Abstract
Methylation of CpG sites in the genome, which is generally conserved during cell replication, is considered to play important roles in cell differentiation and carcinogenesis. However, investigations on changes in methylation status have been limited to known genes. To make a genome-wide search for differentially methylated genes, we developed a methylation-sensitive–representational difference analysis (MS-RDA) method. The representation of the genome was prepared using the methylation-sensitive restriction enzyme HpaII, and the mixture ratio of tester and driver DNAs was optimized to detect differences in methylation status of a single copy per diploid mammalian genome. By performing comparative MS-RDA of one hepatocellular carcinoma and of background liver tissue of one mouse treated with a food carcinogen (2-amino-3,4-dimethylimidazo[4,5-f]quinoline), we were able to identify (i) extensive hypomethylation of long interspersed nuclear element repetitive sequences in a number of hepatocellular carcinomas, (ii) reduction of the gene dosage of their mitochondrial DNA, and (iii) a hypermethylated DNA fragment of unknown origin. Furthermore, by adding the clones obtained in the first MS-RDA to the driver DNA [MS-RDA with elimination of excessive clones (MS-RDA-WEEC)], nine DNA fragments that could not be detected at the first MS-RDA were isolated as differentially methylated DNA fragments. MS-RDA, combined with MS-RDA-WEEC, is thus a promising approach to identify DNA fragments differentially methylated in two DNA sources.
Keywords: genomic subtraction, epigenetic, heterocyclic amine, elimination of excessive clones
The status of mammalian genome methylation regarding 5-methylcytosines at CpG sites is known to be conserved during cell division (1). After DNA replication, DNA methyltransferase methylates cytosines at 5′-CG-3′ sites on the newly synthesized strand in positions symmetric to those on the template strand. This semiconservative nature of CpG methylation on DNA replication allows gene expression or repression specific to developmental stage and tissues and maintains the imprinting status of several genes (2–6). Targeted disruption of DNA methyltransferase has been demonstrated to result in embryonic lethality, showing the critical importance of maintenance of methylation status of genes for embryogenesis and development (1).
Hypomethylation of the overall genome and hypermethylation localized to specific regions are consistent features arising during carcinogenesis (7–9). Hypomethylation of some oncogenes, such as Ha-ras and raf, is associated with their increased expression (8). Hypomethylation also is observed in some repetitive sequences (10, 11) and in genes that do not appear to be directly related to tumor development (10). The role of hypomethylation in carcinogenesis is not fully understood because our knowledge of hypomethylated genomic regions is very limited. Aberrant hypermethylation of tumor suppressor genes, such as VHL, p16/MTS1, and E-cadherin, recently has been indicated to be causally involved in tumors in various origins (12–16). Hypermethylation of the CpG island of the estrogen receptor gene leads to loss of estrogen receptor expression in the course of breast cancer development (17), and demethylation of the island leads to reexpression of the estrogen receptor (18). Aberrant hypermethylation also is known to precede allelic loss of 17p in several cancers and 16q in hepatocellular carcinomas (HCCs) (19, 20). However, gene inactivation and its association with allelic loss may represent only minor aspects of the influence of hypermethylation on carcinogenesis, and further information on the number, size, and distribution of hypermethylated regions is necessary to clarify this issue.
Aberrant methylation during carcinogenesis usually has been investigated by Southern blot analysis of DNA digested with methylation-sensitive restriction enzymes. However, this method is applicable only to known genes and does not allow unknown genes to be assessed. Recently, restriction landmark genomic scanning was shown to be capable of making a genome-wide search of aberrantly methylated DNA (21). However, restriction landmark genomic scanning is technically complex to perform, and the spots identified as differentially methylated are difficult to clone into plasmids. Therefore, a simpler and possibly more efficient method is required.
Representational difference analysis (RDA) is a reliable way to detect differences between two complex genomes (22, 23). With this method, series of subtractive hybridizations are performed using two representations (amplicons) of the two genomes to be compared. An amplicon of a genome is prepared by restriction–digestion of the genomic DNA and PCR amplification of the total digestion product after ligating a universal adaptor. Restriction fragments whose sizes and sequences are suitable for PCR amplification are enriched in the amplicon, and other fragments remain unamplified. Reduction of the complexity of the genome is essential for efficient subtractive hybridization, and excessive reduction of the complexity will result in the loss of target. From this point of view, use of hexanucleotide recognition restriction enzymes has been recommended for RDA because the proportions of the genome represented in the resultant amplicons are 1/10–1/50 of the whole genome. Use of tetranucleotide recognition enzymes has been avoided because the proportion of the genome in amplicons will be too large for efficient subtractive hybridization.
In this study, we established a modification of RDA, methylation-sensitive RDA (MS-RDA), to identify aberrant methylation in the genome. Although HpaII, a methylation-sensitive restriction enzyme, belongs to the tetranucleotide recognition restriction group, most of its recognition sequences are methylated in vivo. Therefore, we expected that amplicons prepared using HpaII would represent appropriate proportions of the genome and that differentially methylated fragments could be successfully identified.
MATERIALS AND METHODS
Tumor Samples and DNA Isolation.
Liver and forestomach tumors were induced by the protocol reported previously (24). In brief, a total of 35 female CDF1 mice were fed 400 ppm of MeIQ (2-amino-3,4-dimethylimidazo[4,5-f]quinoline) in the diet from 7 to 80 weeks of age. Macroscopic tumors were divided into two portions, one used for histological examination and the other frozen for extraction of DNA. Microscopic examination revealed HCCs in 13 mice, adenomas in 13, and preneoplastic liver foci in the remainder.
An HCC (2214T) 13 mm in diameter and a normal portion of the same liver (2214L) were used to isolate differentially methylated DNA fragments. Nine other relatively large HCCs were used for characterization of MS-RDA products. Tissues were homogenized with a tissue homogenizer (Polytron, Kinematica, Lucerne, Switzerland), and DNA was prepared by serial extraction with phenol and chloroform, followed by ethanol precipitation (25).
Control DNA and Methylation of HpaII Sites.
The pN3 plasmid containing human proto-ret cDNA p51 (26) was purified by standard CsCl ultracentrifugation from Escherichia coli transformants and was used as a positive control. The HpaII sites of pN3 were methylated by incubating 20 μg of the plasmid with 100 units of HpaII methylase (NEB, Beverly, MA) and three 80-μM aliquots of fresh S-adenosylmethionine at 4-h intervals. Complete methylation of the HpaII sites in the plasmid was confirmed by digestion with the HpaII restriction enzyme (NEB).
Amplicon Preparation.
To prepare amplicons, DNA was digested with 5 units of HpaII/1 μg of DNA overnight. After phenol extraction and ethanol precipitation, 1 μg of digestion product was ligated to 500 pmol of RHpa adaptor by 800 units of T4 DNA ligase (NEB). RHpa adaptor was prepared by annealing two oligonucleotides, RHpa24 and RHpa11 (Table 1). The ligation product was amplified by 25 cycles of PCR with RHpa24 oligonucleotide as a primer as reported (22).
Table 1.
Sequences of the oligonucleotides used to prepare adaptors and for PCR amplification
| Primer | Sequence |
|---|---|
| RHpa24 | 5′-AGC ACT CTC CAG CCT CTC ACC GAC-3′ |
| RHpa11 | 5′-CGG TCG GTG AG-3′ |
| JHpa24 | 5′-ACC GAC GTC GAC TAT CCA TGA AAC-3′ |
| JHpa11 | 5′-CGG TTT CAT GG-3′ |
| NHpa24 | 5′-AGG CAA CTG TGC TAT CCG AGG GAC-3′ |
| NHpa11 | 5′-CGG TCC CTC GG-3′ |
| SHpa24 | 5′-ACT TCT ACG GCT GAA TTC CGA CAC-3′ |
| SHpa12 | 5′-CGG TGT CGG AAT-3′ |
Competitive Hybridization and Cloning of the MS-RDA Product.
The RHpa adaptor of the tester and driver amplicons was removed by digestion with HpaII and separation with gel filtration chromatography (cDNA spun column; Pharmacia). The J adaptor (500 pmol) was ligated to 1 μg of the tester amplicon with T4 DNA ligase. An appropriate amount of the tester DNA with the J adaptor at its ends was mixed with 40 μg of the driver DNA. The DNA mixture was purified by phenol extraction and ethanol precipitation and dissolved in 4 μl of 3× EE buffer (3 mM EDTA/3 mM N-[2-hydroxyethyl] pipecazine-N′-[3-propanesulfonic acid], pH 8.0), denatured at 96°C for 10 min, and reannealed at 67°C for 16–36 h in the presence of 1 M NaCl. One–tenth of the reannealed product was amplified by PCR with the JHpa24 oligonucleotide as a primer for 10 cycles. Tester/tester and tester/driver double-stranded DNA fragments had J adaptors on both and one end, respectively, and could be amplified exponentially and linearly, respectively. DNA fragments linearly amplified, existing as single-stranded DNA, were digested with 100 units of Mung–Bean nuclease (NEB), and the remaining double-stranded DNA was again amplified by PCR for 20–30 cycles with JHpa24 oligonucleotide.
The second or third cycle of competitive hybridization was performed by switching the adaptor used in the first or the second cycle of competitive hybridization to a new adaptor (N or S for the second cycle; J for the third cycle) (Table 1). Varied amounts of ligation solution were mixed with 40 μg of driver DNA. Denaturing, reannealing, and selective amplification of the self-annealed product were performed as for the first cycle. The final product was blunt-ligated into pBluescript II that had been digested with EcoRV (Toyobo, Osaka) and treated with calf intestinal alkaline phosphatase (Toyobo). After transformation of XL1Blue-competent cells, insert-positive plasmid clones were selected by PCR amplification of the inserts using T3 and T7 primers and restriction–digestion of the PCR product with MspI (NEB), the methylation-insensitive isoschizomer of HpaII. Driver DNA for MS-RDA-WEEC (MS-RDA with elimination of excessive clones) was prepared by PCR amplification of the appropriate clones with the NHpa24 primer, digestion of the PCR products with MspI, and removal of the separated adaptor.
Southern Blot Analysis.
DNA was digested with excess amounts of HpaII or MspI, purified by phenol extraction, and precipitated with ethanol. After quantification of each sample, specified amounts of samples were run in 1.1% agarose (Bio-Rad) or 2% NuSieve agarose (FMC). DNA in the gels was capillary-blotted onto nylon filters (HyBond-N, Amersham) after denaturation in 0.5 M NaOH. Blotted DNA was cross-linked to the filter by UV light using a quantitative cross-linker (Stratalinker, Stratagene).
Probes were prepared by random-labeling 5–100 ng of DNA with [α-32P]dCTP using a MultiPrime labeling kit (Amersham). Hybridization was carried out for 8–12 h in 50% formamide and 0.65 M NaCl at 42°C, and filters were washed four times in 2 × SSC at 50°C. Washed filters were exposed to Kodak XAR film or, for quantification purposes, Fuji imaging plates. The density of each band was measured using arbitrarily defined units reflecting the radiointensity.
Sequencing and Database Searching.
Plasmid DNA (50–500 ng) was sequenced with fluorescein isothiocyanante-labeled T3 or T7 primers and each dideoxy A, C, G, or T mixture (Cy5 Autocycle Sequence kit, Amersham) with incubation at 94°C for 30 s, 55°C for 1 min, and 72°C for 1 min for 30 cycles. Each A, C, G, or T product was run in separate lanes using an automated sequencer (AlfExpress, Pharmacia).
Obtained sequences between the T3 and T7 primers were manually edited to remove the sequences of pBluescript II and adaptors of MS-RDA. Using the edited sequences, an homology search was performed with the blast program at a GenBank Web site.
RESULTS
Establishment of MS-RDA.
Comparison of ethidium bromide staining of genomic DNA digested with HpaII (Fig. 1A, lanes 1 and 2) and MspI (Fig. 1A, lanes 3 and 4) showed the vast majority of HpaII sites in the genome to be methylated. Therefore, despite the wide application of hexanucleotide recognition restriction enzymes for standard RDA, we expected that we could appropriately reduce the complexity of the genome by preparing amplicons with the methylation-sensitive tetranucleotide recognition restriction enzyme HpaII so that competitive hybridization could be efficiently performed.
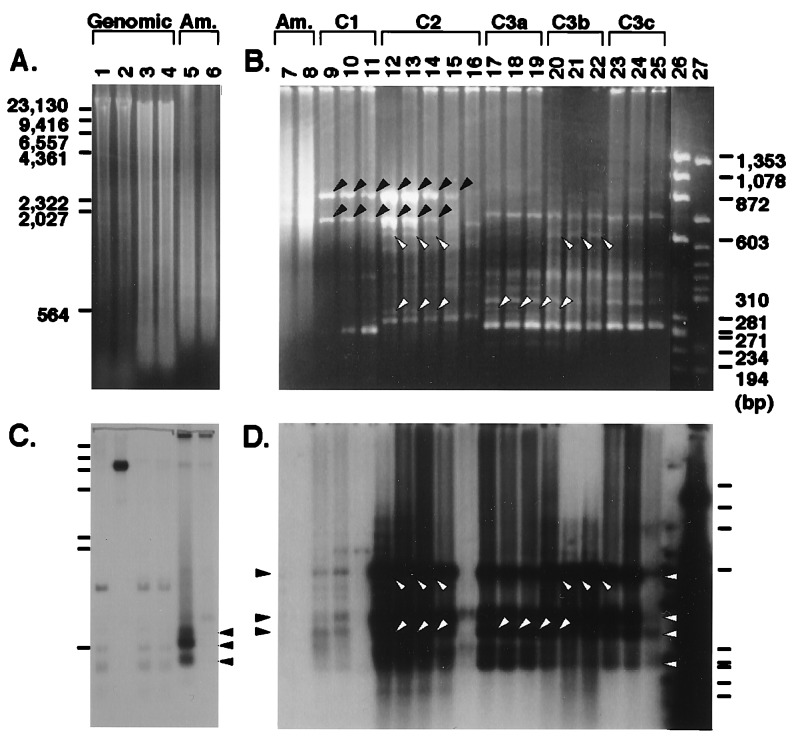
Figure 1.
Optimization of MS-RDA conditions. (A and B) Ethidium bromide staining. (C and D) Southern blot hybridization probed with pN3. (A and C) Amplicons (lanes 5 and 6; 4 μg/lane) were prepared from HpaII digests of mouse liver DNA supplemented with unmethylated pN3 or methylated pN3 (lanes 1 and 2; 10 μg). Lanes 3 and 4: MspI digests of the same DNA with added pN3 (10 μg). Samples were run in 1.1% agarose and blotted. Fragments that were derived from the HpaII digest of pN3 and enriched in the amplicon U are indicated by closed arrowheads (C, lane 5). (B and D) By varying the amounts of tester DNA used for the first, second, and third cycles of competitive hybridization, the optimum allowing detection of a difference of methylation state of one copy per diploid mouse genome was established. Lanes 7 and 8: 2 μg of amplicons prepared from liver total DNA with unmethylated and methylated pN3, respectively (the same as with the amplicon in lanes 5 and 6). Lanes: 9–11, 1 μg of C1-1000, C1-200, and C1-40; 12–16, 1 μg of C2-200, C2-100, C2-40, C2-10, and C2-2; 17–25, 1 μg of C3a-20, C3a-7, C3a-2, C3b-20, C3b-7, C3b-2, C3c-20, C3c-7, and C3c-2; 26: 375 ng of DNA size marker φX174/HaeIII digest; and 27, 100 ng of HpaII digest of pN3. Samples were run in 2% NuSieve/GTG (FMC) agarose and blotted. Repetitive sequences enriched during the competitive hybridizations are indicated by closed arrowheads (B, lanes 9–15). Three target fragments enriched in the amplicons (D, lane 7, shown with closed arrowheads) and another ≈300-bp fragment were enriched by two cycles of competitive hybridization. Target fragments recovered as visible bands are shown by open arrowheads (B and D, lanes 12–14 and 17–22). For a detailed description, see Results. Am., amplicon.
Control exogenous DNA, a 6700-bp plasmid (pN3), was linearized by digestion at a unique HindIII site. An aliquot was methylated by HpaII methylase. Unmethylated or methylated plasmid was added to mouse total DNA at the concentration of one copy per diploid genome (at the weight ratio of 6700/6 × 109). From HpaII digests (Fig. 1A, lanes 1 and 2) of the mouse DNA, unmethylated or methylated pN3 amplicons U (Fig. 1 A and B, lanes 5 and 7) and M (Fig. 1 A and B, lanes 6 and 8) were prepared, respectively. Hybridization of the amplicon U (Fig. 1C, lane 5) and the HpaII digest of total DNA with unmethylated pN3 (Fig. 1C, lane 1) showed that three (shown in Fig. 1C by closed arrowheads) of 17 pN3 fragments (Fig. 1B, lane 27) in the range of 300–600 bp were enriched in the amplicon U and that the average density of the enriched bands was increased 9–23 times, indicating a 1/9–23 reduction of the complexity of the genome.
Competitive hybridization using the HpaII digest of mouse liver DNA tended to enrich two bands (Fig. 1B, closed arrowheads), and these bands were shown to be derived repetitive sequences by cloning of the bands and nucleotide sequencing (data not shown). Therefore, we optimized the amount of tester amplicon so that differential products could be enriched maximally without enrichment of the two repetitive sequences. Ligation solutions of 1 μg, 200 ng, and 40 ng of tester amplicon and the J adaptor were used for the first cycle of competitive hybridization (C1-1000, C1-200, and C1-40, respectively; Fig. 1B, lanes 9–11) with 40 μg of driver amplicon, and the extent of pN3 enrichment was estimated. Southern blot analysis of the products with the pN3 probe revealed that C1-200 yielded the highest amount and the widest range of differential products (Fig. 1D, lane 10). In the C1-200 products, the target sequences were enriched ≈25–45-fold compared with amplicon U, but contaminating repetitive sequences (Fig. 1B, closed arrowheads) were also enriched, judging from ethidium bromide staining (Fig. 1B, lane 10).
After exchanging the J adaptor with the N adaptor, ligation solutions of 200, 100, 40, 10, and 2 ng of C1-200 were used for the second cycle of competitive hybridization with 40 μg of driver DNA (C2-200, C2-100, C2-40, C2-10, and C2-2, respectively; Fig. 1B, lanes 12–16). Target sequences were enriched maximally with C2-200 (Fig. 1B, lane 12; compare hybridization signals in Fig. 1D, lanes 12–16). On the other hand, contaminating repetitive sequences decreased dose-dependently with decrease of tester DNA (compare the intensities of the repetitive sequences shown by closed arrowheads in Fig. 1B). In C2-200, C2-100, C2-40, and C2-10, target sequences were enriched 2400-, 3100-, 2700-, and 1600-fold compared with amplicon U.
After changing back to the J adaptor, the third competitive hybridization was performed with nine kinds of ligation solution (20, 7, and 2 ng of C2-100, C2-40, and C2-10) and 40 μg of driver DNA (C3a-20, C3a-7 and C3a-2; C3b-20, C3b-7 and C3b-2; C3c-20, C3c-7 and C3c-2, respectively; Fig. 1B, lanes 17–25). Target sequences were no more enriched in the third cycle. However, some target sequences were confirmed as visible bands on ethidium bromide staining (Fig. 1B, lanes 17–22, open arrowheads), probably because of reduction of the background smear.
These results showed that differentially methylated DNA fragments at the concentration of one copy per diploid genome can be concentrated ≈6 × 104-fold, sometimes as visible bands on ethidium bromide staining, using 200 ng of tester amplicon for the first cycle of competitive hybridization and 40–100 ng of the C1 product for the second cycle. Performance of the third cycle using the C2-40 or C2-100 product sometimes helps to reduce background smear, but it is not a prerequisite.
Isolation of Aberrantly Methylated DNA Fragments in a Mouse Liver Tumor.
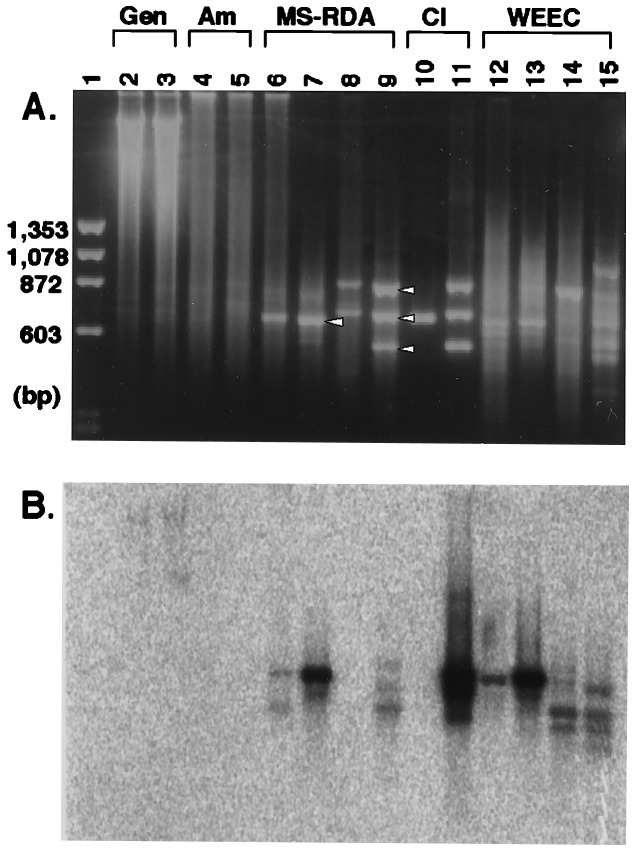
We applied MS-RDA to identify aberrantly methylated DNA fragments in a mouse liver tumor (2214T, histologically diagnosed as a well differentiated HCC) induced by a food carcinogen, MeIQ. DNA was extracted from the liver tumor and normal liver tissue (2214L) of the same mouse. Amplicons (Fig. 2, lanes 4 and 5) were prepared from HpaII digests (Fig. 2, lanes 2 and 3) of the two DNA samples, and two cycles of competitive hybridization were performed using the amplicon from 2214T as tester and the amplicon from 2214L as driver (series T–L; Fig. 2, lanes 6 and 7) and vice versa (series L–T; Fig. 2, lanes 8 and 9). One band was visible on the ethidium bromide-stained gel in the series T–L (Fig. 2, arrowhead, lane 7), and three bands were visible in the series L–T (Fig. 2, arrowheads, lane 9). Those four bands were excised from the gel and cloned into pBluescript II (Table 2).
Figure 2.
Application of MS-RDA and MS-RDA-WEEC to a MeIQ-induced liver tumor. Amplicons (lanes 4 and 5; 2 μg/lane) were prepared from HpaII digests of DNA samples from an MeIQ-induced mouse liver tumor (lane 2; 5 μg) and a normal portion of the same liver (lane 3; 5 μg). Two cycles of competitive hybridization were performed using the amplicon from the tumor as tester and the amplicon from the normal portion as driver (series T–L; lanes 6 and 7; 1 μg/lane) and vice versa (series L–T; lanes 8 and 9). One and three bands were clearly visible after two cycles in series T–L and L–T, respectively (shown by open arrowheads). Three of the four products were high copy sequences, so the one (lane 10; 250 ng) and three clones (lane 11; 250 ng each) were added to respective driver DNA, and homologous fragments in the tester amplicon were eliminated in the course of competitive hybridization (MS-RDA-WEEC). After two cycles of competitive hybridization of both series (T–L, lanes 12 and 13; L–T, lanes 14 and 15), the bands corresponding to the first one and three MS-RDA products were clearly weakened, and other bands appeared. (A) Ethidium bromide staining. (B) The filter was hybridized with clone B10, obtained in series T–L WEEC. Gen., genomic; Am., amplicon; Cl, clone.
Table 2.
Summary of the clones isolated by MS-RDA and MS-RDA-WEEC of 2214T and 2214L
| Tester | Driver | Clone | Length, bp | Incidence in HCCs | Result of the database search |
|---|---|---|---|---|---|
| MS-RDA | |||||
| 2214T | 2214L | hypomethylated in tumors | |||
| A2 | 614 | 10/10 | LINE1 | ||
| 2214L | 2214T | hypermethylated in tumors | |||
| E3 | 580 | 7/8 | unknown | ||
| reduction of gene dosage in tumors | |||||
| D5 | 815 | 4/10 | mitochondrial DNA | ||
| E9 | 653 | 2/5 | mitochondrial DNA | ||
| MS-RDA-WEEC | |||||
| 2214T | 2214L (added with A2) | hypomethylated in tumors | |||
| B10 | 717 | 1/4 | α-enolase | ||
| A8 | 801 | 10/10 | LINE1 | ||
| B3 | 661 | 6/6 | LINE1 | ||
| C5 | 413 | 10/10 | LINE1 | ||
| A7 | 678 | 7/7 | unknown | ||
| B1 | 676 | 10/10 | unknown | ||
| 2214L | 2214T (added with E3, D5, E9) | hypermethylated in tumors | |||
| F8 | 463 | 3/6 | unknown | ||
| E2 | 536 | 3/4 | unknown | ||
| D9 | 518 | 2/4 | unknown |
MS-RDA of 2214T and 2214L yielded one (T–L) and three (L–T) bands, and all of these four bands were demonstrated to be differentially methylated or to have different gene dosage. Six of the 10 clones randomly recoverd in series T–L WEEC were shown to be hypomethylated in 2214T, and three of the 10 clones in series L–T WEEC were shown to be hypermethylated. All of the clones that demonstrated differential methylation were tested using three to nine other liver tumors. Sequencing of the clones and database searching were also carried out, and the results are shown. Clone B10 had 78% homolgy with α-enolase.
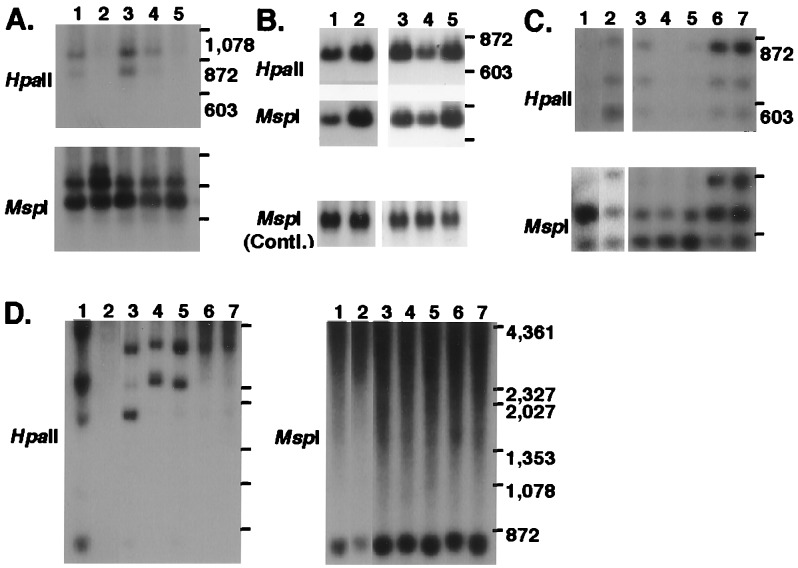
To confirm that those four MS-RDA products really were derived from DNA differentially methylated, the products were used as probes for Southern blot analysis of the total DNA of 2214T and 2214L. The DNA fragment recovered in series T–L (clone A2) detected a band in 2214T but not in 2214L on HpaII digestion, and no difference was detected on MspI digestion (Fig. 3A, lanes 1 and 2), indicating that the band detected by clone A2 was in fact hypomethylated in the tumor. Two of the three bands (clones D5 and E9) recovered in the series L–T demonstrated reduced intensity of bands in 2214T, compared with 2214L, on both HpaII and MspI digestions (Fig. 3B, lanes 1 and 2 for clone D5; data not shown for clone E9), indicating that the gene dosages of the DNA detected by clones D5 and E9 were reduced in the tumor. Clone E3, derived from the remaining band of the series L–T, detected two bands on the MspI digest of 2214T and three bands on that of 2214L, and it detected no bands on the HpaII digest of 2214T and all of the three bands on that of 2214L (Fig. 3C, lanes 1 and 2). These results indicated that the lower two bands detected by E3 on the MspI or HpaII digest of 2214L were hypermethylated in the tumor and that the uppermost band was lost in the tumor.
Figure 3.
Representative results of Southern blot analysis of total DNA from various liver tumors. (A) Probed with clone A2. Lanes: 1, 2214T (wd); 2, 2214L; 3, 2202HCC1 (wd); 4, 2204HCC2 (wd); and 5, 2204NL. (B) Probed with clone D5. Lanes 1–5 as in A. (C) Probed with clone E3. Lanes: 1, 2214T (wd); 2, 2214L; 3, 2202HCC2 (wd); 4, 2204HCC3 (wd); 5, 2204HCC4 (wd); 6, 2205NL; and 7, 2220NL. (D) Probed with A7. Lanes 1–7 as in C. wd, well differentiated HCC; NL, normal liver; Contl., control.
To further determine the significance of these four DNA fragments altered in the liver tumor (2214T), nine HCCs from different mice were examined. Southern blot analysis of DNA from the total of 10 tumors revealed that the DNA detected by A2 was commonly hypomethylated in all cases (see representative results in Fig. 3A, lanes 3 and 4). The reduction of copy number of clones D5 and E9 was observed in four of 10 and two of five liver tumors tested, respectively (Fig. 3B, lane 4 for D5). In seven of eight tumors tested, the lower two bands detected by E3 were hypermethylated, and the uppermost band was lost (Fig. 3C, lanes 3–5).
The isolated four DNA fragments (A2, D5, E9, and E3) were sequenced, and their homology with known DNA sequences was checked by a GenBank database search (Table 2). Clone A2 had 98% homology with the mouse long interspersed nuclear element (LINE1), and clones D5 and E9 had 99% homology with mitochondrial DNA. This finding well coincided with the old findings of a low respiratory capacity of tumor cells (27). Clone E3 had no significant homology with any registered sequences.
MS-RDA-WEEC.
Repetitive sequence and mitochondrial DNA are present at high copy numbers in cells. In addition, our model experiments, in which the initial copy number of pN3 plasmid for MS-RDA was varied, showed that increase of initial copy number accelerates recovery of the plasmid by MS-RDA (data not shown). Therefore, it was suggested that preferential isolation of repetitive sequence and mitochondrial DNA might have hampered isolation of other differentially methylated sequences that were present at one or two copies per genome.
To identify differentially methylated, unique DNA fragments, we introduced the use of clones isolated in the first MS-RDA as driver addendum. Aliquots of 100 ng of isolated clones were added to 40 μg of driver amplicon, and MS-RDA was performed using the J adaptor for the first competitive hybridization and the S adaptor for the second competitive hybridization to avoid amplification of contaminating undigested clones with the N adaptor. In series T–L WEEC (Fig. 2, lanes 12 and 13), in which clone A2 (lane 10) was added to the driver amplicon, the density of the band corresponding to clone A2 was weakened and some other bands became visible. The total product after the second competitive hybridization was randomly cloned into plasmids and used as a probe for Southern blot analysis of HpaII digests of 2214T and 2214L. Among the 10 independent clones tested, six showed hypomethylation (Table 2). In the series L–T WEEC (Fig. 2, lanes 14 and 15), densities of the bands corresponding to clones D5, E9, and E3 (their mixture is shown in Fig. 2, lane 11) were much weakened in a similar way. Among the 10 independent clones obtained with this approach, three showed hypermethylation (Table 2).
Most of the six clones detecting hypomethylation in 2214T showed consistent hypomethylation in three to nine other liver tumors tested (Table 2; Fig. 3D). Of the six clones, one clone turned out to have 78% homology with the α-enolase gene, three were derived from the LINE1 sequence, and two were derived from unknown sequences, having no significant homology with sequences in the database. As for the three hypermethylated clones, hypermethylation also was observed in other liver tumors, but the incidences were 50–75%. None of the three clones had significant homology with any sequences in the database.
DISCUSSION
In the present study, we developed MS-RDA by modifying the standard RDA method using HpaII, a methylation-sensitive tetranucleotide recognition restriction enzyme, for preparation of amplicons. Although tetranucleotide recognition enzymes are known not to reduce the complexity of the genome sufficiently, with the HpaII amplicon, a reduction down to 1/9–23 was achieved because the vast majority of HpaII sites in the genome were methylated and resistant to HpaII digestion (Fig. 1). This reduction of complexity corresponded to that yielded by hexanucleotide recognition enzymes, such as HindIII or BglII (22). Using optimal amounts of driver DNA, 200 ng for the first cycle and 40–100 ng for the second cycle, the MS-RDA method was able to identify differences in methylation status at the level of one copy per diploid genome.
Among the 17 HpaII fragments of pN3, the positive control plasmid, eight fragments were in the range of 200-2000 bp (Fig. 1B, lane 27), being eligible for PCR amplification. However, only three fragments were effectively enriched in the amplicon (Fig. 1, closed arrowheads in lane 5), and these three fragments and one more fragment (a total of four fragments; Fig. 1D, open arrowheads in lane 26) were effectively recovered as differential products after two cycles of competitive hybridization. In addition to the target fragment size, suitability for PCR amplification and adaptor ligation were considered to have affected the final recovery. We estimate that approximately half of the target fragments in the range of 200-2000 bp can be identified by the MS-RDA method.
The methylation status of the genome is mainly regulated in a regional manner, that is, all of the HpaII sites in a several kilobase region are methylated or are not methylated (5, 14, 28). Therefore, when a particular region is differentially methylated in DNA from two different sources, we can expect that some of the HpaII fragments in the region are in the range of 200-2000 bp and that approximately half of them can be recovered by MS-RDA.
We also established a powerful method, RDA-WEEC, to eliminate clones that are present at high copy numbers and tend to be preferentially isolated by RDA. In this study, single copy clones were not isolated in the first MS-RDA because high copy clones were differentially methylated in liver tumor and normal tissue. However, we were able to isolate a number of single copy clones that were differentially methylated by MS-RDA-WEEC. RDA-WEEC has potential application in the elimination of nontarget sequences, such as repetitive sequences in the course of conventional RDA. Actually, we have been able also to efficiently recover polymorphic markers performing RDA-WEEC using pooled DNA (unpublished data).
Seven hypomethylated (series T–L) and four hypermethylated (series L–T) DNA fragments were identified in an MeIQ-induced liver tumor. In the series L–T, we identified also two fragments whose gene dosage was reduced. Hypomethylation of the LINE1 repetitive sequence, ≈6500 bp long, was a very consistent feature of the mouse liver tumors tested. One LINE1-derived clone (clone A2) was identified by MS-RDA, and three other clones (A8, B3, and C5) were identified by MS-RDA-WEEC. Extents of hypomethylation were similar among these four clones (data not shown), so suitability for PCR and adaptor ligation was considered as a cause for the different efficiency of recovery. Two other clones (A7 and B1), identified in MS-RDA-WEEC L–T, detected hypomethylation commonly in all of the liver tumors tested, suggesting that the hypomethylation took place as part of a common pathway involved in hepatocarcinogenesis. Some of the hypomethylation might be causally involved through expression of oncogenes, some might be associated with expression of genes necessary for transformation or responsible for disdifferentiation (29), and some might be simple by-products.
Hypermethylation detected by the four clones (E3, F8, E2, and D9) was also observed in some other liver tumors, but the incidences were variable. Clone E3 detected one deleted DNA fragment and two hypermethylated fragments. It was confirmed that these three fragments existed in each of the parental strains of the CDF1 mouse: BALB/c and DBA (data not shown). Chromosomal mapping of the four clones (E3, F8, E2, and D9) and identification of the flanking genes are under way.
In summary, we have developed two new powerful techniques, MS-RDA and RDA-WEEC. Their application to a mouse liver tumor led to identification of many interesting clones. Therefore, it is to be expected that use of MS-RDA to study other tumors, aging, tissue differentiation, and neurological disorders will yield a great deal of information.
Acknowledgments
We thank Drs. T. Matsushima, R. Inoue, and M. Ochiai for their help in the production of mouse liver tumors and Ms. Y. Sotome for her excellent assistance with the sequencing analysis. This work was supported in part by a Grant-in-Aid from the Ministry of Health and Welfare for the 2nd Term Comprehensive 10-Year Strategy for Cancer Control, Japan, and by a Grant-in-Aid for Cancer Research from the Ministry of Education, Science, and Culture. K.M. and H.O. are recipients of Research Resident Fellowships from the Japanese Foundation for Promotion of Cancer Research.
ABBREVIATIONS
- HCC
hepatocellular carcinoma
- RDA
representational difference analysis
- MS-RDA
methylation-sensitive RDA
- RDA-WEEC
RDA with elimination of excessive clones
- MeIQ
2-amino-3,4-dimethylimidazo[4,5-f]quinoline
- LINE
long interspersed nuclear element
References
- 1.Li E, Bestor T H, Jaenisch R. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 2.Guillaudeux T, D’Almeida M, Girr M, Rodriguez A M, Pontarotti P, Fauchet R, Le Bouteiller P. Am J Reprod Immunol. 1993;30:228–238. doi: 10.1111/j.1600-0897.1993.tb00624.x. [DOI] [PubMed] [Google Scholar]
- 3.Kudo S, Fukuda M. J Biol Chem. 1995;270:13298–13302. doi: 10.1074/jbc.270.22.13298. [DOI] [PubMed] [Google Scholar]
- 4.Thakur M K, Kaur J. Cell Mol Biol. 1992;38:525–532. [PubMed] [Google Scholar]
- 5.Li E, Beard C, Jaenisch R. Nature (London) 1993;366:362–365. doi: 10.1038/366362a0. [DOI] [PubMed] [Google Scholar]
- 6.Kirillov A, Kistler B, Mostoslavsky R, Cedar H, Wirth T, Bergman Y. Nat Genet. 1996;13:435–441. doi: 10.1038/ng0895-435. [DOI] [PubMed] [Google Scholar]
- 7.el-Deiry W S, Nelkin B D, Celano P, Yen R W, Falco J P, Hamilton S R, Baylin S B. Proc Natl Acad Sci USA. 1991;88:3470–3474. doi: 10.1073/pnas.88.8.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Counts J L, Goodman J I. Mol Carcinog. 1994;11:185–188. doi: 10.1002/mc.2940110402. [DOI] [PubMed] [Google Scholar]
- 9.Counts J L, Goodman J I. Cell. 1995;83:13–15. doi: 10.1016/0092-8674(95)90228-7. [DOI] [PubMed] [Google Scholar]
- 10.Goelz S E, Vogelstein B, Hamilton S R, Feinberg A P. Science. 1985;228:187–190. doi: 10.1126/science.2579435. [DOI] [PubMed] [Google Scholar]
- 11.Dante R, Dante-Paire J, Rigal D, Roizes G. Anticancer Res. 1992;12:559–563. [PubMed] [Google Scholar]
- 12.Gonzalez-Zulueta M, Bender C M, Yang A S, Nguyen T, Beart R W, Van Tornout J M, Jones P A. Cancer Res. 1995;55:4531–4535. [PubMed] [Google Scholar]
- 13.Herman J G, Merlo A, Mao L, Lapidus R G, Issa J P, Davidson N E, Sidransky D, Baylin S B. Cancer Res. 1995;55:4525–4530. [PubMed] [Google Scholar]
- 14.Herman J G, Latif F, Weng Y, Lerman M I, Zbar B, Liu S, Samid D, Duan D S, Gnarra J R, Linehan W M, Baylin S B. Proc Natl Acad Sci USA. 1994;91:9700–9704. doi: 10.1073/pnas.91.21.9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshiura K, Kanai Y, Ochiai A, Shimoyama Y, Sugimura T, Hirohashi S. Proc Natl Acad Sci USA. 1995;92:7416–7419. doi: 10.1073/pnas.92.16.7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graff J R, Herman J G, Lapidus R G, Chopra H, Xu R, Jarrard D F, Isaacs W B, Pitha P M, Davidson N E, Baylin S B. Cancer Res. 1995;55:5195–5199. [PubMed] [Google Scholar]
- 17.Ottaviano Y L, Issa J P, Parl F F, Smith H S, Baylin S B, Davidson N E. Cancer Res. 1994;54:2552–2555. [PubMed] [Google Scholar]
- 18.Ferguson A T, Lapidus R G, Baylin S B, Davidson N E. Cancer Res. 1995;55:2279–2283. [PubMed] [Google Scholar]
- 19.Makos M, Nelkin B D, Reiter R E, Gnarra J R, Brooks J, Isaacs W, Linehan M, Baylin S B. Cancer Res. 1993;53:2719–2722. [PubMed] [Google Scholar]
- 20.Kanai Y, Ushijima S, Tsuda H, Sakamoto M, Sugimura T, Hirohashi S. Jpn J Cancer Res. 1996;87:1210–1217. doi: 10.1111/j.1349-7006.1996.tb03135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawai J, Hirose K, Fushiki S, Hirotsune S, Ozawa N, Hara A, Hayashizaki Y, Watanabe S. Mol Cell Biol. 1994;14:7421–7427. doi: 10.1128/mcb.14.11.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lisitsyn N, Lisitsyn N, Wigler M. Science. 1993;259:946–951. doi: 10.1126/science.8438152. [DOI] [PubMed] [Google Scholar]
- 23.Toyota M, Canzian F, Ushijima T, Hosoya Y, Kuramoto T, Serikawa T, Imai K, SUgimura T, Nagao M. Proc Natl Acad Sci USA. 1996;93:3914–3919. doi: 10.1073/pnas.93.9.3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohgaki H, Hasegawa H, Suenaga M, Kato T, Sato S, Takayama S, Sugimura T. Carcinogenesis. 1986;7:1889–1893. doi: 10.1093/carcin/7.11.1889. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. pp. 9.14–9.23. [Google Scholar]
- 26.Tahira T, Ishizaka Y, Itoh F, Sugimura T, Nagao M. Oncogene. 1990;5:97–102. [PubMed] [Google Scholar]
- 27.Warburg O H. Ueber den Stoffwechsel der Tumoren. Berlin: Springer; 1926. [Google Scholar]
- 28.Kochanek S, Renz D, Doerfler W. EMBO J. 1993;12:1141–1151. doi: 10.1002/j.1460-2075.1993.tb05755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugimura T. In: Chemical Tumor Problems. Nakahara W, editor. Tokyo: Japanese Society for the Promotion of Science; 1970. pp. 269–284. [Google Scholar]