 1 × 104 HUVE (solid lines) and HME (dashed lines) cells were used per well in 24-well plates (5 × 103 cells per cm2), with six replicates for each sample. For cell proliferation assays, 5 × 104 cells were used per 35-mm dish (5 × 103 cells per cm2), with three replicates for each sample. Data shown are the percent increases over controls.
An Anti-Angiogenin Monoclonal Antibody Inhibits the Activity of Angiogenin.
Angiogenin-induced [3H]thymidine incorporation and cell proliferation of HUVE cells were inhibited by 26-2F, an anti-angiogenin monoclonal antibody (22), but not by CCL130, a nonimmune control antibody (Table 2). The effect of 20 ng/ml angiogenin, which induced a 33% increase in [3H]thymidine incorporation and a 16% increase in cell proliferation, was completely inhibited by 10 μg/ml of 26-2F. Mixtures of angiogenin with 26-2F had no stimulating effect, but with CCL 130, there was a 37% (16,000 ± 700 cpm) and a 14% (16,800 ± 200 cells) increase. Neither bFGF nor VEGF was affected by 26-2F. At 5 and 20 ng/ml, VEGF and bFGF stimulated a 48% and a 715% increase of [3H]thymidine incorporation and a 139% and a 122% increase in cell proliferation, respectively. In the presence of 10 μg/ml 26-2F, increases induced by VEGF and bFGF were 53% and 717% and 120% and 101%, respectively, not significantly different from those seen in the absence of 26-2F.
Table 2.
Effect of anti-angiogenin monoclonal antibody on the activity of angiogenin in HUVE cells
| Samples | [3H]thymidine incorporation, cpm (% increase) | Proliferation, no. of cells (% increase) |
|---|---|---|
| Control | 11,700 ± 1,300 (100) | 14,800 ± 800 (100) |
| Angiogenin | 15,600 ± 1,100 (133) | 17,200 ± 300 (116) |
| 26-2F | 11,700 ± 1,500 (100) | 14,900 ± 200 (101) |
| Angiogen plus 26-2F | 11,500 ± 1,200 (98) | 15,100 ± 1,000 (102) |
| VEGF | 17,300 ± 600 (148) | 35,300 ± 1,000 (239) |
| VEGF plus 26-2F | 17,900 ± 400 (153) | 32,500 ± 1,200 (220) |
| bFGF | 95,300 ± 500 (815) | 32,900 ± 300 (222) |
| bFGF plus 26-2F | 95,600 ± 800 (817) | 29,800 ± 300 (201) |
HUVE cells were plated at 15,000 cells per 35-mm culture dish (1,500 cells per cm2). Concentrations of angiogenin, VEGF, FGF, and 26-2F were 20 ng/ml, 5 ng/ml, 20 ng/ml, and 10 μg/ml, respectively.
Factors That Affect the Activity of Angiogenin in Endothelial Cells.
Cell density. Cell density was the most important factor that influenced angiogenin-induced [3H]thymidine incorporation and cell proliferation. Endothelial cells responded to angiogenin only when they were in sparse culture. The responsiveness diminished when the cell density exceeded 2 × 104 cells per cm2. On the other hand, cells cannot be too sparse (<103 per cm2), since the resulting high signal/noise precludes meaningful results. The optimal density for [3H]thymidine incorporation and cell proliferation assays was 6 × 103–1.5 × 104 and 4–8 × 103 cells per cm2, respectively.
Attachment factors.
The type of attachment factor employed was another critical item. In the [3H]thymidine incorporation assay, best results were obtained without the use of any attachment factor. However, for successful cell proliferation assays, culture dishes should be coated with fibronectin or the attachment factors from Cell Systems. Polylysine and angiogenin itself as attachment factors did not provide any advantage over fibronectin in either assay.
FBS.
FBS completely inhibited the activity of angiogenin (Table 3). FBS itself stimulated a 67% increase in [3H]thymidine incorporation into HME cells. When angiogenin was added in the presence of 1% FBS, the stimulation, 67%, was the same as in the absence of angiogenin. In contrast, the same concentration of angiogenin added to cells in HE-SFM resulted in a stimulation of 19%.
Table 3.
Effect of FBS on angiogenin-stimulated [3H]thymidine incorporation in HME cells
| Samples | [3H]Thymidine incorporation, cpm | % increase |
|---|---|---|
| Control | 5,700 ± 60 | 100 |
| Angiogenin | 6,800 ± 200 | 119 |
| FBS | 9,500 ± 400 | 167 |
| FBS plus angiogenin | 9,500 ± 700 | 167 |
HME cells were plated at 1 × 104 cells per well in 24-well plates (5 × 103 cells per cm2). FBS was at 1% and was present during both starvation and stimulation.
Exogenous bFGF.
Angiogenin activity was not masked by exogenous bFGF (Table 4). Angiogenin alone stimulated 33 and 16% increases and bFGF alone stimulated 182 and 36% increases in HUVE and HME cell proliferation, respectively. A mixture of angiogenin and bFGF stimulated 265 and 62% increases, which were 29 and 19% greater than for samples treated just with bFGF. The percent increase stimulated by angiogenin in the presence of bFGF was very close to that seen in its absence.
Table 4.
Effects of bFGF on angiogenin-stimulated proliferation of human endothelial cells
| Samples | Cells
|
|||
|---|---|---|---|---|
| HUVE
|
HME
|
|||
| Cell no. | % increase | Cell no. | % increase | |
| Control | 69,900 ± 1,000 | 100 | 49,900 ± 1,400 | 100 |
| Angiogenin | 93,000 ± 4,000 | 133 | 57,900 ± 1,400 | 116 |
| bFGF | 197,000 ± 3,600 | 282 | 67,700 ± 1,600 | 136 |
| bFGF plus angiogenin | 255,000 ± 5,000 | 365 | 80,800 ± 1,100 | 162 |
HUVE and HME cells, at 7 × 104 and 5 × 104 per 35-mm dish, were stimulated by angiogenin (1 μg/ml), bFGF (2 ng/ml), or a mixture of the two.
RNase A.
As shown in Table 5, angiogenin-stimulated proliferation of HUVE cells was not inhibited by the homologous protein, RNase A. The percent increases induced by 1 μg/ml angiogenin in the absence or presence of 10 μg/ml RNase A were 25 and 21%, respectively, while 10 μg/ml RNase A itself had no effect on cell proliferation under these conditions.
Table 5.
Effect of RNase A on angiogenin-stimulated proliferation of HUVE cells
| Samples | Cell no. | % increase |
|---|---|---|
| Control | 192,300 ± 600 | 100 |
| Angiogenin | 240,200 ± 1,900 | 125 |
| RNase A | 197,700 ± 300 | 103 |
| Angiogenin plus RNase A | 232,300 ± 1,000 | 121 |
Experiments were carried out in T25 flasks containing 1.5 × 105 cells per flask (6 × 103 cells per cm2). Three flasks were used for each sample. Concentrations of angiogenin and RNase A were 1 and 10 μg/ml, respectively.
Endogenous FGFs.
Endothelial cells are known to secrete aFGF and bFGF (23). We therefore investigated the effects of endogenous FGFs on the activity of angiogenin. As shown in Table 6, an anti-aFGF antibody had little effect, while an anti-bFGF monoclonal antibody significantly reduced spontaneous [3H]thymidine incorporation of both HME and HUVE cells. These results suggest a critical role of endogenous bFGF but not aFGF in survival and minimum growth of endothelial cells under serum-free conditions. Nevertheless, the angiogenin-induced [3H]thymidine incorporation in both cell types was not inhibited by anti-aFGF antibodies, anti-bFGF antibodies, or a combination of the two. The increases stimulated by angiogenin relative to the corresponding controls in the presence of anti-aFGF, anti-bFGF, and both antibodies were 14, 21, and 30% in HUVE cells and 15, 27, and 37% in HME cells, respectively. These results were not significantly different from those obtained in the absence of anti-FGF antibodies, 34 and 24% for HUVE and HME cells, respectively.
Table 6.
Effect of anti-FGF antibodies on angiogenin-stimulated [3H]thymidine incorporation in human endothelial cells
| Antibody | Cells
|
|||
|---|---|---|---|---|
| HUVE
|
HME
|
|||
| [3H]thymidine incorporation, cpm | % increase | [3H]thymidine incorporation, cpm | % increase | |
| Control | ||||
| − | 4,400 ± 200 | 100 | 9,700 ± 300 | 100 |
| + | 5,900 ± 50 | 134 | 12,000 ± 300 | 124 |
| aFGF IgG | ||||
| − | 5,100 ± 100 | 100 | 10,500 ± 400 | 100 |
| + | 5,800 ± 100 | 114 | 12,100 ± 500 | 115 |
| bFGF IgG | ||||
| − | 3,400 ± 600 | 100 | 5,900 ± 300 | 100 |
| + | 4,100 ± 500 | 121 | 7,500 ± 400 | 127 |
| aFGF IgG plus | ||||
| bFGF IgG | ||||
| − | 3,000 ± 200 | 100 | 5,900 ± 800 | 100 |
| + | 3,900 ± 100 | 130 | 8,100 ± 400 | 137 |
HUVE and HME cells were plated at 1.6 × 104 cells per well in 24-well plates (8 × 103 cells per cm2). Concentrations of angiogenin, anti-aFGF, and anti-bFGF antibody were 1, 10, and 10 μg/ml, respectively. −, Absence of angiogenin; +, presence of angiogenin.
Identification of a Putative Angiogenin Receptor on the Surface of Endothelial Cells.
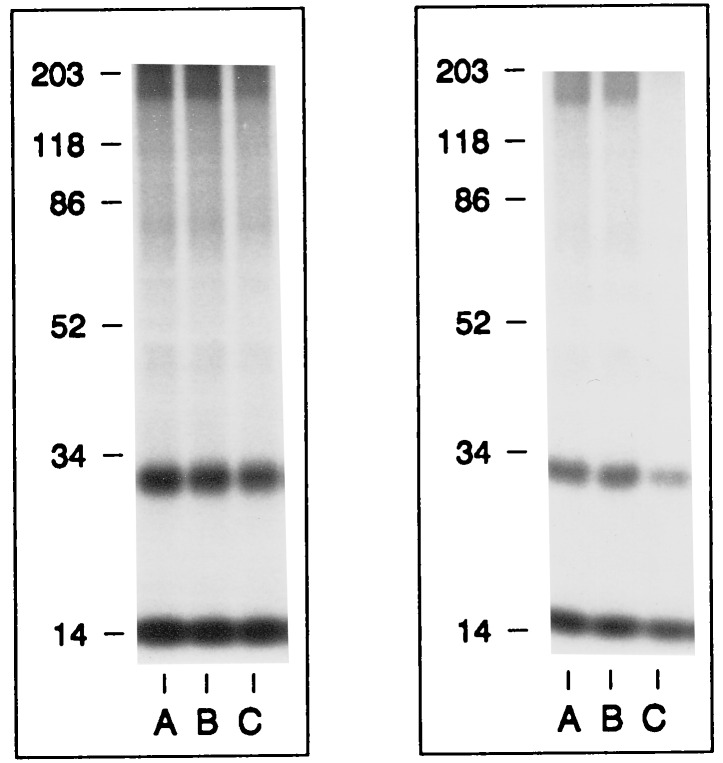
The response of endothelial cells to angiogenin in [3H]thymidine incorporation and cell proliferation indicates that a cellular receptor for angiogenin must be expressed under such conditions. To identify this receptor, 125I-labeled angiogenin was used to bind to the surface of endothelial cells under sparse culture conditions where they exhibit a positive response in both assays. Sulfo-DSS, a water-soluble, membrane-impermeable, bifunctional chemical crosslinker, was used to crosslink 125I-labeled angiogenin to its putative receptor. It is known that with subconfluent endothelial cells, angiogenin binds to cell surface actin and that the angiogenin–actin complex dissociates from the cell surface (14). If actin was to be expressed on the endothelial cell surface under the conditions used above, it might prevent angiogenin from binding to other cell surface receptors, especially when low concentrations of angiogenin or short incubation times are used. Therefore, the cells were first incubated with 50 ng/ml of 125I-labeled angiogenin to deplete them of any cell surface actin. After the first incubation, the cells were reincubated with 50 ng/ml of fresh 125I-labeled angiogenin and then crosslinked by Sulfo-DSS. As shown in Fig. 2 (Left), SDS/PAGE and autoradiography revealed a crosslinked band of ≈180 kDa with HUVE (lane A), HUAE (lane B), and HME (lane C) cells, indicating that a ≈170-kDa angiogenin-binding protein is expressed on the surface of three types of endothelial cells. In this experiment, 125I-labeled angiogenin–actin complex was not observed, since only cell monolayers were analyzed. Further, Sulfo-DSS is known to be an inefficient crosslinker for actin–angiogenin (14).
Figure 2.
Binding and crosslinking of angiogenin to a 170-kDa protein on the surface of endothelial cells. (Left) 125I-labeled angiogenin was crosslinked to the surface of HUVE (lane A), HUAE (lane B), and HME (lane C) cells. (Right) Inhibition of binding and crosslinking between 125I-labeled angiogenin and the 170-kDa putative receptor (lane A) by 10-fold excess of unlabeled RNase A (lane B) and angiogenin (lane C). Molecular weight standard is indicated at the left of each panel. Bands at 14 kDa and 28 kDa are the free monomeric and dimeric forms of 125I-labeled angiogenin, respectively. Angiogenin dimers are formed in the process of iodination by an unknown mechanism.
Binding of 125I-labeled angiogenin to the 170-kDa protein (Fig. 2 Right, lane A) was inhibited by a 10-fold excess of unlabeled angiogenin (lane C), but not by RNase A (lane B), indicating that the 170-kDa protein is specific for angiogenin.
Purification of the 170-kDa Putative Angiogenin Receptor.
HUVE cells were cultured under angiogenin-responsive conditions as described, and their surface molecules were biotinylated and enriched by affinity chromatography on monomeric avidin–Sepharose. After successive adsorption by DNase I–Sepharose and RNase A–Sepharose to remove actin and other nonspecific binding proteins, the biotinylated putative angiogenin receptor was purified by affinity chromatography on an angiogenin–Sepharose column. The final product was analyzed by SDS/PAGE and detected with streptavidin–alkaline phosphatase after transfer to a Biodyne B nylon membrane. As shown in Fig. 3, a single band of ≈170 kDa was observed, consistent with the molecular weight estimation from the crosslinking experiments. Approximately 10 ng of protein was obtained from cells grown in a single T162 flask.
Figure 3.

SDS/PAGE analysis of the putative angiogenin receptor. Lanes A and B were the samples from a deactivated Sepharose and an angiogenin–Sepharose affinity column, respectively.
DISCUSSION
Angiogenesis is a complex process that starts with an angiogenic stimulus and ends with the formation of new blood vessels. Among the many angiogenic proteins known, angiogenin is the only one that was originally isolated from human tumor cells based solely on its ability to induce blood vessel formation (1). Much work has been done on the structure–function relationships of angiogenin by means of chemical modification (24, 25), site-directed mutagenesis (26, 27), and x-ray crystallography (28). Monoclonal antibodies raised against angiogenin (22) have been shown to suppress significantly the establishment of human colon adenocarcinoma cells implanted into athymic mice (15). However, the mechanism by which angiogenin stimulates angiogenesis has not been elucidated mainly due to a lack of evidence that it is mitogenic and the inability to identify its cellular receptor.
Meanwhile, there has been compelling evidence pointing to endothelial cells as the target cells for angiogenin: (i) it induces blood vessel proliferation in the chicken chorioallantoic membrane and rabbit cornea (1) and meniscus (14); (ii) it binds to endothelial cells but not to fibroblasts (4); (iii) it induces a second messenger response in endothelial cells (5); (iv) it undergoes nuclear translocation in endothelial cells but not in fibroblasts (10); and (v) it induces endothelial cell invasion through binding to its cell surface-binding protein (8). The mitogenic response and receptor expression, two apparently interrelated phenomena, may both depend on endothelial cell status.
To identify the specific circumstances under which human endothelial cells might respond to exogenous angiogenin by expressing a cellular receptor for the protein, we systematically investigated [3H]thymidine incorporation and cell proliferation over a range of culture conditions. The single most important element that determined whether endothelial cells were responsive to angiogenin or not was cell density. Other details such as the cell passage number, confluence status, attachment factors, and starvation and stimulation times had some influence, but were not as critical as cell density. Angiogenin did not stimulate [3H]thymidine incorporation or cell proliferation in confluent endothelial cells. Only sparse (<104 cells per cm2) cells were reactive. Some differences were observed between the optimal cell density for [3H]thymidine incorporation and that for cell proliferation assays, presumably due to the different nature of the two assays. Multiple steps of washing, precipitation, and solubilization are required in the [3H]thymidine incorporation assay, which inevitably leads to detachment and loss of cells during the process. This also results in a higher signal/noise ratio with cell densities <5 × 103 cells per cm2. The cell proliferation assay is a simpler procedure and meaningful results can be obtained with cell densities as low as 4 × 103 cells per cm2.
The mitogenic and proliferative activities of angiogenin are relatively low compared with those of bFGF and VEGF. Angiogenin never stimulated a >80% increase in either [3H]thymidine incorporation or cell proliferation under all the different conditions examined. Prolonged incubation was not practical, since extensive cell death occurred after 2 days in HE-SFM. This might be different from the in vivo situation, where angiogenin may cause continual proliferation as long as cells are sparse. Once they reach a certain degree of confluence, the cells may lose their responsiveness and become insensitive to angiogenin. This can result either from receptor down-regulation and cell desensitization or from disassembly of the cell proliferation machinery used by angiogenin. In any event, the increased [3H]thymidine incorporation and cell proliferation stimulated by angiogenin were statistically significant. Hence, angiogenin is a real, albeit weak, mitogen for human endothelial cells when they are cultured under the conditions specified.
Two lines of evidence indicated that the action of angiogenin in endothelial cells is specific. First was the observation that RNase A, a close homolog of angiogenin but one which is not angiogenic, was inactive under the same conditions and did not inhibit angiogenin-induced [3H]thymidine incorporation and cell proliferation. Second was the neutralization of the activity of angiogenin by an anti-angiogenin monoclonal antibody but not by a nonimmune control antibody. Further, the same concentration of the anti-angiogenin antibody did not inhibit the activities of bFGF and VEGF in both types of assay. These data also indicate that the endogenous angiogenin secreted by endothelial cells is neither necessary for nor inhibitory to the actions of bFGF and VEGF.
Growth factors such as bFGF and aFGF are known to be secreted by endothelial cells and act in an autocrine or paracrine manner to stimulate proliferation of these cells (23). Neutralization of endogenous FGFs by their antibodies neither potentiates nor incapacitates the activity of angiogenin toward endothelial cells. It is interesting to note that endothelial cells, when cultured in serum-free conditions, do not require endogenous aFGF to maintain minimum growth or survival since addition of anti-aFGF antibodies did not have much effect on spontaneous incorporation of [3H]thymidine. Anti-bFGF antibodies, however, markedly decreased spontaneous incorporation of [3H]thymidine (Table 4), indicating a critical role of endogenous bFGF on the growth and survival of endothelial cells. Nevertheless, there was no synergistic effect when the cells were stimulated simultaneously with bFGF and angiogenin. They also did not antagonize each other in the [3H]thymidine incorporation assay. The results lead to the conclusion that angiogenin and fibroblast growth factor stimulate cell growth independently, probably through interaction with distinct cellular receptors.
Endothelial cells, which are responsive to angiogenin in [3H]thymidine incorporation and cell proliferation assays, express a 170-kDa angiogenin-binding protein on their surface, as revealed by chemical crosslinking experiments. Expression of this protein is correlated with the activity of angiogenin in both assays. No crosslinked band was detected when cell density exceeded 2 × 104 cells per cm2, consistent with the finding that angiogenin was not active toward cells maintained at a density >2 × 104 cells per cm2. These data suggest that the 170-kDa protein may serve as a functional receptor for angiogenin and mediate angiogenin-stimulated proliferation of endothelial cells. We are currently investigating whether this protein is involved in nuclear translocation of angiogenin.
It appears that the putative receptor and actin are not expressed concurrently on the endothelial cell surface. Previous studies showed that in subconfluent cells (5–8 × 104 cells per cm2, which is substantially higher than the density used in this study), no high-molecular weight molecules were found to bind angiogenin but that cell surface actin is expressed and binds angiogenin specifically (17). When the 170-kDa putative angiogenin receptor was expressed in sparsely cultured cells, no angiogenin–actin complex was detected in the crosslinked samples from either cell monolayers or supernatants of two incubations with 125I-labeled angiogenin (data not shown). The results seem to suggest that actin and the 170-kDa putative receptor participate in certain specific cellular functions at different stages in the process of angiogenin-induced angiogenesis. Binding of angiogenin to cell surface actin has been demonstrated to result in activation of a cell-associated protease system and cell invasion (8). After the cells are activated and start to migrate and invade into the basement membrane, the local density of the cells in the vicinity of the migrating cells decreases, and this may trigger the expression of the 170-kDa putative angiogenin receptor on the remaining adjacent cells. These cells become responsive to stimulation by angiogenin and will therefore divide to fill the space created by the migrating cells. The expression of the receptor may then be turned off when the gap is filled and cells become more dense. Such density-dependent receptor expression may regulate the angiogenin-induced growth of the new capillary network.
ABBREVIATIONS
- VEGF
vascular endothelial growth factor
- aFGF and bFGF
acidic and basic fibroblast growth factor, respectively
- HME
human microvascular endothelial
- HUAE
human umbilical arterial endothelial
- HUVE
human umbilical venous endothelial
- Sulfo-DSS
disulfosuccinimidyl suberate
- HE-SFM
human endothelial serum-free medium
- FBS
fetal bovine serum
References
- 1.Fett J W, Strydom D J, Lobb R R, Alderman E M, Bethune J L, Riordan J F, Vallee B L. Biochemistry. 1985;24:5480–5486. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J, Shing Y. J Biol Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- 3.Moscatelli D, Rifkin D B. Biochim Biophys Acta. 1988;948:67–85. doi: 10.1016/0304-419x(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 4.Badet J, Soncin F, Guitton J D, Lamare O, Cartwright T, Barritault D. Proc Natl Acad Sci USA. 1989;86:8427–8431. doi: 10.1073/pnas.86.21.8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bicknell R, Vallee B L. Proc Natl Acad Sci USA. 1988;85:5961–5965. doi: 10.1073/pnas.85.16.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soncin F. Proc Natl Acad Sci USA. 1992;89:2232–2236. doi: 10.1073/pnas.89.6.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu G-f, Riordan J F. Biochem Biophys Res Commun. 1993;197:682–687. doi: 10.1006/bbrc.1993.2533. [DOI] [PubMed] [Google Scholar]
- 8.Hu G-f, Riordan J F, Vallee B L. Proc Natl Acad Sci USA. 1994;91:12096–12100. doi: 10.1073/pnas.91.25.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jimi S-I, Ito K-I, Kohno K, Ono M, Kuwano M, Itagaki Y, Isikawa H. Biochem Biophys Res Commun. 1995;211:476–483. doi: 10.1006/bbrc.1995.1838. [DOI] [PubMed] [Google Scholar]
- 10.Moroianu J, Riordan J F. Proc Natl Acad Sci USA. 1994;91:1677–1681. doi: 10.1073/pnas.91.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moroianu J, Riordan J F. Biochem Biophys Res Commun. 1994;203:1765–1772. doi: 10.1006/bbrc.1994.2391. [DOI] [PubMed] [Google Scholar]
- 12.Shimoyama S, Gansauge F, Gansauge S, Negri G, Oohara T, Beger H G. Cancer Res. 1996;56:2703–2706. [PubMed] [Google Scholar]
- 13.Bläser J, Triebl S, Kopp C, Tschesche H. Eur J Clin Chem Clin Biochem. 1993;31:513–516. doi: 10.1515/cclm.1993.31.8.513. [DOI] [PubMed] [Google Scholar]
- 14.King T V, Vallee B L. J Bone Jt Surg Br Vol. 1991;73:587–590. doi: 10.1302/0301-620X.73B4.1712788. [DOI] [PubMed] [Google Scholar]
- 15.Olson K A, Fett J W, French T C, Key M E, Vallee B L. Proc Natl Acad Sci USA. 1995;92:442–446. doi: 10.1073/pnas.92.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu G-f, Chang S-I, Riordan J F, Vallee B L. Proc Natl Acad Sci USA. 1991;88:2227–2231. doi: 10.1073/pnas.88.6.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu G-f, Strydom D J, Fett J W, Riordan J F, Vallee B L. Proc Natl Acad Sci USA. 1993;90:1217–1221. doi: 10.1073/pnas.90.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folkman J, Klagsbrun M. Science. 1987;235:442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- 19.Chamoux M, Dehouck M P, Fruchart J C, Spik G, Montreuil J, Cecchelli R. Biochem Biophys Res Commun. 1991;176:833–839. doi: 10.1016/s0006-291x(05)80261-x. [DOI] [PubMed] [Google Scholar]
- 20.Sholley M M, Ferguson G P, Seibel H R, Montour J L, Wilson J D. Lab Invest. 1984;51:624–634. [PubMed] [Google Scholar]
- 21.Shapiro R, Harper J W, Fox E A, Jansen H-W, Hein F, Uhlmann E. Anal Biochem. 1988;175:450–461. doi: 10.1016/0003-2697(88)90569-6. [DOI] [PubMed] [Google Scholar]
- 22.Fett J W, Olson K A, Rybak S M. Biochemistry. 1995;33:5421–5427. doi: 10.1021/bi00184a010. [DOI] [PubMed] [Google Scholar]
- 23.Folkman J, Klagsbrun M, Sasse J, Wadniski M, Ingber D, Voldavsky I. Am J Pathol. 1988;130:393–400. [PMC free article] [PubMed] [Google Scholar]
- 24.Harper J W, Vallee B L. Proc Natl Acad Sci USA. 1988;85:7139–7143. doi: 10.1073/pnas.85.19.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shapiro R, Fox E A, Riordan J F. Biochemistry. 1989;28:1726–1732. doi: 10.1021/bi00430a045. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro R, Vallee B L. Biochemistry. 1992;31:12477–12485. doi: 10.1021/bi00164a026. [DOI] [PubMed] [Google Scholar]
- 27.Curran T P, Shapiro R, Riordan J F. Biochemistry. 1983;32:2307–2313. doi: 10.1021/bi00060a023. [DOI] [PubMed] [Google Scholar]
- 28.Acharya K R, Shapiro R, Allen S C, Riordan J F, Vallee B L. Proc Natl Acad Sci USA. 1994;91:2915–2919. doi: 10.1073/pnas.91.8.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]