Abstract
The E2F family of transcription factors plays a key role in regulating cell-cycle progression. Accordingly, E2F is itself tightly controlled by a series of transcriptional and posttranscriptional events. Here we provide evidence that E2F1 protein levels are regulated by the ubiquitin–proteasome-dependent degradation pathway. An analysis of E2F1 mutants identified a conserved carboxyl-terminal region, which is required for eliciting ubiquitination and protein turnover. Fusion of this E2F1 carboxyl-terminal sequence to a heterologous protein, GAL4, resulted in destabilization of GAL4. Previous studies identified an overlapping region of E2F1 that facilitates complex formation with retinoblastoma tumor suppressor protein, pRB, and we found that pRB blocks ubiquitination and stabilizes E2F1. These results suggest a new mechanism for controlling the cell-cycle regulatory activity of E2F1.
Keywords: cell cycle
E2F is involved in regulating cell-cycle progression at least in part by controlling the expression of genes that are implicated in or directly linked to cell proliferative responses. Notably, E2F can elicit both activation and repression of promoters containing E2F binding elements (1–5). As a dimeric complex with a partner DP protein, E2F family members bind DNA and activate transcription through an acidic, carboxyl-terminal activation domain (6–9). However, complex formation of E2F/DP with the tumor suppressor protein, pRB, has been shown to elicit transrepression of promoters bearing E2F elements (2–4). Evidence that this repression of cell proliferative genes is an important function of E2F in controlling cell growth is suggested from recent studies demonstrating that inactivation of E2F1 leads to increased cell proliferation and tumor formation in mice (10, 11).
The execution of these known functions of E2F is controlled through precise temporal control mechanisms involving both transcriptional and posttranscriptional pathways. Transcription of one E2F family member, E2F1, has been shown to be induced as cells traverse G1 and is down-regulated some time after the G1/S transition (9). In addition, E2F activity is modulated through precisely timed phosphorylation events mediated by specific cyclin dependent kinase–cyclin complexes. E2F1 is inactivated at S/G2 either by direct phosphorylation or by modification of its heterodimeric partner DP, which abrogates E2F/DP DNA binding activity (12, 13). In addition, while certain members of the E2F family (E2F1, E2F2, and E2F3) are complexed with pRB during G1, pRB becomes phosphorylated as cells approach the G1/S transition and dissociates from E2F (5).
The timely degradation of certain transiently induced and/or oscillatory proteins is fundamentally linked to the regulation of such genes. Indeed, key regulators of the cell-cycle machinery, such as cyclins and cyclin-dependent kinase inhibitors, are short-lived proteins that are degraded through the ubiquitin–proteasome pathway (14, 15). Similarly, E2F1 expression is cell-cycle regulated, and we show here processing of E2F1 through a ubiquitin-dependent proteolytic pathway. In addition, we demonstrate that complex formation with pRB blocks E2F1 degradation suggesting at least one means through which E2F1 turnover is modulated.
MATERIALS AND METHODS
Cell Culture and DNA Transfection.
U2OS cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 μg/ml streptomycin, and 100 units/ml penicillin (culture medium) in a humidified atmosphere at 37°C with 5% CO2/95% air. Transfections were carried out on 10-cm dishes for 16 hr by the calcium phosphate method (16). Transfected cells were washed twice with PBS and either refed or split. Transfections included 4 μg of pRc-CMV-HA-DP1 (where indicated) and pRc-CMV-HA-E2F1 (or the indicated E2F1 mutants) plus 20 μg of pcDNA3 (Invitrogen) or pcDNA3-pRB (or pRB mutants). Transfections for GAL4 studies included 4 μg of pUHD10-GAL4-HA or pUHD10-GAL4-HA-E2F plasmids, 2 μg of pUHD15–1 (required for driving promoter in pUHD10) plus 20 μg of control or pRB expression vectors. For experiments shown in Fig. 1C, 4 μg of pRc-CMV-HA-E2F1, 4 μg of pRc-CMV-HA-DP1, or 8 μg of corresponding control vector were cotransfected with 4 μg of HA-Ub, His6-Ub, or control plasmid and 20 μg of carrier plasmid [Bluescript (Stratagene)]. In-frame GAL4-HA-E2F1 sequences were cloned into the pUHD10–3 (17) expression vector. Expression from these plasmids was accomplished by cotransfection with the pUHD15–1 transactivator vector (17). pcDNA3-RB constructs were cloned by transferring the indicated HA-pRB sequences from the pSG5-HA-pRB plasmid (a generous gift of William Sellers, Dana–Farber Cancer Institute) vector into pcDNA3. The plasmids encoding HA-E2F1 and HA-DP1 (12) were generous gifts from Wilhelm Krek (Fredrich Miescher Institut, Basel).
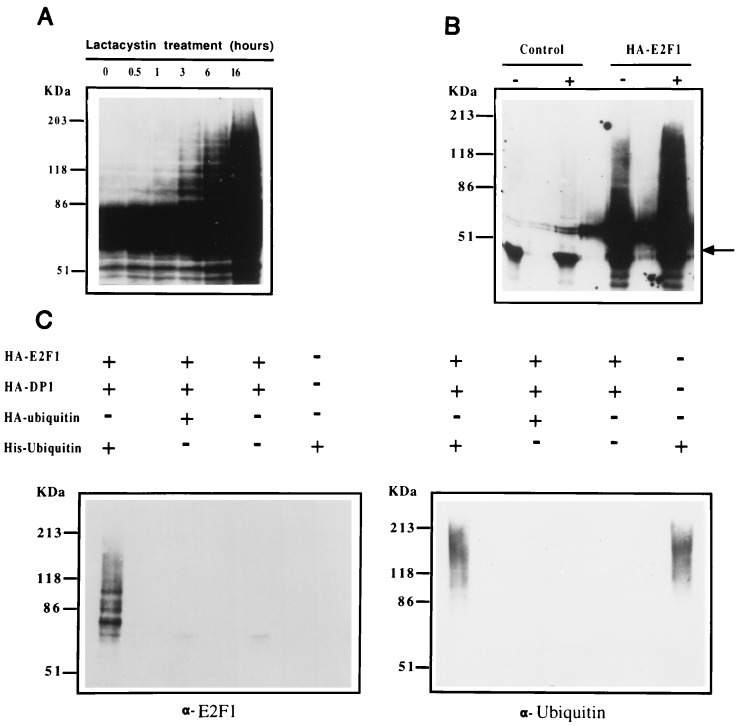
Figure 1.
E2F1 expression is modulated through the ubiquitin–proteasome pathway in vivo. (A) U2OS osteosarcoma cells were cotransfected with HA-E2F1 and HA-DP1 and treated for the indicated periods of time with 10 μM lactacystin. Aliquots from cell extracts were immunoblotted with the anti-E2F1 p98 polyclonal antibody (9). No signal was detected in the control experiment with mock-transfected cells (data not shown). (B) Control or HA-E2F1 expression plasmids were transfected into U2OS cells. Sixteen hours after transfection, cells were split and one-half were treated with 10 μM lactacystin for 3 hr. Extracts from both treated (+) and untreated (−) cells were prepared under denaturing conditions and immunoprecipitated with anti-HA 12CA5 mAb followed by immunoblotting with anti-E2F1 p98 antibody. The arrow points to a nonspecific band that likely corresponds to the immunoglobulin heavy chain. (C) U2OS cells were transfected with the indicated expression plasmids. Lactacystin (10 μM) was added for the last 3 hr of culture. Cells were lysed under denaturing conditions and extracts were purified over nickel-NTA beads. The purified material was assayed by immunoblot analysis employing anti-E2F1 p98 and anti-ubiquitin antibodies (Left and Right, respectively).
Immunoprecipitation and Immunoblotting.
Twenty-four hours after removal of the precipitate, transfected cells were washed twice with PBS, trypsinized, and washed twice again with PBS. For immunoblotting, cells were resuspended in 2× sample buffer [50 mM Tris·HCl, pH 6.8/6% SDS/20% (vol/vol) glycerol/0.02% bromophenol blue/5% (vol/vol) 2-mercaptoethanol]. For immunoprecipitation under denaturing conditions, cells were resuspended in 0.5 ml of 25 mM Tris·HCl (pH 6.8)/1.5% SDS per 100-mm dish and boiled for 15 min. Samples were then diluted in 8 vol of EBC/BSA (50 mM Tris·HCl/180 mM NaCl/0.5% Nonidet P-40/0.5% BSA) supplemented with protease inhibitors (1 mM phenylmethylsulfonyl fluoride/1 μg/ml antipain/1 μg/ml leupeptin). Extracts were precleared for 30 min at 4°C with protein A-Sepharose beads (Pharmacia) and then incubated with 2 μg of anti-E2F1 SQ41 plus 50 μl of 187.1 anti-mouse κ chain mAb for 2 hr at 4°C. Forty microliters of a 50% (wt/vol) slurry of protein A-Sepharose beads were then added and the mixture was rocked for 60 min at 4°C, washed four times in EBC/BSA buffer, plus once in 50 mM Tris·HCl (pH 6.8). Immunobeads were boiled in sample buffer and the samples analyzed electrophoretically. For nondenaturing conditions, cells were resuspended in EBC/BSA buffer and incubated for 15 min on ice. After vortexing, samples were clarified by centrifugation and precleared as described above. Samples were incubated in the presence of 1 μl of anti-HA 12CA5 mAb for 2 hr at 4°C. After incubation for 1 hr with 40 μl of 50% (wt/vol) slurry of protein A-Sepharose, samples were washed and processed as described above.
Nickel-NTA-Agarose Purification.
Transfected cells were lysed in 1 ml of buffer A (6 M guanidinium-HCl/0.1 M Na2HPO4/NaH2PO4, pH 8.0/10 mM imidazole) per 100-mm dish 24 hr after removal of the precipitate. The lysate was sonicated for 30 sec to reduce viscosity and then mixed on a rotator with 50 μl (settled volume) of nickel-NTA-agarose (Qiagen, Chatsworth, CA) for 3 hr at room temperature. The beads were washed three times with 1 ml of buffer A, twice with 1 ml of buffer A diluted in 25mM with Tris-HCl (pH 6.8)/20 mM imidazole 1:4, and twice with 1 ml of 25mM Tris-HCl (pH 6.8)/20 mM imidazole. Purified proteins were eluted by boiling the beads in 2× sample buffer supplemented with 200 mM imidazole and analyzed by immunoblotting.
Antibodies and Reagents.
Anti-E2F1 SQ41 mAb and p98 polyclonal antibody have been reported (9). Anti-E2F1 SQ71 mAb was generated as described for SQ41 and was provided by James DeCaprio (Dana–Farber Cancer Institute). Affinity-purified anti-HA 12CA5 mAb and anti-ubiquitin polyclonal antibody were obtained from Babco (Richmond, CA) and Sigma, respectively. Lactacystin was obtained from Elias Corey (Harvard University, Cambridge, MA).
RESULTS
Degradation of E2F1 Through the Ubiquitin–Proteasome Pathway.
Eukaryotic cells contain multiple proteolytic systems, including the lysosomal proteases, calpains, the ATP-ubiquitin–proteasome-dependent pathway, and an ATP-independent nonlysosomal process (18). Since the ubiquitin–proteasome pathway is involved in the control of other transiently expressed proteins, we investigated whether E2F1 protein levels are regulated via this mechanism. In this pathway, substrates are conjugated to ubiquitin and the formation of a multiubiquitin chain earmarks it for recognition by the 26S multicatalytic proteinase complex (19, 20). Because recognition and degradation of multiubiquitinated substrates by the proteasome complex is rapid, these species are transient and are rarely detected.
To determine whether multiubiquitinated forms of E2F1 can be observed in vivo, U2OS cells were transfected with an E2F1 expression vector and treated with the highly specific, irreversible, proteasome inhibitor, lactacystin (21). Western blot analysis of E2F1 following a lactacystin time course revealed a ladder of higher molecular weight E2F1 species, first observed after only 3 hr of treatment (Fig. 1 A). This ladder is even more evident with longer lactacystin treatments (Fig. 1A). To address whether this ladder could be observed in the absence of lactacystin, nontreated or lactacystin-treated transfectants were enriched by immunoprecipitation with a hemagglutinin (HA)-specific antibody prior to E2F1 immunoblot analysis (Fig. 1B). A ladder of higher molecular weight forms of E2F1 is observed in nonlactacystin-treated cells and increased levels are observed in lactacystin-treated cultures (Fig. 1B).
To more directly assess whether E2F1 can form ubiquitin conjugates, cells were cotransfected with an E2F1 expression vector and plasmids driving the expression of either His6-epitope-tagged ubiquitin or HA-tagged ubiquitin (His6-Ub and HA-Ub, respectively), or the corresponding control vector (22). Cells were collected following 3 hr of lactacystin treatment, and extracts were prepared under denaturing conditions and purified on nickel-NTA columns (23). Denaturing conditions were used to prevent copurification of noncovalently associated proteins and to prevent degradation or deubiquitination of protein–ubiquitin conjugates. Immunoblot analysis of column eluates with an anti-E2F1 antibody revealed a ladder of E2F1-immunoreactive proteins in the His6-Ub eluate, but not in the HA-Ub or control eluates (Fig. 1C Left). In addition, E2F1 reactivity was not detected in eluates from cells transfected with the His6-Ub vector alone, although these extracts contained fair amounts of ubiquitinated proteins (Fig. 1C Right).
Deletion of the E2F1 Carboxyl-Terminal Region Prevents Ubiquitination and Protein Turnover.
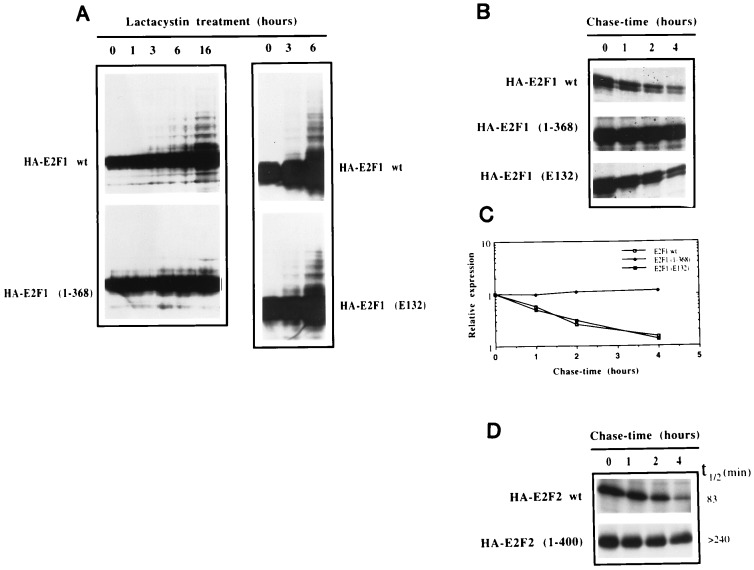
An analysis of E2F1 mutants revealed that deletion of the carboxyl-terminal activation/pRb binding domain [E2F1(1–368)] resulted in a protein that only poorly forms a high molecular weight ladder in the presence of lactacystin (Fig. 2A and data not shown). This carboxyl-terminal sequence contains no lysine residues, suggesting that it may function to target ubiquitination at amino-terminal lysine residues. Alternatively, these data could be explained by proposing that the transactivation function of E2F1 is required for inducing some factor that elicits its’ own degradation. However, an E2F1 mutant that is defective for DNA binding E2F1 (E132) is modified like the wild-type protein (Fig. 2A), demonstrating that transactivation is not required for this activity. In accordance with these studies, pulse-chase analysis of E2F1, E2F1 (1–368), and E2F1 (E132) showed that E2F1 (1–368) is a much more stable protein than wild-type E2F1 or E2F1 (E132) (Fig. 2 B and C). Whereas the half-life of both wild-type E2F1 and E2F1 (E132) is ≈70 min, the half-life of E2F1 (1–368) is more than 4 hr. Together, these data suggest that the carboxyl-terminal region of E2F1 contains sequences that target E2F1 for ubiquitin–proteasome-dependent degradation. This carboxyl-terminal region is highly conserved among E2F family members and truncation of the analogous sequence from a second E2F family member, E2F2, likewise results in a significantly more stable protein (Fig. 2D). This raises the possibility that other members of the E2F family are similarly regulated; however, a further analysis of this issue is clearly warranted.
Figure 2.
Deletion of the transactivation domain inhibits ubiquitination and stabilizes E2F1. (A) U2OS cells were transfected with the indicated plasmids together with HA-DP1 and treated with 10 μM lactacystin for the indicated periods of time. Aliquots from extracts were subjected to immunoblot analysis employing the anti-E2F1 mAb, SQ71. (B) U2OS cells were transfected with the indicated plasmids and split into four different plates the following day. Two days after transfection, cells were methionine-starved for 30 min and then pulsed for 60 min with [35S]methionine. Cells were then washed twice with PBS and chased for the indicated periods of time with culture medium. Extracts were prepared under denaturing conditions and immunoprecipitated with the anti-E2F1 SQ41 mAb. Samples were analyzed by SDS/PAGE and autoradiography. (C) The experiment shown in Fig. 2B was analyzed using a PhosphorImager to quantitate the amounts of wild-type and mutant derivatives of E2F1 that were immunoprecipitated at the different chase-time points. The abundance at each time point was calculated relative to the abundance at 0 hr. For each construct, the line graph was adjusted to an exponential model using a graphing program (Cricket graph). (D) Pulse-chase experiments were performed as described in Fig. 2B, except that the anti-HA 12CA5 mAb was used for immunoprecipitations. Half-life (t1/2) determinations were calculated as in Fig. 2C.
The E2F1 Carboxyl-Terminal Region Contains a Degradation Targeting Sequence.
The ability of the carboxyl-terminal region of E2F1 to serve as proteasome targeting sequences was further examined by assessing whether this region can confer decreased stability to a heterologous protein. For these studies, expression vectors containing the DNA binding domain of the yeast transactivator, GAL4, cloned in-frame with a HA tag and different portions of E2F1 were transfected into U2OS cells and pulse-chase experiments were performed. As shown in Fig. 3A, fusion of carboxyl-terminal E2F1 sequences (amino acids 368–437 and 389–437) resulted in a greater than 2-fold decrease in the half-life of GAL4. In contrast, fusion of amino acids 1–368 of E2F1 did not result in destabilization of the fusion protein but instead appeared to have some stabilizing activity (Fig. 3A).
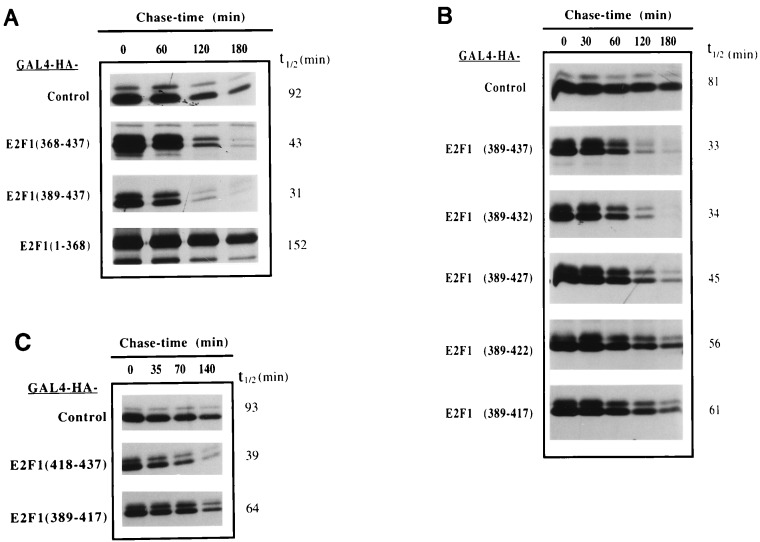
Figure 3.
The E2F1 transactivation/pRb binding domain contains a degradation targeting sequence. U2OS cells were transfected with the indicated GAL4 or GAL4-E2F1 expression plasmids and split the day after into four or five plates. Pulse-chase experiments were carried out as described in Fig. 2. Extracts were prepared under nondenaturing conditions and immunoprecipitated with the anti-HA 12CA5 mAb. Samples were analyzed by SDS/PAGE and half-life (t1/2) determination was performed as described in Fig. 2.
To further refine E2F1 degradation targeting sequences, half-life studies were performed on a series of five amino acid carboxyl-terminal GAL4-E2F1 deletion mutants (Fig. 3B). Deletion of residues 433–437 did not increase the stability of the chimera. Processive deletions up to amino acid 417 resulted in slight-to-moderate incremental increases in fusion protein stability and the half-life of GAL4-E2F1 (389–417) is close to that of GAL4 alone. Moreover, fusion of E2F1 residues 418–437 to GAL4 resulted in a protein that is nearly as unstable as GAL4-E2F1 (389–437) (Fig. 3C). These results suggest that key targeting sequences lie between amino acids 418–432, although flanking sequences may also provide some destabilizing activity.
Inhibition of E2F1 Ubiquitination and Degradation by the Retinoblastoma Tumor Suppressor Protein, pRB.
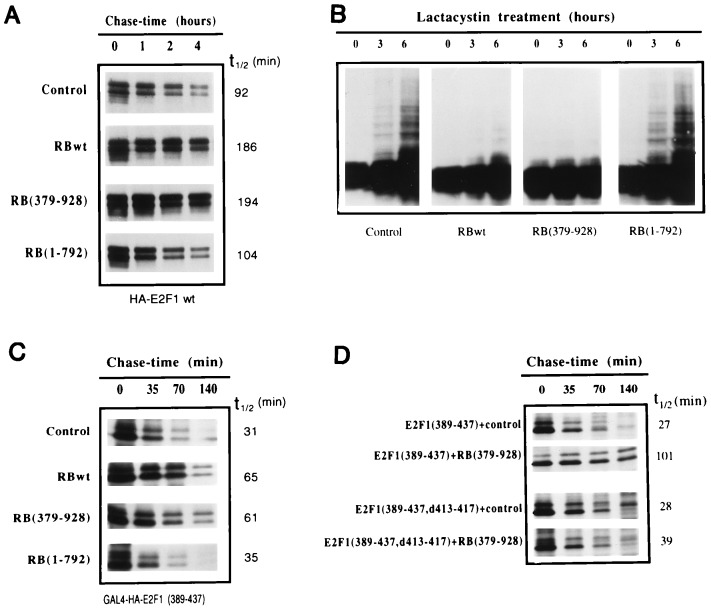
A previous study identified a minimal 18 amino acid pRB binding region spanning residues 409 to 426 of E2F1 (24), which clearly overlaps key degradation targeting sequences. The observation that these two mapping studies did not identify exactly coincident sequences suggests that pRB is not likely to be involved in eliciting protein degradation. However, the overlapping nature of these domains suggest that pRB binding may at least influence E2F1 turnover. To address this issue, an E2F1 expression vector was cotransfected with either a control or a pRB expression vector and E2F1 pulse-chase experiments were performed. As shown in Fig. 4A, pRB increased the half-life of E2F1 about two-fold as compared with cells cotransfected with the control vector. Similarly, an amino-terminal pRB deletion mutant, pRB(379–928), which can still interact with E2F1 (16) increases the half-life of E2F1 (Fig. 4A). In contrast, a pRB mutant in which carboxyl-terminal sequences required for binding to E2F1 (16) are deleted [pRB(1–792)] does not significantly affect the half-life of E2F1 (Fig. 4A). Consistent with these results, pRBwt and pRB(379–928) significantly inhibit the formation of high molecular weight forms of E2F1 observed in the presence of lactacystin, whereas pRB(1–792) does not (Fig. 4B). These data show that pRB can protect E2F1 from ubiquitination and proteolytic degradation.
Figure 4.
The tumor suppressor protein pRb blocks ubiquitination and increases the half-life of E2F1. (A) The indicated pRb expression plasmids were cotransfected with HA-E2F1 and HA-DP1 into U2OS cells. The following day, cells were split into four plates for pulse-chase analysis. Extracts were prepared under denaturing conditions and immunoprecipitated with the SQ41 anti-E2F1 mAb. Samples were analyzed by SDS/PAGE and autoradiography. Half-lives (t1/2) were calculated as indicated in Fig. 2. (B) Expression plasmids for HA-E2F1, together with HA-DP1 and the indicated pRB constructs, were cotransfected into U2OS cells. Lactacystin (10 μM) was added for the indicated periods of time. Aliquots from cell extracts were immunoblotted with anti-E2F1 SQ71 mAb. (C) The indicated pRb expression plasmids were cotransfected with GAL4-HA-E2F1(389–437) into U2OS cells. The following day, cells were split into four plates for pulse-chase analysis. Extracts were prepared under nondenaturing conditions and immunoprecipitated with the 12CA5 anti-HA mAb. (D) GAL4-E2F1(389–437) or the pRb binding mutant GAL4-E2F1(389–437,d413–417) plasmids were cotransfected with a control or a pRb(379–928) expression vector into U2OS cells. Cells were cotransfected with non-HA-tagged pRB expression vectors so that coimmunoprecipitation/interaction studies could be performed.
Similar results were obtained in the context of GAL4 chimeras. Cotransfection of pRBwt or pRB(379–928) increases the half-life of GAL4-E2F1(389–437), whereas pRB(1–792) does not (Fig. 4C). Furthermore, while the half-life of GAL4-E2F1(389–437) is increased by pRB(379–928), GAL4-E2F1(389–437,d413–417), which interacts poorly with pRB by virtue of a five amino acid deletion (25), is only weakly stabilized by pRB (Fig. 4D). Therefore, the inhibition of protein turnover by pRB is not the result of a general effect on the ubiquitin–proteasome machinery but is instead likely to be primarily the result of a direct interaction with E2F1. Notably, some pRB was found to associate with GAL4-E2F1(389–437,d413–417) in immunoprecipitation experiments (data not shown). This may explain the partial stabilization observed with this mutant. Together these studies provide evidence that a direct interaction of pRB with E2F1 is required for blocking ubiquitination and proteasome processing of E2F1.
DISCUSSION
E2F1 activity is strictly controlled throughout the cell cycle via a complex set of regulatory processes. Here we show that E2F1 can be targeted to the ubiquitin–proteasome-dependent proteolytic machinery. Targeting of E2F1 for ubiquitination occurs through the carboxyl-terminal region containing the activation/pRB binding domains and we provide genetic data suggesting that pRB inhibits E2F1 ubiquitination and turnover through a mechanism involving direct interactions between these two factors. The overlapping nature of key pRB interaction sequences with ubiquitination targeting sequences of E2F1 suggests a simple occlusion model for inhibition of E2F1 degradation, whereby pRB blocks cellular ubiquitination machinery from recognizing E2F1.
Recent reports demonstrating that homozygous mutation of E2F1 in mice leads to increased proliferation and tumor formation in certain tissues (10, 11) raises the possibility that the repressor function of E2F family members, complexed with their cognate pRB family member, is a crucial means of controlling the expression of cell-cycle promoting genes. Stabilization of E2F factors by pRB family members would serve to enforce this repressor activity ensuring that genes involved in cell-cycle progression are not inappropriately expressed. Conversely, previous studies have demonstrated that overexpression of E2F1 can lead to unscheduled entry of cells into S-phase and/or the induction of apoptosis (10, 11, 26–32). The rapid processing of uncomplexed E2F1 at certain points in the cell cycle, G2/M for example, could therefore be a mechanism for controlling E2F1 activity and cell fate. Experiments are underway to assess the cell-cycle regulation of endogenous E2F1 proteolytic processing.
Here we provide evidence that the active/uncomplexed form of E2F1 is subjected to rapid proteolytic processing. As such, one would predict that the dissociation of pRb/E2F1 complexes by the adenovirus E1A, simian virus 40 large T, or human papillomavirus E7 viral oncoproteins would be counterproductive, since this should unmask E2F1 ubiquitin–proteasome targeting. However, a recent study demonstrated that in addition to being able to dissociate E2F/pRb complexes, E1A provides a stabilizing activity that inhibits the turnover of E2F1 (33). It was also shown that E1A can interact with a member of the ubiquitin-conjugating enzymes, UBC9, which plays a role in progression through the S- and G2 phase of the cell cycle in yeast (33). However, it is not yet clear whether this UBC9 binding activity plays a role in the stabilizing activity of E1A. Nevertheless, it will be interesting to learn whether other viral oncoproteins that are known to disrupt E2F-pRB interactions similarly alter the ubiquitin–proteasome machinery in a way that leads to stabilization of free E2F.
Observations similar to those described here have been made by two other groups (34, 35). The former study demonstrated that the pRB related protein, p107, mediates stabilization of a distinct E2F family member, E2F4. This observation, the finding that key ubiquitin targeting sequences lie in a highly conserved region of E2F1, and the analysis showing that the carboxyl terminus of E2F2 mediates destabilization, all suggest that this mode of regulation may be a commonly used mechanism for modulating E2F activity.
While mapping studies dissociated ubiquitin–proteasome targeting from pRB binding, we have so far been unable to clearly dissect ubiquitin–proteasome targeting from the transcriptional activation function of the E2F1 carboxyl-terminal region. This leaves open the question of whether a functional relationship exists between these two activities. For example, do certain enzymes involved in directing ubiquitin conjugation play some role in promoting transcriptional activation? Addressing the relationship between E2F1 transcriptional activation and ubiquitin–proteasome targeting will require information regarding specific cofactors involved in these processes.
Acknowledgments
We thank William Sellers for kindly providing pRB expression vectors, Wilhelm Krek for gifts of pRC-HA-E2F1, pRC-HA-E2F2, and pRC-HA-DP-1 expression plasmids, Mathias Treier and Dirk Bohmann for tagged ubiquitin expression vectors, and James DeCaprio for SQ41 and SQ71 anti-E2F1 mAbs. We also thank William Sellers, Ratna Valamudi, James DeCaprio, Eduardo Folco, and Antonio Rodriguez for invaluable advice during this project. Additional thanks to Jack Strominger for continued support and encouragement. This work was supported by a Human Frontier Science Program Organization postdoctoral fellowship, to M.R.C., National Institutes of Health Grant NIH-R29 GM48045 and a Leukemia Society of America Special Fellowship to E.K.F., and National Institutes of Health Grant NIH-CA47554.
ABBREVIATION
- HA
hemagglutinin
References
- 1.Nevins J R. Science. 1992;258:424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- 2.Weintraub S J, Prater C A, Dean D C. Nature (London) 1992;358:259–261. doi: 10.1038/358259a0. [DOI] [PubMed] [Google Scholar]
- 3.Weintraub S J, Chow K N B, Luo R X, Zhang S H, He S, Dean D C. Nature (London) 1995;375:812–815. doi: 10.1038/375812a0. [DOI] [PubMed] [Google Scholar]
- 4.Sellers W R, Rodgers J W, Kaelin W G. Proc Natl Acad Sci USA. 1995;92:11544–11548. doi: 10.1073/pnas.92.25.11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinberg R A. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 6.Krek W, Livingston D M, Shirodkar S. Science. 1993;262:1557–1560. doi: 10.1126/science.8248803. [DOI] [PubMed] [Google Scholar]
- 7.Wu C-L, Zukerberg L R, Ngwu C, Harlow E, Lees J A. Mol Cell Biol. 1995;15:2536–2546. doi: 10.1128/mcb.15.5.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helin K, Harlow E. J Virol. 1994;68:5027–5035. doi: 10.1128/jvi.68.8.5027-5035.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaelin W G, Krek W, Sellers W R, DeCaprio J A, Ajchenbaum F, Fuchs C S, Chittenden T, Li Y, Farnham P J, Blanar M A, Livingston D M, Flemington E K. Cell. 1992;70:351–364. doi: 10.1016/0092-8674(92)90108-o. [DOI] [PubMed] [Google Scholar]
- 10.Field S J, Tsai F-Y, Kuo F, Zubiaga A M, Kaelin W G, Livingston D M, Orkin S H, Greenberg M E. Cell. 1996;85:549–561. doi: 10.1016/s0092-8674(00)81255-6. [DOI] [PubMed] [Google Scholar]
- 11.Yamasaki L, Jacks T, Bronson R, Goillot E, Harlow E, Dyson N J. Cell. 1996;85:537–548. doi: 10.1016/s0092-8674(00)81254-4. [DOI] [PubMed] [Google Scholar]
- 12.Krek W, Ewen M E, Shirodkar S, Arany Z, Kaelin W G, Livingston D M. Cell. 1994;78:161–172. doi: 10.1016/0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 13.Dynlacht B D, Flores O, Lees J A, Harlow E. Genes Dev. 1994;8:1772–1786. doi: 10.1101/gad.8.15.1772. [DOI] [PubMed] [Google Scholar]
- 14.Barinaga M. Science. 1995;269:631–632. doi: 10.1126/science.7624789. [DOI] [PubMed] [Google Scholar]
- 15.Glotzer M, Murray A W, Kirschner M W. Nature (London) 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- 16.Qin X, Chittenden T, Livingston D M, Kaelin W G. Genes Dev. 1992;6:953–964. doi: 10.1101/gad.6.6.953. [DOI] [PubMed] [Google Scholar]
- 17.Gossen M, Bujard H. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrett A J. Ann NY Acad Sci. 1992;674:1–15. doi: 10.1111/j.1749-6632.1992.tb27472.x. [DOI] [PubMed] [Google Scholar]
- 19.Jentsch S, Schlenker S. Cell. 1995;82:881–884. doi: 10.1016/0092-8674(95)90021-7. [DOI] [PubMed] [Google Scholar]
- 20.Hochstrasser M. Curr Opin Cell Biol. 1995;7:215–223. doi: 10.1016/0955-0674(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 21.Fenteany G, Standaert R F, Lane W S, Shoi S, Corey E J, Schreiber S L. Science. 1995;268:726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- 22.Treier M, Staszewski L M, Bohmann D. Cell. 1994;78:787–798. doi: 10.1016/s0092-8674(94)90502-9. [DOI] [PubMed] [Google Scholar]
- 23.Hochuli E, Bannwarth W, Dobeli H, Gentz R, Stuber D. Bio/Technology. 1988;6:1321–1325. [Google Scholar]
- 24.Helin K, Lees J A, Vidal M, Dyson N, Harlow E, Fattaey A. Cell. 1992;70:337–350. doi: 10.1016/0092-8674(92)90107-n. [DOI] [PubMed] [Google Scholar]
- 25.Flemington E K, Speck S H, Kaelin W G. Proc Natl Acad Sci USA. 1993;90:6914–6918. doi: 10.1073/pnas.90.15.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin X Q, Livingston D M, Kaelin W G, Jr, Adams P D. Proc Natl Acad Sci USA. 1994;91:10918–10922. doi: 10.1073/pnas.91.23.10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shan B, Lee W H. Mol Cell Biol. 1994;14:8166–8173. doi: 10.1128/mcb.14.12.8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu X, Levine A J. Proc Natl Acad Sci USA. 1994;91:3602–3606. doi: 10.1073/pnas.91.9.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kowalic T F, DeGregori J, Schwarz J K, Nevins J R. J Virol. 1995;69:2491–2500. doi: 10.1128/jvi.69.4.2491-2500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krek W, Xu G, Livingston D. Cell. 1995;83:1149–1158. doi: 10.1016/0092-8674(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 31.Johnson D G, Cress W D, Jakoi L, Nevins J R. Proc Natl Acad Sci USA. 1994;91:12823–12827. doi: 10.1073/pnas.91.26.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu G, Livingston D M, Krek W. Proc Natl Acad Sci USA. 1995;92:1357–1361. doi: 10.1073/pnas.92.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hateboer G, Hijmans E M, Nooij J B D, Schlenker S, Jentsch S, Bernards R. J Biol Chem. 1996;271:25906–25911. doi: 10.1074/jbc.271.42.25906. [DOI] [PubMed] [Google Scholar]
- 34.Hateboer G, Derkhoven R M, Bernards R, Beijersbergen R L. Genes Dev. 1996;10:2960–2970. doi: 10.1101/gad.10.23.2960. [DOI] [PubMed] [Google Scholar]
- 35.Hofmann F, Martelli F, Livingston D M, Wang Z. Genes Dev. 1996;10:2949–2959. doi: 10.1101/gad.10.23.2949. [DOI] [PubMed] [Google Scholar]